Abstract
BACKGROUND
The epididymis performs an important role in the maturation of spermatozoa including their acquisition of progressive motility and fertilizing ability. However, the molecular mechanisms that govern these maturational events are still poorly defined. This review focuses on recent progress in our understanding of epididymal function including its development, role of the luminal microenvironment in sperm maturation, regulation and novel mechanisms the epididymis utilizes to carry out some of its functions.
METHODS
A systematic search of Pubmed was carried out using the search term ‘epididymis’. Articles that were published in the English language until the end of August 2008 and that focused on the specific topics described above were included. Additional papers cited in the primary reference were also included.
RESULTS
While the majority of these findings were the result of studies in animal models, recent studies in the human epididymis are also presented including gene profiling studies to examine regionalized expression in normal epididymides as well as in those from vasectomized patients.
CONCLUSIONS
Significant progress has been made in our understanding of epididymal function providing new insights that ultimately could improve human health. The data also indicate that the human epididymis plays an important role in sperm maturation but has unique properties compared with animal models.
Keywords: epididymis, sperm maturation, human, rodent
Introduction
Spermatozoa leaving the testis and entering the long convoluted tubule known as the epididymis are non-functional gametes. It is only during transit through the epididymis that spermatozoa undergo maturation and acquire progressive motility and the ability to fertilize ova. Because spermatozoa are, for the most part, synthetically inactive, maturation involves the interaction of spermatozoa with proteins that are synthesized and secreted in a region-dependent manner from the epididymal epithelium. Despite considerable effort, the molecular and biochemical events that are integral for epididymal sperm maturation are unknown.
The importance of understanding epididymal function and sperm maturation is emphasized by the fact that up to 40% of infertile men exhibit idiopathic infertility that may reflect sperm maturational disorders. Unfortunately, owing to the lack of alternative therapies, these patients and their partners require assisted reproductive techniques (ART) such as intracytoplasmic sperm injection (ICSI), which utilizes spermatozoa independent of maturational status, to achieve a pregnancy. Although effective, because natural selection processes that prevent suboptimal spermatozoa from fertilizing ova are bypassed, there can be increased risks of genetic abnormalities being transmitted to the offspring (Cox et al., 2002; Merlob et al., 2005; Georgiou et al., 2006; Fedder et al., 2007; Sanchez-Albisua et al., 2007). If the mechanisms of sperm maturation were established, it is possible that sperm could be matured in vitro providing an alternative therapy to current reproductive technologies.
The significance of the lack of understanding regarding the functional role of the epididymis in sperm maturation is also underscored by the lack of contraceptives for men. Although much work has been put into developing hormonal methods that interfere with sperm production in the testis, these approaches have been hampered by cumbersome regimes, extended times before efficacy is achieved, and possible side effects of the administered hormones. Interest has shifted to include identifying epididymal molecules that could serve as targets for non- steroidal-based male contraceptives with the idea that sperm production would occur normally but the spermatozoa would be non-functional. Clearly, if we are to improve human health by developing new and better ways to improve as well as prevent fertility, further research into the epididymis is needed.
The purpose of this review is to provide a general background of the epididymis followed by a brief overview of recent progress in the field including advances in our understanding of the epididymal epithelium and its regulation, composition and function of the luminal fluid, as well as changes occurring in spermatozoa during epididymal transit. Because of the limited availability of epididymal tissue from healthy men of reproductive age, the lack of appropriate in vitro models, and the constraint to manipulate the human epididymis experimentally, the majority of these studies have been carried out in rodent models. However, as discussed below, genomic and proteomic analyses of the human epididymis have revealed valuable new information which lends support to the view that, although there may be species differences with regard to where in the epididymis spermatozoa acquire their functions, the human epididymis does serve a role in the functional maturation of spermatozoa.
Materials and Methods
A systematic search of Pubmed was carried out using the search term ‘epididymis’. Articles that were published in the English language until the end of August 2008 and that focused on the specific topics described above were included. Additional papers cited in the primary reference were also taken into account. This review incorporates background material presented in a previous review (Robaire et al., 2006) but includes recent advancements as well as topics not covered or presented with a different emphasis such as the human epididymis and components of the epididymal luminal environment.
Results
Epididymal development
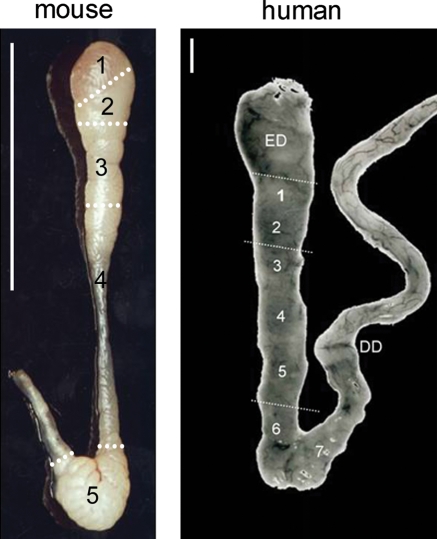
On the basis of histological and ultrastructural differences, the epididymis can be grossly divided into three regions including the caput (head), corpus (body) and cauda (tail) epididymidis. The most proximal epididymal region, in some species such as the mouse, is also known as the initial segment (Fig. 1). Each epididymal region carries out distinctive functions with the caput and corpus carrying out early and late sperm maturational events, respectively, while the cauda region primarily serves as a storage site for functionally mature spermatozoa.
Figure 1.
Mouse and human epididymides.
Mouse: 1, initial segment, proximal caput; 2, midcaput; 3, distal caput; 4, corpus; 5, cauda. Adapted (regional divisions by the author) from Trevor Cooper, University of Muenster (Yeung and Cooper, 2002), with permission of author and publisher, Springer. Human: ED, efferent ducts; 1, anterior caput; 2, posterior caput; 3, anterior corpus; 4, midcorpus; 5, posterior corpus; 6, anterior cauda; 7, posterior cauda; DD, ductus deferens. Reprinted by permission of the author (Dacheux et al., 2006), UMR INRA-CNRS and publisher, Elsevier Limited. Scale bar, 1 cm.
The epididymis is derived from the Wolffian duct and at birth consists mainly of mesenchymal tissue. The epididymis undergoes considerable remodeling including duct elongation and convolution so that by puberty the epididymis has acquired its fully differentiated state consisting of a highly tortuous tubule lined by epithelial cells (Rodriguez et al., 2002). The development of a fully differentiated epithelium is dependent not only on androgens but also requires the influence of luminal factors from the testis (Rodriguez et al., 2002). Considering that the adult epididymis exhibits region-specific characteristics, it is not surprising that homeobox genes, such as Hox genes that control segmental patterning during development, are expressed during epididymal development and participate in the appearance of segment-specific differences (Rao and Wilkinson, 2002). Although studies have established circulating androgens and luminal factors as playing a necessary role in the development of the epididymis, less is known of other factors that mediate the series of morphogenic events that result in the formation of the adult epididymis (Lei et al., 2003; Zhang et al., 2004).
Epithelial–mesenchymal interactions
Several studies have implicated epithelial-mesenchymal interactions as integral for epididymal morphogenesis. Indeed, early studies showed when proximal regions of Wolffian duct epithelium were cultured on seminal vesicle mesenchyme, the epithelium differentiated into seminal vesicle epithelium (Higgins et al., 1989). Bone morphogenetic proteins (BMP), members of the transforming growth factor β (TGFβ) superfamily, and their receptors may be involved in this interaction since disruption of the Bmp4, Bmp7 and Bm8a and Bmp8b genes results in epididymal degeneration that is region-specific (Zhao et al., 1998, 2001; Chen et al., 1999; Hu et al., 2004). C-ros, (ROS1), a member of the tyrosine kinase receptor family, may also play an essential role in epididymal development, since mice lacking the rosI gene lack the initial segment and are sterile (Sonnenberg-Riethmacher et al., 1996). Because during kidney development, ROS1 is thought to regulate the extracellular matrix, a storage site for growth factors (Liu et al., 1996), a similar mechanism of action may also occur in the epididymis. Mice with mutations in the SH2 domain protein tyrosine phosphatase (SHP-1) gene [‘motheaten’ (me), ‘viable motheaten’ (mev)] exhibit an aberrant proximal epididymal region similar to that in the c-ros knockout (Keilhack et al., 2001). Since SHP-1 and c-ROS are coexpressed in the epididymis and interact in vitro, SHP-1 was proposed to function as a regulator of c-ros signaling (Keilhack et al., 2001).
LGR4, a leucine-rich repeat domain containing G protein-coupled receptor (GPCR) 4 also appears critical for epididymal development since in the LGR4 (Lgr4) knockout mouse the epididymal tubule, especially in the caput, fails to elongate and convolute and the resulting duct is surrounded by a thick condensation of mesenchymal cells (Mendive et al., 2006). The abnormal arrangement of the epithelium and mesenchyme in the LGR4 knockout suggested that altered interactions between these two compartments may cause the phenotype (Mendive et al., 2006). The LGR4 hypomorphic mutant mouse (Lgr4Gt) also exhibits altered post-natal development of the epididymis that lacks the initial segment. Examination of the epididymal ultrastructure demonstrated disruption of the extracellular matrix with an increase in laminin (Hoshii et al., 2007). Thus Lgr4, as postulated for c-ros, may regulate epididymal morphogenesis via maintenance of the extracellular matrix.
Most recently, inhibin beta A, a mesenchyme-derived paracrine factor, was shown to control the coiling of the epithelium in the anterior Wolffian duct, the Anlage of the adult epididymis, thus providing evidence in vivo that interactions between the epithelial and mesenchyme compartments are essential for proper development of the epididymis (Tomaszewski et al., 2007). These studies also demonstrated that the regulation of epididymal coiling was not directly controlled by androgens since the Inhba knockout embryos exhibited normal androgenic parameters.
Epididymal cell structure and function
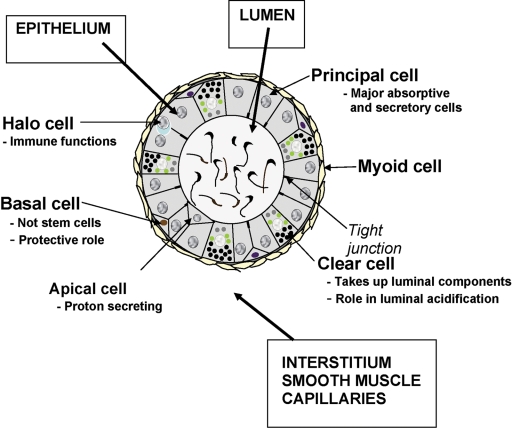
The adult epididymis consists of a pseudostratified epithelium of several cell types including principal, basal, clear, narrow, apical and halo cells (Fig. 2). The primary cell type throughout the tubule is the principal cell which constitutes ∼80% of the epithelium and is, by far, the most studied since it is responsible for the bulk of the proteins that are secreted into the lumen. Less is known regarding the function of the remaining cell types; however, narrow, apical and clear cells contain the vacuolar H+-ATPase and secrete protons into the lumen and thus participate in its acidification (Pietrement et al., 2006; Kujala et al., 2007), while clear cells are also endocytic cells and may be responsible for clearance of proteins from the epididymal lumen. Basal cells do not access the luminal compartment and are in close association with the overlying principal cells, as indicated by the presence of basal cell cytoplasmic extensions between principal cells, and thus may regulate its functions (Veri et al., 1993; Seiler et al., 1999). Halo cells appear to be the primary immune cell in the epididymis, while apical cells may also endocytose luminal components. It is likely that the individual cell types within the epithelium may perform separate as well as integrated functions within the epididymis. In support of this view, recent studies demonstrated that basal cells regulate principal cell electrolyte transport by releasing paracrine factors, specifically by the release of prostaglandin PGE2 (Cheung et al., 2005). Thus, cell–cell interactions within the epithelium can directly affect the luminal environment and ultimately sperm maturation. The principal cells also form tight junctions with one another and as such form the blood–epididymis barrier. This barrier creates an immunoprotective site within the epididymal lumen that is necessary for sperm maturation. Several androgen-dependent transmembrane proteins including occludin and claudins contribute to the formation of these tight junctions (Cyr et al., 2007). Gap junctions formed by a family of integral proteins known as connexins, are also present between adjacent principal cells both at their apical and lateral surfaces. These structures, which consist of aligned intercellular pores, allow the transport of molecules <1 kDa.
Figure 2.
Schematic diagram of the cellular organization in a representative cross-section of the rat epididymis. Modified and reprinted with permission of the author (Robaire et al., 2003), McGill University and publisher, The Van Doren Co, Charlottesville, VA.
Region-specific microenvironments
Within the principal cells, gene expression and subsequently protein synthesis and secretion are tightly regulated and region-specific such that neighboring cells may express very different subsets of genes and gene products. This region-dependent expression contributes to the distinctive luminal protein profile within each epididymal region which is thought to be integral for sperm maturation. While previously it was thought that varying patterns of gene expression along the tubule was loosely associated with different epididymal regions, Turner et al. (2003 demonstrated that the presence of connective tissue septa further subdivides the caput, corpus and cauda epididymidis into discrete intra-regional segments and that region-specific gene expression may in fact be highly ordered and compartmentalized within these precise segments. By using size exclusion dyes and radiolabeled molecules, these authors further demonstrated that the connective tissue septa may also act as barriers restricting the movement of molecules from the interstitial space of one segment to the next. This would allow segment-specific paracrine signaling to occur between stromal and epithelial cells that could regulate the tightly controlled segment-specific expression of genes. Supporting this view, microperifusion studies have demonstrated that the effects of perifused growth factors including epidermal growth factor (EGF), fibroblast growth factor (FGF2) and vascular endothelial growth factor (VEGFA) on epithelial cell mitogen-activated protein kinase (MAPK) signaling was restricted to the perifused region only and not neighboring epididymal segments, presumably reflecting a functional barrier created by the connective tissue septa. However, when growth factors were simultaneously perifused with collagenase, that degrades components of the connective tissue septa, MAPK signaling was activated in both the perifused and adjacent epididymal segments (Turner et al., 2007).
Thus, the epididymal tubule is a highly ordered and segmented organ with each segment representing a unique physiological compartment. Each compartment possesses distinctive gene expression profiles within the epithelium that dictate segment-specific secretion of proteins into the luminal fluid directly or indirectly affecting sperm maturation. Segment-specific expression of genes encoding signaling molecules, regulatory proteins, transporters and receptors also contribute to the formation of special microenvironments by allowing the epithelium to respond uniquely to different stimuli such as hormones and other regulatory factors. Identifying and determining the function of segment-specific proteins is of paramount importance for understanding epididymal sperm maturation. For this reason a number of gene profiling studies have been carried out in attempts to identify genes exhibiting regionalized expression in the epididymis (Jervis and Robaire, 2001; Penttinen et al., 2003; Hsia and Cornwall, 2004; Johnston et al., 2005; Oh et al., 2006; Zhang et al., 2006; Jelinsky et al., 2007; Thimon et al., 2007; Li et al., 2008;) as well as proteomic studies to identify proteins (Chaurand et al., 2003; Dacheux et al., 2006; Yuan et al., 2006). These studies have yielded large datasets including novel sequences as well as genes with known identities but previously not known to be expressed by the epididymis. Because of the vast amount of data associated with these studies and the availability of the data to the public, specific genes will not be discussed here, other than to mention that gene sequences included those as potential proteases and protease inhibitors, defensins, transporters, transcription factors, as well as genes associated with metabolism, cell signaling and part of the antioxidant system.
Regulation of epididymal cell function
Control by luminal fluid
The epididymis is highly dependent on androgens, primarily dihydrotestosterone, for function and will be reduced to 25% of its normal weight following castration. Restoration of circulating testosterone reverses the cellular changes in the caput, corpus and cauda epididymidis but not in the initial segment (Ezer and Robaire, 2002). Early studies suggested that the initial segment was controlled by additional regulatory factors since ligation of the efferent ducts, which connect the testis to the epididymis and are the passageway for spermatozoa and luminal components, resulted in a profound regression of the initial segment region (Fawcett and Hoffer, 1979). Because efferent duct ligation does not affect circulating androgen levels, these studies suggested that the maintenance of initial segment morphology required components in the luminal fluid, i.e. lumicrine regulation, including spermatozoa (Scheer and Robaire, 1980; Garrett et al., 1990; Hinton et al., 1998; Turner and Riley, 1999). Gene expression studies together with expression profiling revealed a subset of initial segment-expressed genes, including those encoding secretory proteins that are down-regulated following efferent duct ligation such Cst8 (CRES), Ggt_pr4 (GGT), Gpx5 (GPX), Lcn8 (MEP17), Etv4 (PEA3) and others suggesting that luminal factors are not only needed for the maintenance of initial segment morphology but for function as well (Scheer and Robaire, 1980; Garrett et al., 1990; Cornwall et al., 1992; Hinton et al., 1998; Hsia and Cornwall, 2003; Avram et al., 2004; Sipila et al., 2006). Because the mRNAs for several of these genes were profoundly decreased within hours after efferent duct ligation, argues for a direct effect of the loss of testicular factors on gene expression rather than indirect effects due to regression of the epithelium (Hinton et al., 1998) (Cornwall, unpublished observations).
Control by luminal proteins
While it is not known if one or many testicular factors are required to maintain initial segment function, studies by Lan et al. (1998) suggested that bFGF may be one such factor. Administration in vitro of FGF2 but not EGF to efferent duct ligated rats restored Ggt_pr4 mRNA, protein and activity in the initial segment to control levels. Furthermore, these investigators proposed that FGF may elicit its effects on Ggt_pr4 gene expression via activation of the ras-raf-MAPK pathway and downstream activation of the ETV4 transcription factor (Hinton et al., 1998; Lan et al., 1998). Most recently, studies by these investigators suggested, not surprisingly, that not all testis-regulated genes respond in the same way to changes in ETV4 transcriptional activity. The administration of a ETV5-dominant negative plasmid by in vivo electroporation to the rat initial segment, resulted in the down-regulation of ETV5, ETV4 and ETV1 mRNAs in the initial segment as well as putative target genes Ggt_pr4, Srd5a1 (steroid 5 alpha reductase) and Gpx5 (Yang et al., 2006). However, although the testis-regulated genes Cst8 and Lcn8 contain ETS binding sites within their promoters, they did not respond to the dominant negative, suggesting that there either may be several testicular factors each differentially regulating specific subsets of genes or that one or a few testicular factors may mediate different downstream effects via the activation of multiple signaling pathways and subsequent effector molecules (Yang et al., 2006). In support of alternative signaling pathways that mediate initial segment function, the mRNAs of Cst8 and the related cystatin E2 as well as Lcn8 and Lcn9, all of which depend on luminal factors for expression, are profoundly down-regulated in mice lacking the HE6/Gpr64 gene, a member of the LNB-7TM subfamily of GPCR expressed in the epithelium of both the efferent ducts and initial segment (Davies et al., 2007). Because preliminary studies suggested HE6/Gpr64 interacted with a profilin-like molecule, known regulators of the cytoskeleton, HE6/Gpr64 may regulate the microenvironment of the initial segment by its association with adaptor/scaffolding proteins ultimately affecting signal transduction pathways and downstream target genes (Kirchhoff et al., 2006, 2008). Indeed, mice lacking the HE6/Gpr64 gene are infertile owing to dysregulation of fluid resorption in the efferent ducts (Davies et al., 2004).
Control by lipids
Other molecules that appear critical for the maintenance of the epididymal epithelium and subsequently sperm maturation include oxysterols, derivatives of cholesterol. Mice lacking the nuclear oxysterol receptor (LXR) α and β isoforms exhibited a disruption of the caput epididymidis characterized by a localized disruption of the epithelium, especially in the proximal caput regions, including the accumulation of lipids, and an accumulation of amorphous material in the epididymal lumen (Frenoux et al., 2004; Saez et al., 2007). Spermatozoa within the lumen were structurally abnormal with detached heads and tail angulation. Curiously, the effects were not observed until rather abruptly around 6 months of age suggesting that compensatory mechanisms may for a time prevent the expression of the phenotype due to the loss of LXR. The LXRs bind DNA as obligate heterodimers with retinoid X receptors and control the elimination of cholesterol by regulating genes involved in its catabolism, transport and uptake. Thus in addition to proteinaceous factors, lipids also play an integral role in the regulation of epididymal function.
Control by spermatozoa
Other studies suggest that in addition to factors in the fluid, sperm cells themselves may regulate the epididymal epithelium (Garrett et al., 1990). Ejaculated bovine spermatozoa washed free of seminal plasma and incubated with primary cultures of caput, corpus and cauda epididymidal cells affect the epithelial secretory profile in an epididymal region and temperature-dependent manner (Reyes-Moreno et al., 2008). Although further studies are needed to determine whether similar results are obtained with testicular or epididymal spermatozoa, these studies provide tantalizing evidence that cell–cell communication between spermatozoa and the epithelium may direct epididymal function.
Signaling molecules and transcription factors
Several molecules that play roles in the developing embryo are also expressed in the adult epididymis and may regulate epididymal functions. These include the Rhox (reproductive homeobox X-linked) and ladybird-like homeobox genes (Lbx) and sonic hedgehog (Shh). Homeobox genes encode transcription factors that typically regulate developmental events including limb development and organogenesis but can also be expressed in adult tissues. The Rhox genes are a cluster of over 30 genes that are expressed in a cell type-specific manner in reproductive tissues including the epididymis (Maclean et al., 2005). Rhox 5, in particular, may function in sperm maturation since spermatozoa from mice lacking Rhox5 exhibited reduced fertility in part because of impaired sperm motility. Because the individual Rhox family members exhibit different region-specific expression patterns in the epididymis, as well as diverse amino acid motifs that interact with the DNA, it suggests that they regulate a broad range of downstream target genes and biological functions. The homeobox gene Lbx2, typically known to be expressed in the nervous system, is expressed in a region-dependent manner in the epididymis and thus may also contribute to the development of the epididymis as well as regulation of adult epididymal function (Moisan et al., 2008). Hedgehog proteins are extracellular signaling molecules that play roles in the regulation of patterning processes during embryonic development. Shh is also expressed in the adult epididymis (Turner et al., 2006). Inhibition of the Shh pathway by the administration of cyclopamine reduced the ability of mouse cauda epididymidal spermatozoa to initiate motility following dilution suggesting that Shh was important for sperm motility maturation (Turner et al., 2006).
The forkhead transcription factors carry out multiple roles in the epididymis. Several studies suggest that Foxa family members such as Foxa2 [Fox(forkhead box) subclass A], play a role in steroid hormone-responsive gene promoters including that for lipocalin 5 (Lcn5) (Yu et al., 2006). The epididymal expression of Foxi1 is also necessary for normal sperm function since spermatozoa from mice lacking Foxi1 showed a high incidence of tail angulation and a decreased ability to migrate through the female tract resulting in decreased fertility (Blomqvist et al., 2006). Foxi1 in the epididymal narrow and clear cells regulates the expression of the B1-subunit of the vacuolar H+-ATPase proton pump as well as carbonic anhydrase II and the chloride/bicarbonate transporter pendrin. Because acidification of the epididymal lumen requires the function of the vacuolar H+-ATPase proton pump and is necessary for sperm maturation (Yeung et al., 2004; Pastor-Soler et al., 2005), the fertility defect in mice lacking Foxi1 may reflect impaired epididymal sperm maturation as a result of increased luminal pH.
Epididymal luminal environment
The majority of studies in the epididymis have focused on identifying specific epididymal secretory proteins and their functional roles in sperm maturation ultimately to identify novel targets for male contraception or provide new treatments for male infertility. However, equally important as these individual proteins, is understanding the complex epididymal luminal milieu as a whole, since perturbations in the microenvironment that surrounds the sperm cell could affect maturation. Indeed, alterations in the luminal pH affect sperm maturation (Yeung et al., 2004; Pastor-Soler et al., 2005). The epididymal lumen is also rich in inorganic ions and small organic molecules which create an environment that is hyperosmotic relative to serum (Turner, 2002). During epididymal transit, spermatozoa may acquire the capacity to regulate their cell volume, possibly by the uptake of luminal components that function as intracellular osmolyte reserves, so that upon exposure to the iso-osmotic secretions of the female tract osmotic shock does not occur (Cooper and Yeung, 2003; Cooper, 2007). Osmolites may also function within the epididymal lumen to affect protein folding or interactions.
The epididymal lumen contains perhaps the most complex fluid found in any exocrine gland resulting from the continuous changes in composition as well as the presence of components in unusually high concentrations for reasons not yet known, or those not present in other body fluids (Dacheux and Dacheux, 2002). The caput epididymidis is the most metabolically active region secreting 70–80% of the total overall protein secretion in the epididymal lumen. Moreover, by the time spermatozoa migrate into this region, 99% of the fluid accompanying them has been resorbed, resulting not only in a profound concentration of spermatozoa but luminal components as well (Clulow et al., 1998). While such an environment may be integral for sperm maturation, an environment low in water content creates a situation of macromolecular crowding which places unique stresses on luminal proteins that can alter their behavior and lead to protein misfolding and aggregation (Minton, 2005).
Other stressors such as inappropriate ionic strength, oxidative stress pH and temperature extremes are also known to promote the unfolding of fully folded native proteins and the formation of misfolded, aggregated proteins which can be cytotoxic. Considering that this same epididymal environment must protect spermatozoa and allow maturation, it is likely that mechanisms are in place to prevent or remove aggregated proteins.
Several recently published reports provide evidence that the epididymal fluid does not just consist of a large pool of soluble proteins in their native conformations, but rather also contains proteinaceous aggregate structures of varying molecular mass. In particular, the amyloidogenic prion protein is present in the epididymal lumen both in insoluble exosome-like membranous vesicles (epididymosomes) (Ecroyd et al., 2004), and in a soluble highmolecular mass lipophilic complex in association with hydrophobic proteins (Ecroyd et al., 2005). The chaperone clusterin, which is known to interact with hydrophobic proteins to maintain their solubility, is also detected in the soluble prion protein complex. This suggests that these structures may be a means to maintain proteins in their soluble state either to prevent aggregation and precipitation and enable clearance or to allow hydrophobic proteins to be transferred between cells such as the epididymal epithelium and spermatozoa. It must also be considered that high molecular mass proteinaceous structures in the epididymal lumen carry out biological functions.
Other evidence for the presence of protein aggregates in the epididymal lumen comes from studies examining molecular chaperones in the reproductive tract. Both heat-shock protein 1 (HSPD1, HSP60) and tumor rejection antigen 1 (TRA1, a member of the heat-shock protein 90 family) colocalize to large electron-dense bodies in the epididymal lumen. These structures seem not to be membrane-bound and are larger than epididymosomes suggesting unique structures (Asquith et al., 2005). Because proposed functions of TRA1 include the folding of denatured proteins as well as multimer assembly (Nigam et al., 1994), its function in epididymal lumen may be as a means of extracellular quality control, specifically to refold proteins from non-native (denatured or misfolded and potentially aggregation prone) to native conformations or alternatively to facilitate clearance from the epididymal lumen.
Our studies of the cystatin CRES in the epididymal luminal fluid demonstrated that it was present not only in monomeric forms but in soluble SDS-sensitive and SDS-resistant forms as well as insoluble forms as defined by its precipitation following high-speed centrifugation (von Horsten et al., 2007). Within the epididymis CRES is synthesized and secreted by the proximal caput epididymidal epithelium, accumulates in the lumen of the midcaput, but abruptly disappears from the distal caput epididymidal lumen (Cornwall and Hann, 1995). Although the mechanism(s) for the sudden disappearance of CRES is not known, its self-aggregation to high-molecular mass forms may contribute to the inability to detect the monomeric forms of CRES in distal epididymal regions. As found for cystatin C, which is a proven amyloidogenic protein, studies of CRES demonstrated that it also forms amyloid in vitro, raising the possibility of amyloid formation within the epididymal lumen (von Horsten et al., 2007).
Because amyloid structures can be cytotoxic and associated with disease, it is likely that the epididymis has mechanisms to guard against the cytotoxicities of protein aggregates. While intracellular mechanisms to control aggregated proteins are well-characterized, to date little is known in any organ system mechanisms that control proteins that may aggregate once they are secreted into the extracellular space. Our studies of CRES demonstrated that it is a substrate for transglutaminase cross-linking and that following exposure to transglutaminase, CRES aggregation was not of the amyloid-type (von Horsten et al., 2007). Thus, transglutaminase cross-linking may be one mechanism the epididymis utilizes to regulate the formation of potentially cytotoxic aggregate structures.
Until recently, evidence did not support a role for the ubiquitin/proteosome pathway in extracellular control in any organ system with the exception of studies in the epididymis showing that ubiquitin was associated with structures in the luminal fluid (Fraile et al., 1996; Sutovsky et al., 2001). Most recently, a biologically active proteosome was identified in the human alveolar space in the lung (Sixt et al., 2007), while further studies in the epididymis demonstrated that components of the ubiquitin pathway including ubiquitin activating enzyme E1, ubiquitin carrier enzyme E2 and ubiquitin C-terminal hydrolase PGP9.5/UCHL1 are present and active within the epididymal luminal fluid (Baska et al., 2008). Thus in the epididymis, pathways that normally function within the cell may also occur in the extracellular environment and as a result provide the mechanisms to appropriately maintain the luminal environment ultimately protecting the maturing spermatozoa. Indeed, spermatozoa from mice lacking the ubiquitin ligase Herc4 exhibit angulated tails, reduced motility and reduced fertility supporting a role for the ubiquitin pathway in sperm maturation (Rodriguez and Stewart, 2007).
Epididymosomes
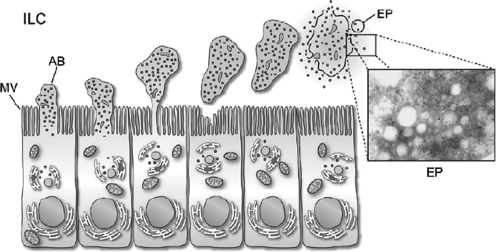
The epididymis utilizes common as well as unique mechanisms to deliver secretory proteins to the sperm surface. Most secreted proteins possess the typical signal sequences indicating trafficking through the Golgi and subsequent packaging and release from secretory granules (merocrine secretion). However, several studies have shown that epididymal spermatozoa also acquire proteins that lack signal sequences suggesting an unusual secretion pathway in the epithelium. Differential extraction of spermatozoa indicates some proteins act like integral membrane proteins and, in fact, are thought to be anchored to the sperm plasma membrane by a glycosylphosphatidylinositol anchor (Kirchhoff and Hale, 1996; Cooper, 1998). In the epididymal lumen, several of these proteins are associated with membranous vesicles known as epididymosomes. While previously thought to be an artefact of fixation, these small membrane-bound vesicles originate from the epididymal epithelial cells in a process known as apocrine secretion. This type of secretion involves the formation of apical blebs containing various sized vesicles from the epithelial cells and once the blebs have detached they are thought to fragment and release the small vesicles (Aumuller et al., 1999) (Fig. 3). Although similar type vesicles have been known for some time to be secreted by the prostate (prostasomes) and present in the semen where they have proposed roles as protection for sperm against complement, enhancement of motility and stabilization of the sperm membrane, Yanagimachi et al. (1985) was the first to describe such vesicles in the epididymal lumen and showed interactions of these vesicles with spermatozoa.
Figure 3.
Schematic diagram of apocrine secretion in principal cells of the epididymis. Inset, electron micrograph of epididymosomes. AB, apical bleb; EP, epididymosome; ILC, intraluminal compartment; MV, microvilli. Reprinted with permission of the author, University of Laval and the publisher Blackwell publishing (Sullivan et al., 2007).
Analysis of proteins associated with the epididymosomes reveals protein profiles quite different from that of proteins in the lumen. Proteins associated with epididymosomes include P26h, believed to be involved in zona pellucida binding, HE5, macrophage migration inhibitory factor, ubiquitin and glutathione peroxidase, all of which have been shown to be transferred to spermatozoa in the epididymis (Kirchhoff and Hale, 1996; Sutovsky et al., 2001; Frenette et al., 2003; Saez et al., 2003). However, not all proteins associated with epididymosomes are transferred to spermatozoa suggesting that only some proteins have the ability to be transferred or that complete fusion and transfer of vesicles to spermatozoa does not occur (Sullivan et al., 2005). Studies examining epididymosomes in the cauda fluid from the ovine epididymis identified different subsets of proteins present in these vesicles including dipeptidyl peptidase V, neprilysin, mannosidase and actin, but observed no interactions of such vesicles with spermatozoa also suggesting that transfer of proteins may be by a subtle exchange rather than complete fusion (Gatti et al., 2005). It is also possible that epididymosomes are heterogeneous with different protein compositions depending on downstream functions.
The functional significance of epididymosomes in sperm maturation remains to be elucidated. It is possible that they evolved to ensure the safe delivery of some proteins to the sperm cell and perhaps to particular sperm domains without possible damage by luminal proteases. Alternatively, given the complexities of sperm maturation and the vast multitude of cellular and extracellular events that the epididymis must carry out for maturation to occur, it is also possible that the epididymis has developed new strategies to deliver cellular proteins and their associated functions to the sperm surface rather than synthesize a secretory protein that carries out the same function as its cellular form. It is also possible that, in addition to the delivery of proteins to the sperm surface, the epididymosomes act as signaling centres or scaffolds within the luminal compartment affecting protein function independently of that associated with spermatozoa. Indeed, the epididymosomes are believed to contain lipid raft-like structures which in somatic and germ cells are thought to function as sites of signaling complexes (Brown and London, 1998). Finally, it is possible that epididymosomes contribute to the clearance of proteins from the lumen by binding and delivering proteins to cells for endocytosis.
Sperm maturation
As spermatozoa migrate from the proximal to the distal regions of the epididymis, they undergo a series of morphological, biochemical and physiological changes with the end result being spermatozoa that have acquired the function of progressive motility and the ability to fertilize an ovum. Microscopic studies have demonstrated that during epididymal transport, spermatozoa undergo remodeling that includes changes in the dimension and appearance of the acrosome and nucleus, in some species migration of the cytoplasmic droplet along the tail, as well as structural changes in intracellular organelles (Olson et al., 2002).
The sperm plasma membrane, a highly compartmentalized structure, in particular, is modified during transit with changes in overall phospholipids and cholesterol (Jones et al., 2007). Spatially separated lipids and proteins are re-organized during maturation possibly allowing the formation of signaling complexes critical for fertilization. Lipid rafts have been described in mouse, guinea pig, human, and porcine spermatozoa (Travis et al., 2001; Cross, 2004; Shadan et al., 2004) and may function as large multimolecular signaling assemblies that re-organize and aggregate during capacitation inducing signaling cascades. In many species, a reduction in sperm cholesterol is one of the first steps that triggers signal transduction cascades during capacitation including tyrosine phosphorylation of sperm proteins. Removal of cholesterol from immature testicular spermatozoa does not induce tyrosine phosphorylation suggesting that downstream signaling molecules have not yet assembled. The failure of immature spermatozoa to undergo tyrosine phosphorylation has also been proposed to reflect their inability to generate sufficient ATP required for subsequent phosphorylation events (Aitken et al., 2007).
Mass spectrometry has also been used to compare the protein profiles of immature caput spermatozoa with that of mature cauda spermatozoa. These studies revealed a number of proteins that increased with epididymal maturation including several that are phosphorylated such as glucose-regulating protein, heat-shock protein 70, actin, β-tubulin, lactic acid dehydrogenase and the mitochondrial proteins aconitase and β subunit F1 ATPase (Aitken et al., 2007). Thus, the ability of specific sperm proteins to be phosphorylated may represent a key maturational step possibly as a result of the development of signaling complexes.
The topography of the sperm surface also changes in a domain-specific manner during epididymal transit. Atomic force microscopy demonstrated that particles ranging in size from 20 to 60 nm were associated with the acrosomal cap, equatorial segment and post-acrosomal region, and varied depending on whether the spermatozoa were isolated from the initial segment or caudal regions (Takano and Abe, 2004; Jones et al., 2007). These studies were interpreted as reflecting changes in protein associations with the sperm surface as part of the maturational process. Indeed, the protein composition of the spermatozoon changes as the cells mature with some proteins disappearing, others being modified, while others change their cellular localization. The biochemical alterations in sperm proteins reflect either those proteins that were synthesized during spermatogenesis or those that were secreted by the epididymal epithelial cells and interact with the maturing sperm. Sperm proteins that were produced during spermatogenesis and are modified such as by deglycosylation or proteolytic processing during epididymal transit include ADAM family members fertilin and cyritestin, CE9, α-mannosidase and many others (Table I). Other sperm proteins including SPAM1 and β-galactosyltransferase exhibit new localization patterns during epididymal transit which may in part be triggered by proteolytic processing (Phelps et al., 1990). Epididymal proteins that are known to interact with spermatozoa and thus may be involved in their maturation include the CRISP family proteins, P26h, P34h, SPAG11, Eppin and others (Table I).
Table I.
Epididymal sperm proteins
| Sperm proteins modified or relocalized during epididymal transit | Epididymal proteins that interact with spermatozoa |
|---|---|
| Spam11 | CRISP111 |
| ADAM22, ADAM33, ADAM154, ADAM245 | P26h12 |
| α-mannosidase6 | Clusterin13 |
| CE97 | HE114, HE215, HE416, HE517, HE1218 |
| β-galactosidase8 | HEL7519 |
| Basigin9 | SPAG1120 |
| α-enolase10 | Eppin21 |
| Grp78/Hsp7010 | Cystatin 1122 |
| Endoplasmin10 | SED123 |
| Phosphatidylethanolamine binding protein10 | |
| Lactate dehydrogenase 310 | |
| Testis lipid-binding protein10 | |
| Cytokeratin10 | |
| β-subunit F1-ATPase10 |
1(Phelps et al., 1990); 2(Lum and Blobel, 1997); 3(Frayne et al., 1998); 4(Pasten-Hidalgo et al., 2008); 5(Zhu et al., 2001); 6(Tulsiani et al., 1995); 7(Nehme et al., 1993); 8(Scully et al., 1987); 9(Saxena et al., 2002); 10(Baker et al., 2005); 11(Cohen et al., 2000); 12(Legare et al., 1999); 13(Sylvester et al., 1991); 14(Kirchhoff et al., 1996); 15(Osterhoff et al., 1994); 16(Kirchhoff et al., 1991); 17(Kirchhoff and Hale, 1996); 18(Saalmann et al., 2001); 19(Lin et al., 2008); 20(Yenugu et al., 2006); 21(Richardson et al., 2001); 22(Hamil et al., 2002); 23(Ensslin and Shur, 2003).
Human epididymis
As in other mammalian species, the human epididymis is a long convoluted tubule that serves as a conduit for the transport of spermatozoa from the testis to the vas deferens and is the site where spermatozoa mature and acquire their functions of progressive motility and fertility. Unfortunately, very little is known about the human epididymis and its role in the sperm maturation process owing to the lack of studies that have utilized normal human tissue. Normal epididymal tissue can only be obtained from organ donors or patients undergoing treatment for testicular cancer. Until recently, the majority of studies examining human epididymal function used samples from patients after surgical reversal of chronic epididymal obstruction. In some cases, following the anastomosis of the vas deferens to the proximal regions of the epididymis, sperm motility and fertility was observed resulting in the conclusion that, in human, transit through the epididymis was not required for sperm maturation (Silber 1980, 1989a, b, c; Silber et al., 1990). In contrast, other studies clearly indicated a role for the human epididymis in sperm maturation including those that demonstrated during vasoepididymostomy, the pregnancy rates were higher the more distal that the anastomosis was (72% at the corpus, 43% at the caput) (Silber, 1989a). Also, in patients with a congenital absence of the vas deferens, those that had a longer epididymis resulted in a higher fertilization and pregnancy rate during IVF than those with a shorter epididymis (Patrizio et al., 1994). The few studies of normal human epididymis showed that, compared with cauda spermatozoa, spermatozoa from the caput or efferent ducts showed limited motility and fertility and were unable to undergo an ionophore-stimulated acrosome reaction, further demonstrating a role for the epididymis in human sperm maturation (Hinrichsen and Blaquier, 1980; Yeung et al., 1993, 1997). Although the results of these early studies in the human epididymis were paradoxical, more recent studies suggest that the unusual observation that spermatozoa from proximal regions of previously obstructed epididymides were fertile is thought to reflect changes that occur in the epididymal epithelium as a result of the obstruction (Cooper, 1990). Indeed, although the motility of ejaculated sperm is initially poor following vasoepididymostomy at the level of the caput region, it can profoundly improve over the course of 1–2 years suggesting that following the surgery, the caput epididymidal epithelium may undergo compensatory changes allowing the maturation of sperm motility (Silber, 1980). Taken together, it is important that further studies are carried out to understand the normal functions of the human epididymis if new therapies for infertility as well as contraceptive targets are to be identified.
Human epididymis: structure and function
The human epididymis is distinct from most other mammalian species by the fact that its most proximal region, the caput, is largely composed of efferent ducts most of which unite to form a common duct from which the epididymis originates (Yeung et al., 1991) (Fig. 1). Within this region, at least seven types of tubules have been identified, each characterized by a different epithelium. Although tall cells with long stereocilia were observed and thought to represent an ‘initial segment’-type epithelium, this was not recognized as a discrete region as is apparent in rodent species (Fig. 1). The human epididymis is also different from other species in that it lacks a pronounced cauda region and thus is a poor reservoir for spermatozoa (Bedford, 1994). Since sperm transit time through the epididymis is rapid in human (2–6 days) (Amann and Howards, 1980) compared with rodent (10–13 days), sperm maturation may also occur more quickly and thus perhaps prolonged storage is not required. Alternatively, compared with other species, sperm maturation in human may be a more simplified process.
In a broad sense, the human epididymis is functionally similar to that of other mammalian species. The efferent ducts and epididymis follows a biphasic pattern of development likely reflecting the androgen status of the developing embryo and at puberty (De Miguel et al., 1998). The microvasculature of the human epididymis also follows the same general pattern as in the epididymis of other mammals (Kormano and Reijonen, 1976). Furthermore, from the few functional studies that have been carried out on spermatozoa from different regions of the human epididymis, it is evident that during epididymal transit spermatozoa show maturation-associated changes in motility, fertility and morphology (Hinrichsen and Blaquier, 1980; Yeung et al., 1997; Soler et al., 2000). Finally, as in other animal models, the human epididymal epithelium secretes epididymosomes (Thimon et al., 2008).
Human epididymal proteins
Several epididymal luminal proteins that have been studied in other mammals and implicated in sperm maturation have been identified in the lumen of the human epididymis including the CRISP (D/E) proteins (Kratzschmar et al., 1996), P34h short-chain dehydrogenase/reductase (Boue et al., 1994, 1996), beta-hexosaminidase (Miranda et al., 1995), clusterin (O’Bryan et al., 1994), ADAM 7 (Liu et al., 2000) and others (Lasserre et al., 2001). By the use of a tissue-specific cloning strategy, several novel genes were first identified in the human epididymis that were then studied further in other mammalian species. These include HE1, encoding a putative cholesterol binding protein; HE2, a β-defensin; HE3, with unknown function; HE4, a member of the four disulfide core or whey acidic protein (WAP) family of proteinase inhibitors; HE5, CD52 implicated in human immunological infertility; and HE6, a new member of the LNB-7TM subfamily of GPCR (Kirchhoff, 2002).
In addition to examination of known epididymal mRNAs and proteins, gene profiling studies have been performed in the normal human epididymis to determine region-specific gene expression. These studies revealed that as found in other mammals, gene expression was regionalized throughout the human epididymis with the caput region exhibiting the highest percentage of region-specific genes (Zhang et al., 2006; Dube et al., 2007; Thimon et al., 2007). However, these studies also revealed differences between the rodent and human epididymis in that several genes exhibited distinct expression profiles. ADAM7 shown to exhibit caput-specific expression in the mouse epididymis (Cornwall and Hsia, 1997) was absent from the human caput region and primarily expressed in the corpus region (Thimon et al., 2007). Other genes that are primarily expressed in the caput region of rodents but in other regions in the human included CRISP1. Similarly, in separate studies the c-ros receptor-type protein tyrosine kinase mRNA and protein are expressed only in the mouse initial segment and proximal caput epididymal region, while in the human epididymis mRNA and protein are present throughout the epididymis except for the proximal caput epididymal region (Legare and Sullivan, 2004). These studies suggest that the expression and likely function of several genes have shifted to different regions in the human epididymis compared with that in the mouse and may reflect the morphological differences between the epididymides of the two species. This also may indicate distinctions between humans and rodents with regard to where in the epididymis spermatozoa acquire their functions which may reflect their different reproductive strategies. In humans, maturation may occur in more proximal regions than in rodent species. In support of such a difference, are proteomic studies examining the profiles of proteins secreted by different regions of the human epididymis. These studies showed that, unlike the rodent epididymis, which exhibited region-dependent changes in the secreted proteins, the human epididymis only had minor changes between the proteins examined (Dacheux et al., 2006). Because these studies only focused on abundant proteins including CRISP, actin, calmodulin, cystatin C, superoxide dismutase, HE3, clusterin and others, it may be that the region-specific functions for these proteins are less important in the human than mouse while proteins that are region-specific were in low abundance and not detected in this study. It is also possible that major differences in secretory activity are confined to within the most proximal region which in the human consists of efferent ducts of varying epithelia. The different types of tubules in this region could perform some region-specific functions characteristic of more distal regions in the rodent epididymis.
Human epididymis: clinical relevance
Several recent studies demonstrated that changes in epididymal gene expression occurred following vasectomy. This would support the previously observed ultrastructural changes including decreases in epithelial cell and stereocilia height and tubular lumen following vasectomy in humans (Rajalakshmi et al., 1993). In particular, vasectomy resulted in the down-regulation of HE1 mRNA in the human epididymis with no effect on HE2 or HE5 mRNA or proteins (Legare et al., 2004). In contrast to changes in the levels of expression, other genes exhibited new sites of expression following vasectomy. The P34h mRNA, typically expressed in the human corpus epididymidis, was detected only in the proximal caput region after vasectomy (Legare et al., 2001). Similarly in rodent species, gene and protein expression profiles change after vasectomy including that for CRISP1 which suggests the possibility of altered epididymal functions (Turner et al., 1999). Whether this represents an orchestrated response to the stasis of the luminal flow following the surgical procedure or deregulation of epididymal gene expression is not known. However, these studies suggest that conditions such as vasectomy and likely the reversal procedures including vasovasostomy and vasoepididymostomy can have major effects on epididymal cell functions which could positively or negatively affect sperm maturation.
Finally, there is evidence that gene expression profiles are altered in the epididymides of infertile men compared with fertile men, further emphasizing the biological relevance of the epididymis in human reproduction. Profiling studies that compared gene expression in the epididymides of normal men with that of men with non-obstructive azoospermia revealed that 414 genes exhibited a 2-fold or more change in expression in the caput epididymidis of the infertile men (Dube et al., 2008), and those that were the most up-regulated in the infertile men were several transcription factors and signaling molecules (4- to 9-fold changes) and CRISP1 which showed an 11-fold increase in the epididymides of infertile men. Genes that were down-regulated in the caput epididymidis included several that encoded proteins involved in the immune response, protein folding and proteolysis, ubiquitination, sperm motility and DNA packaging and organization. Interestingly, although the genes encoding tight junctional proteins claudin 10 and TJP1 were not altered in the infertile epididymis, their proteins showed altered localization in the epididymal epithelium suggesting effects of the condition on the permeability of the blood–epididymis barrier (Dube et al., 2007). Because the epididymides were obtained from patients that exhibited non-obstructive azoospermia and thus lack spermatozoa in the epididymis, the altered gene expression in the epididymal epithelium could reflect the lack of important signals that come from the spermatozoa or associated molecules in the luminal fluid that are required for the lumicrine regulation of epididymal gene expression as described previously in the rodent epididymis.
Taken together, studies to date support a role for the human epididymis in sperm maturation. Furthermore, the tissue- and region-specific expression of genes in the human epididymis suggests similar mechanisms of sperm maturation as in rodent species. However, it is also clear that the human epididymis has unique properties which likely have contributed to the confusion regarding the interpretation of its biological role in sperm maturation.
Summary
The epididymis is an amazingly complex tubule consisting of individual microenvironments that serve to mature spermatozoa functionally. Understanding the intricacies of epididymal epithelial cell function and its regulation, the ever-changing luminal microenvironment, and the processes that affect sperm function are essential for the development of new therapies for infertility as well as for contraceptive development. In a broader sense, studying the epididymis has the potential to be of profound significance for understanding basic biological mechanisms including mechanisms of transcriptional control, secretion and turnover of proteins in the extracellular space including mechanisms of quality control for misfolded proteins, and cell–cell communication and cell signaling.
Funding
Funded in part by National Institutes of Health (HD33903, HD44669).
References
- Aitken RJ, Nixon B, Lin M, Koppers AJ, Lee YH, Baker MA. Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian J Androl. 2007;9:554–564. doi: 10.1111/j.1745-7262.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol. 1980;124:211–215. doi: 10.1016/s0022-5347(17)55377-x. [DOI] [PubMed] [Google Scholar]
- Asquith KL, Harman AJ, McLaughlin EA, Nixon B, Aitken RJ. Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol Reprod. 2005;72:328–337. doi: 10.1095/biolreprod.104.034470. [DOI] [PubMed] [Google Scholar]
- Aumuller G, Wilhelm B, Seitz J. Apocrine secretion—fact or artifact? Ann Anat. 1999;181:437–446. doi: 10.1016/S0940-9602(99)80020-X. [DOI] [PubMed] [Google Scholar]
- Avram C, Yeung CH, Nieschlag E, Cooper TG. Regulation of the initial segment of the murine epididymis by dihydrotestosterone and testicular exocrine secretions studied by expression of specific proteins and gene expression. Cell Tissue Res. 2004;317:13–22. doi: 10.1007/s00441-004-0902-x. [DOI] [PubMed] [Google Scholar]
- Baker MA, Witherdin R, Hetherington L, Cunningham-Smith K, Aitken RJ. Identification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresis. Proteomics. 2005;5:1003–1012. doi: 10.1002/pmic.200401100. [DOI] [PubMed] [Google Scholar]
- Baska KM, Manandhar G, Feng D, Agca Y, Tengowski MW, Sutovsky M, Yi YJ, Sutovsky P. Mechanism of extracellular ubiquitination in the mammalian epididymis. J Cell Physiol. 2008;215:684–696. doi: 10.1002/jcp.21349. [DOI] [PubMed] [Google Scholar]
- Bedford JM. The status and the state of the human epididymis. Hum Reprod. 1994;9:2187–2199. doi: 10.1093/oxfordjournals.humrep.a138416. [DOI] [PubMed] [Google Scholar]
- Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. Embo J. 2006;25:4131–4141. doi: 10.1038/sj.emboj.7601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boue F, Berube B, De Lamirande E, Gagnon C, Sullivan R. Human sperm-zona pellucida interaction is inhibited by an antiserum against a hamster sperm protein. Biol Reprod. 1994;51:577–587. doi: 10.1095/biolreprod51.4.577. [DOI] [PubMed] [Google Scholar]
- Boue F, Blais J, Sullivan R. Surface localization of P34H an epididymal protein, during maturation, capacitation, and acrosome reaction of human spermatozoa. Biol Reprod. 1996;54:1009–1017. doi: 10.1095/biolreprod54.5.1009. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Chaurand P, Fouchecourt S, DaGue BB, Xu BJ, Reyzer ML, Orgebin-Crist MC, Caprioli RM. Profiling and imaging proteins in the mouse epididymis by imaging mass spectrometry. Proteomics. 2003;3:2221–2239. doi: 10.1002/pmic.200300474. [DOI] [PubMed] [Google Scholar]
- Chen MY, Carpenter D, Zhao GQ. Expression of bone morphogenetic protein 7 in murine epididymis is developmentally regulated. Biol Reprod. 1999;60:1503–1508. doi: 10.1095/biolreprod60.6.1503. [DOI] [PubMed] [Google Scholar]
- Cheung KH, Leung GP, Leung MC, Shum WW, Zhou WL, Wong PY. Cell–cell interaction underlies formation of fluid in the male reproductive tract of the rat. J Gen Physiol. 2005;125:443–454. doi: 10.1085/jgp.200409205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA, Man SY. Fluid and electrolyte reabsorption in the ductuli efferentes testis. J Reprod Fertil Suppl. 1998;53:1–14. [PubMed] [Google Scholar]
- Cohen DJ, Rochwerger L, Ellerman DA, Morgenfeld MM, Busso D, Cuasnicu PS. Relationship between the association of rat epididymal protein ‘DE’ with spermatozoa and the behavior and function of the protein. Mol Reprod Dev. 2000;56:180–188. doi: 10.1002/(SICI)1098-2795(200006)56:2<180::AID-MRD9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cooper TG. In defense of a function for the human epididymis. Fertil Steril. 1990;54:965–975. doi: 10.1016/s0015-0282(16)53988-0. [DOI] [PubMed] [Google Scholar]
- Cooper TG. Interactions between epididymal secretions and spermatozoa. J Reprod Fertil Suppl. 1998;53:119–136. [PubMed] [Google Scholar]
- Cooper TG. Sperm maturation in the epididymis: a new look at an old problem. Asian J Androl. 2007;9:533–539. doi: 10.1111/j.1745-7262.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61:28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Hann SR. Transient appearance of CRES protein during spermatogenesis and caput epididymal sperm maturation. Mol Reprod Dev. 1995;41:37–46. doi: 10.1002/mrd.1080410107. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Hsia N. ADAM7, a member of the ADAM (a disintegrin and metalloprotease) gene family is specifically expressed in the mouse anterior pituitary and epididymis. Endocrinology. 1997;138:4262–4272. doi: 10.1210/endo.138.10.5468. [DOI] [PubMed] [Google Scholar]
- Cornwall GA, Orgebin-Crist MC, Hann SR. The CRES gene: a unique testis-regulated gene related to the cystatin family is highly restricted in its expression to the proximal region of the mouse epididymis. Mol Endocrinol. 1992;6:1653–1664. doi: 10.1210/mend.6.10.1280328. [DOI] [PubMed] [Google Scholar]
- Cox GF, Burger J, Lip V, Mau UA, Sperling K, Wu BL, Horsthemke B. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross NL. Reorganization of lipid rafts during capacitation of human sperm. Biol Reprod. 2004;71:1367–1373. doi: 10.1095/biolreprod.104.030502. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Gregory M, Dube E, Dufresne J, Chan PT, Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J Androl. 2007;9:463–475. doi: 10.1111/j.1745-7262.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Dacheux JL, Dacheux F. Protein secretion in the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 151–168. [Google Scholar]
- Dacheux JL, Belghazi M, Lanson Y, Dacheux F. Human epididymal secretome and proteome. Mol Cell Endocrinol. 2006;250:36–42. doi: 10.1016/j.mce.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Davies B, Baumann C, Kirchhoff C, Ivell R, Nubbemeyer R, Habenicht UF, Theuring F, Gottwald U. Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Mol Cell Biol. 2004;24:8642–8648. doi: 10.1128/MCB.24.19.8642-8648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Behnen M, Cappallo-Obermann H, Spiess AN, Theuring F, Kirchhoff C. Novel epididymis-specific mRNAs downregulated by HE6/Gpr64 receptor gene disruption. Mol Reprod Dev. 2007;74:539–553. doi: 10.1002/mrd.20636. [DOI] [PubMed] [Google Scholar]
- De Miguel MP, Marino JM, Martinez-Garcia F, Nistal M, Paniagua R, Regadera J. Pre- and post-natal growth of the human ductus epididymidis—a morphometric study. Reprod Fertil Dev. 1998;10:271–277. doi: 10.1071/r98059. [DOI] [PubMed] [Google Scholar]
- Dube E, Chan PT, Hermo L, Cyr DG. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis. Biol Reprod. 2007;76:1034–1044. doi: 10.1095/biolreprod.106.059246. [DOI] [PubMed] [Google Scholar]
- Dube E, Hermo L, Chan PT, Cyr DG. Alterations in gene expression in the caput epididymides of nonobstructive azoospermic men. Biol Reprod. 2008;78:342–351. doi: 10.1095/biolreprod.107.062760. [DOI] [PubMed] [Google Scholar]
- Ecroyd H, Sarradin P, Dacheux JL, Gatti JL. Compartmentalization of prion isoforms within the reproductive tract of the ram. Biol Reprod. 2004;71:993–1001. doi: 10.1095/biolreprod.104.029801. [DOI] [PubMed] [Google Scholar]
- Ecroyd H, Belghazi M, Dacheux JL, Gatti JL. The epididymal soluble prion protein forms a high-molecular-mass complex in association with hydrophobic proteins. Biochem J. 2005;392:211–219. doi: 10.1042/BJ20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405–417. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- Ezer N, Robaire B. Androgenic regulation of the structure and functions of the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 297–316. [Google Scholar]
- Fawcett DW, Hoffer AP. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod. 1979;20:162–181. doi: 10.1095/biolreprod20.2.162. [DOI] [PubMed] [Google Scholar]
- Fedder J, Gabrielsen A, Humaidan P, Erb K, Ernst E, Loft A. Malformation rate and sex ratio in 412 children conceived with epididymal or testicular sperm. Hum Reprod. 2007;22:1080–1085. doi: 10.1093/humrep/del488. [DOI] [PubMed] [Google Scholar]
- Fraile B, Martin R, De Miguel MP, Arenas MI, Bethencourt FR, Peinado F, Paniagua R, Santamaria L. Light and electron microscopic immunohistochemical localization of protein gene product 9.5 and ubiquitin immunoreactivities in the human epididymis and vas deferens. Biol Reprod. 1996;55:291–297. doi: 10.1095/biolreprod55.2.291. [DOI] [PubMed] [Google Scholar]
- Frayne J, Jury JA, Barker HL, Hall L. The MDC family of proteins and their processing during epididymal transit. J Reprod Fertil Suppl. 1998;53:149–155. [PubMed] [Google Scholar]
- Frenette G, Lessard C, Madore E, Fortier MA, Sullivan R. Aldose reductase and macrophage migration inhibitory factor are associated with epididymosomes and spermatozoa in the bovine epididymis. Biol Reprod. 2003;69:1586–1592. doi: 10.1095/biolreprod.103.019216. [DOI] [PubMed] [Google Scholar]
- Frenoux JM, Vernet P, Volle DH, Britan A, Saez F, Kocer A, Henry-Berger J, Mangelsdorf DJ, Lobaccaro JM, Drevet JR. Nuclear oxysterol receptors, LXRs, are involved in the maintenance of mouse caput epididymidis structure and functions. J Mol Endocrinol. 2004;33:361–375. doi: 10.1677/jme.1.01515. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Garrett SH, Douglass J. A spermatozoa-associated factor regulates proenkephalin gene expression in the rat epididymis. Mol Endocrinol. 1990;4:108–118. doi: 10.1210/mend-4-1-108. [DOI] [PubMed] [Google Scholar]
- Gatti JL, Metayer S, Belghazi M, Dacheux F, Dacheux JL. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol Reprod. 2005;72:1452–1465. doi: 10.1095/biolreprod.104.036426. [DOI] [PubMed] [Google Scholar]
- Georgiou I, Syrrou M, Pardalidis N, et al. Genetic and epigenetic risks of intracytoplasmic sperm injection method Asian. J Androl. 2006;8:643–673. doi: 10.1111/j.1745-7262.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Hamil KG, Liu Q, Sivashanmugam P, et al. Cystatin 11: a new member of the cystatin type 2 family. Endocrinology. 2002;143:2787–2796. doi: 10.1210/endo.143.7.8925. [DOI] [PubMed] [Google Scholar]
- Higgins SJ, Young P, Brody JR, Cunha GR. Induction of functional cytodifferentiation in the epithelium of tissue recombinants. I. Homotypic seminal vesicle recombinants. Development. 1989;106:219–234. doi: 10.1242/dev.106.2.219. [DOI] [PubMed] [Google Scholar]
- Hinrichsen MJ, Blaquier JA. Evidence supporting the existence of sperm maturation in the human epididymis. J Reprod Fertil. 1980;60:291–294. doi: 10.1530/jrf.0.0600291. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57. [PubMed] [Google Scholar]
- Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, Yamamura K. LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod. 2007;76:303–313. doi: 10.1095/biolreprod.106.054619. [DOI] [PubMed] [Google Scholar]
- Hsia N, Cornwall GA. Cres2 and Cres3: new members of the cystatin- related epididymal spermatogenic subgroup of family 2 cystatins. Endocrinology. 2003;144:909–915. doi: 10.1210/en.2002-220890. [DOI] [PubMed] [Google Scholar]
- Hsia N, Cornwall GA. DNA microarray analysis of region-specific gene expression in the mouse epididymis. Biol Reprod. 2004;70:448–457. doi: 10.1095/biolreprod.103.021493. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, Mishina Y, Garbers DL, Zhao GQ. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004;276:158–171. doi: 10.1016/j.ydbio.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Jelinsky SA, Turner TT, Bang HJ, Finger JN, Solarz MK, Wilson E, Brown EL, Kopf GS, Johnston DS. The rat epididymal transcriptome: comparison of segmental gene expression in the rat and mouse epididymides. Biol Reprod. 2007;76:561–570. doi: 10.1095/biolreprod.106.057323. [DOI] [PubMed] [Google Scholar]
- Jervis KM, Robaire B. Dynamic changes in gene expression along the rat epididymis. Biol Reprod. 2001;65:696–703. doi: 10.1095/biolreprod65.3.696. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, Kopf GS, Turner TT. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–413. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- Jones R, James PS, Howes L, Bruckbauer A, Klenerman D. Supramolecular organization of the sperm plasma membrane during maturation and capacitation. Asian J Androl. 2007;9:438–444. doi: 10.1111/j.1745-7262.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Keilhack H, Muller M, Bohmer SA, et al. Negative regulation of Ros receptor tyrosine kinase signaling. An epithelial function of the SH2 domain protein tyrosine phosphatase SHP-1. J Cell Biol. 2001;152:325–334. doi: 10.1083/jcb.152.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff C. Specific gene expression in the human and non-human primate epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 201–218. [Google Scholar]
- Kirchhoff C, Hale G. Cell-to-cell transfer of glycosylphosphatidylinositol- anchored membrane proteins during sperm maturation. Mol Hum Reprod. 1996;2:177–184. doi: 10.1093/molehr/2.3.177. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C, Osterhoff C, Young L. Molecular cloning and characterization of HE1, a major secretory protein of the human epididymis. Biol Reprod. 1996;54:847–856. doi: 10.1095/biolreprod54.4.847. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45:350–357. doi: 10.1095/biolreprod45.2.350. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C, Obermann H, Behnen M, Davies B. Role of epididymal receptor HE6 in the regulation of sperm microenvironment. Mol Cell Endocrinol. 2006;250:43–48. doi: 10.1016/j.mce.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Kirchhoff C, Osterhoff C, Samalecos A. HE6/GPR64 adhesion receptor co-localizes with apical and subapical F-actin scaffold in male excurrent duct epithelia. Reproduction. 2008;136:235–245. doi: 10.1530/REP-08-0078. [DOI] [PubMed] [Google Scholar]
- Kormano M, Reijonen K. Microvascular structure of the human epididymis. Am J Anat. 1976;145:23–27. doi: 10.1002/aja.1001450103. [DOI] [PubMed] [Google Scholar]
- Kratzschmar J, Haendler B, Eberspaecher U, Roosterman D, Donner P, Schleuning WD. The human cysteine-rich secretory protein (CRISP) family. Primary structure and tissue distribution of CRISP-1, CRISP-2 and CRISP-3. Eur J Biochem. 1996;236:827–836. doi: 10.1111/j.1432-1033.1996.t01-1-00827.x. [DOI] [PubMed] [Google Scholar]
- Kujala M, Hihnala S, Tienari J, Kaunisto K, Hastbacka J, Holmberg C, Kere J, Hoglund P. Expression of ion transport-associated proteins in human efferent and epididymal ducts. Reproduction. 2007;133:775–784. doi: 10.1530/rep.1.00964. [DOI] [PubMed] [Google Scholar]
- Lan ZJ, Labus JC, Hinton BT. Regulation of gamma-glutamyl transpeptidase catalytic activity and protein level in the initial segment of the rat epididymis by testicular factors: role of basic fibroblast growth factor. Biol Reprod. 1998;58:197–206. doi: 10.1095/biolreprod58.1.197. [DOI] [PubMed] [Google Scholar]
- Lasserre A, Barrozo R, Tezon JG, Miranda PV, Vazquez-Levin MH. Human epididymal proteins and sperm function during fertilization: un update. Biol Res. 2001;34:165–178. doi: 10.4067/s0716-97602001000300004. [DOI] [PubMed] [Google Scholar]
- Legare C, Sullivan R. Expression and localization of c-ros oncogene along the human excurrent duct. Mol Hum Reprod. 2004;10:697–703. doi: 10.1093/molehr/gah087. [DOI] [PubMed] [Google Scholar]
- Legare C, Berube B, Boue F, Lefievre L, Morales CR, El-Alfy M, Sullivan R. Hamster sperm antigen P26h is a phosphatidylinositol-anchored protein. Mol Reprod Dev. 1999;52:225–233. doi: 10.1002/(SICI)1098-2795(199902)52:2<225::AID-MRD14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Legare C, Thabet M, Picard S, Sullivan R. Effect of vasectomy on P34H messenger ribonucleic acid expression along the human excurrent duct: a reflection on the function of the human epididymis. Biol Reprod. 2001;64:720–727. doi: 10.1095/biolreprod64.2.720. [DOI] [PubMed] [Google Scholar]
- Legare C, Verville N, Sullivan R. Vasectomy influences expression of HE1 but not HE2 and HE5 genes in human epididymis. J Androl. 2004;25:30–43. doi: 10.1002/j.1939-4640.2004.tb02756.x. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Zou W, Mishra S, Li X, Rao Ch V. Epididymal phenotype in luteinizing hormone receptor knockout animals and its response to testosterone replacement therapy. Biol Reprod. 2003;68:888–895. doi: 10.1095/biolreprod.102.009738. [DOI] [PubMed] [Google Scholar]
- Li JY, Wang HY, Liu J, Liu Q, Zhang JS, Wan FC, Liu FJ, Jin SH, Zhang YL. Transcriptome analysis of a cDNA library from adult human epididymis. DNA Res. 2008;15:115–122. doi: 10.1093/dnares/dsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YQ, Li JY, Wang HY, Liu J, Zhang CL, Wang WT, Liu J, Li N, Jin SH. Cloning and identification of a novel sperm binding protein, HEL-75, with antibacterial activity and expressed in the human epididymis. Hum Reprod. 2008;23:2086–2094. doi: 10.1093/humrep/den084. [DOI] [PubMed] [Google Scholar]
- Liu ZZ, Wada J, Kumar A, Carone FA, Takahashi M, Kanwar YS. Comparative role of phosphotyrosine kinase domains of c-ros and c-ret protooncogenes in metanephric development with respect to growth factors and matrix morphogens. Dev Biol. 1996;178:133–148. doi: 10.1006/dbio.1996.0204. [DOI] [PubMed] [Google Scholar]
- Liu HW, Lin YC, Chao CF, Chang SY, Sun GH. GP-83 and GP-39, two glycoproteins secreted by human epididymis are conjugated to spermatozoa during maturation. Mol Hum Reprod. 2000;6:422–428. doi: 10.1093/molehr/6.5.422. [DOI] [PubMed] [Google Scholar]
- Lum L, Blobel CP. Evidence for distinct serine protease activities with a potential role in processing the sperm protein fertilin. Dev Biol. 1997;191:131–145. doi: 10.1006/dbio.1997.8609. [DOI] [PubMed] [Google Scholar]
- Maclean JA, 2nd, Chen MA, Wayne CM, Bruce SR, Rao M, Meistrich ML, Macleod C, Wilkinson MF. Rhox: a new homeobox gene cluster. Cell. 2005;120:369–382. doi: 10.1016/j.cell.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, Vassart G. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol. 2006;290:421–434. doi: 10.1016/j.ydbio.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Merlob P, Sapir O, Sulkes J, Fisch B. The prevalence of major congenital malformations during two periods of time, 1986-1994 and 1995-2002 in newborns conceived by assisted reproduction technology. Eur J Med Genet. 2005;48:5–11. doi: 10.1016/j.ejmg.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Minton AP. Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations. J Pharm Sci. 2005;94:1668–1675. doi: 10.1002/jps.20417. [DOI] [PubMed] [Google Scholar]
- Miranda PV, Brandelli A, Tezon JG. Characterization of beta-N- acetylglucosaminidase from human epididymis. Int J Androl. 1995;18:263–270. [PubMed] [Google Scholar]
- Moisan V, Bomgardner D, Tremblay JJ. Expression of the Ladybird-like homeobox 2 transcription factor in the developing mouse testis and epididymis. BMC Dev Biol. 2008;8:22. doi: 10.1186/1471-213X-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme CL, Cesario MM, Myles DG, Koppel DE, Bartles JR. Breaching the diffusion barrier that compartmentalizes the transmembrane glycoprotein CE9 to the posterior-tail plasma membrane domain of the rat spermatozoon. J Cell Biol. 1993;120:687–694. doi: 10.1083/jcb.120.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman M. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- O’Bryan MK, Mallidis C, Murphy BF, Baker HW. Immunohistological localization of clusterin in the male genital tract in humans and marmosets. Biol Reprod. 1994;50:502–509. doi: 10.1095/biolreprod50.3.502. [DOI] [PubMed] [Google Scholar]
- Oh J, Lee J, Woo JM, Choi E, Park I, Han C, Baek N, Lee H, Kim do H, Cho C. Systematic identification and integrative analysis of novel genes expressed specifically or predominantly in mouse epididymis. BMC Genomics. 2006;7:314. doi: 10.1186/1471-2164-7-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson GE, NagDas SK, Winfrey VP. Structural differentiation of spermatozoa during post-testicular maturation. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 371–387. [Google Scholar]
- Osterhoff C, Kirchhoff C, Krull N, Ivell R. Molecular cloning and characterization of a novel human sperm antigen (HE2) specifically expressed in the proximal epididymis. Biol Reprod. 1994;50:516–525. doi: 10.1095/biolreprod50.3.516. [DOI] [PubMed] [Google Scholar]
- Pasten-Hidalgo K, Hernandez-Rivas R, Roa-Espitia AL, Sanchez-Gutierrez M, Martinez-Perez F, Monrroy AO, Hernandez-Gonzalez EO, Mujica A. Presence, processing, and localization of mouse ADAM15 during sperm maturation and the role of its disintegrin domain during sperm-egg binding. Reproduction. 2008;136:41–51. doi: 10.1530/REP-07-0300. [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N, Pietrement C, Breton S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology (Bethesda) 2005;20:417–428. doi: 10.1152/physiol.00036.2005. [DOI] [PubMed] [Google Scholar]
- Patrizio P, Ord T, Silber SJ, Asch RH. Correlation between epididymal length and fertilization rate in men with congenital absence of the vas deferens. Fertil Steril. 1994;61:265–268. doi: 10.1016/s0015-0282(16)56515-7. [DOI] [PubMed] [Google Scholar]
- Penttinen J, Pujianto DA, Sipila P, Huhtaniemi I, Poutanen M. Discovery in silico and characterization in vitro of novel genes exclusively expressed in the mouse epididymis. Mol Endocrinol. 2003;17:2138–2151. doi: 10.1210/me.2003-0008. [DOI] [PubMed] [Google Scholar]
- Phelps BM, Koppel DE, Primakoff P, Myles DG. Evidence that proteolysis of the surface is an initial step in the mechanism of formation of sperm cell surface domains. J Cell Biol. 1990;111:1839–1847. doi: 10.1083/jcb.111.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrement C, Sun-Wada GH, Silva ND, McKee M, Marshansky V, Brown D, Futai M, Breton S. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod. 2006;74:185–194. doi: 10.1095/biolreprod.105.043752. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi M, Kumar BV, Kapur MM, Pal PC. Ultrastructural changes in the efferent duct and epididymis of men with obstructive infertility. Anat Rec. 1993;237:199–207. doi: 10.1002/ar.1092370207. [DOI] [PubMed] [Google Scholar]
- Rao M, Wilkinson MF. Homeobox genes and the male reproductive system. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 269–283. [Google Scholar]
- Reyes-Moreno C, Laflamme J, Frenette G, Sirard MA, Sullivan R. Spermatozoa modulate epididymal cell proliferation and protein secretion in vitro. Mol Reprod Dev. 2008;75:512–520. doi: 10.1002/mrd.20751. [DOI] [PubMed] [Google Scholar]
- Richardson RT, Sivashanmugam P, Hall SH, Hamil KG, Moore PA, Ruben SM, French FS, O’Rand M. Cloning and sequencing of human Eppin: a novel family of protease inhibitors expressed in the epididymis and testis. Gene. 2001;270:93–102. doi: 10.1016/s0378-1119(01)00462-0. [DOI] [PubMed] [Google Scholar]
- Robaire B, Jervis KM, Ezer N. Cell dynamics and cell death in the epididymal epithelium. In: Hinton BT, Turner TT, editors. The Third International Conference on the Epididymis. Charlottesville, VA: The Van Doren Company; 2003. pp. 35–49. [Google Scholar]
- Robaire B, Hinton BT, Orgebin-Crist MC. The epididymis. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. 3rd edn. New York: Elsevier; 2006. pp. 1071–1148. [Google Scholar]
- Rodriguez CI, Stewart CL. Disruption of the ubiquitin ligase HERC4 causes defects in spermatozoon maturation and impaired fertility. Dev Biol. 2007;312:501–508. doi: 10.1016/j.ydbio.2007.09.053. [DOI] [PubMed] [Google Scholar]
- Rodriguez CM, Kirby JL, Hinton BT. The development of the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 251–267. [Google Scholar]
- Saalmann A, Munz S, Ellerbrock K, Ivell R, Kirchhoff C. Novel sperm-binding proteins of epididymal origin contain four fibronectin type II-modules. Mol Reprod Dev. 2001;58:88–100. doi: 10.1002/1098-2795(200101)58:1<88::AID-MRD12>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Saez F, Frenette G, Sullivan R. Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J Androl. 2003;24:149–154. doi: 10.1002/j.1939-4640.2003.tb02653.x. [DOI] [PubMed] [Google Scholar]
- Saez F, Chabory E, Cadet R, Vernet P, Baron S, Lobaccaro JM, Drevet JR. Liver X receptors and epididymal epithelium physiology. Asian J Androl. 2007;9:574–582. doi: 10.1111/j.1745-7262.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Albisua I, Borell-Kost S, Mau-Holzmann UA, Licht P, Krageloh-Mann I. Increased frequency of severe major anomalies in children conceived by intracytoplasmic sperm injection. Dev Med Child Neurol. 2007;49:129–134. doi: 10.1111/j.1469-8749.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- Saxena DK, Oh-Oka T, Kadomatsu K, Muramatsu T, Toshimori K. Behaviour of a sperm surface transmembrane glycoprotein basigin during epididymal maturation and its role in fertilization in mice. Reproduction. 2002;123:435–444. doi: 10.1530/rep.0.1230435. [DOI] [PubMed] [Google Scholar]
- Scheer H, Robaire B. Steroid delta 4-5 alpha-reductase and 3 alpha-hydroxysteroid dehydrogenase in the rat epididymis during development. Endocrinology. 1980;107:948–953. doi: 10.1210/endo-107-4-948. [DOI] [PubMed] [Google Scholar]
- Scully NF, Shaper JH, Shur BD. Spatial and temporal expression of cell surface galactosyltransferase during mouse spermatogenesis and epididymal maturation. Dev Biol. 1987;124:111–124. doi: 10.1016/0012-1606(87)90464-7. [DOI] [PubMed] [Google Scholar]
- Seiler P, Cooper TG, Yeung CH, Nieschlag E. Regional variation in macrophage antigen expression by murine epididymal basal cells and their regulation by testicular factors. J Androl. 1999;20:738–746. [PubMed] [Google Scholar]
- Shadan S, James PS, Howes EA, Jones R. Cholesterol efflux alters lipid raft stability and distribution during capacitation of boar spermatozoa. Biol Reprod. 2004;71:253–265. doi: 10.1095/biolreprod.103.026435. [DOI] [PubMed] [Google Scholar]
- Silber SJ. Vasoepididymostomy to the head of the epididymis: recovery of normal spermatozoal motility. Fertil Steril. 1980;34:149–153. [PubMed] [Google Scholar]
- Silber SJ. Results of microsurgical vasoepididymostomy: role of epididymis in sperm maturation. Hum Reprod. 1989;a 4:298–303. doi: 10.1093/oxfordjournals.humrep.a136892. [DOI] [PubMed] [Google Scholar]
- Silber SJ. Apparent fertility of human spermatozoa from the caput epididymidis. J Androl. 1989;b 10:263–269. doi: 10.1002/j.1939-4640.1989.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Silber SJ. Role of epididymis in sperm maturation. Urology. 1989;c 33:47–51. doi: 10.1016/0090-4295(89)90066-6. [DOI] [PubMed] [Google Scholar]
- Silber SJ, Ord T, Balmaceda J, Patrizio P, Asch RH. Congenital absence of the vas deferens. The fertilizing capacity of human epididymal sperm. N Engl J Med. 1990;323:1788–1792. doi: 10.1056/NEJM199012273232602. [DOI] [PubMed] [Google Scholar]
- Sipila P, Pujianto DA, Shariatmadari R, Nikkila J, Lehtoranta M, Huhtaniemi IT, Poutanen M. Differential endocrine regulation of genes enriched in initial segment and distal caput of the mouse epididymis as revealed by genome-wide expression profiling. Biol Reprod. 2006;75:240–251. doi: 10.1095/biolreprod.105.047811. [DOI] [PubMed] [Google Scholar]
- Sixt SU, Beiderlinden M, Jennissen HP, Peters J. Extracellular proteasome in the human alveolar space: a new housekeeping enzyme? Am J Physiol Lung Cell Mol Physiol. 2007;292:L1280–L1288. doi: 10.1152/ajplung.00140.2006. [DOI] [PubMed] [Google Scholar]
- Soler C, Perez-Sanchez F, Schulze H, Bergmann M, Oberpenning F, Yeung C, Cooper TG. Objective evaluation of the morphology of human epididymal sperm heads. Int J Androl. 2000;23:77–84. doi: 10.1046/j.1365-2605.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Godecke S, Birchmeier C. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev. 1996;10:1184–1193. doi: 10.1101/gad.10.10.1184. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Saez F, Girouard J, Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis. 2005;35:1–10. doi: 10.1016/j.bcmd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–491. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno R, Ramalho-Santos J, Dominko T, Thompson WE, Schatten G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J Cell Sci. 2001;114:1665–1675. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- Sylvester SR, Morales C, Oko R, Griswold MD. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biol Reprod. 1991;45:195–207. doi: 10.1095/biolreprod45.1.195. [DOI] [PubMed] [Google Scholar]
- Takano H, Abe K. AFM study of surface structure changes in mouse spermatozoa associated with maturation. Methods Mol Biol. 2004;242:85–94. doi: 10.1385/1-59259-647-9:85. [DOI] [PubMed] [Google Scholar]
- Thimon V, Koukoui O, Calvo E, Sullivan R. Region-specific gene expression profiling along the human epididymis. Mol Hum Reprod. 2007;13:691–704. doi: 10.1093/molehr/gam051. [DOI] [PubMed] [Google Scholar]
- Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–1707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J, Joseph A, Archambeault D, Yao HH. Essential roles of inhibin beta A in mouse epididymal coiling. Proc Natl Acad Sci USA. 2007;104:11322–11327. doi: 10.1073/pnas.0703445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis AJ, Merdiushev T, Vargas LA, Jones BH, Purdon MA, Nipper RW, Galatioto J, Moss SB, Hunnicutt GR, Kopf GS. Expression and localization of caveolin-1, and the presence of membrane rafts, in mouse and Guinea pig spermatozoa. Dev Biol. 2001;240:599–610. doi: 10.1006/dbio.2001.0475. [DOI] [PubMed] [Google Scholar]
- Tulsiani DR, NagDas SK, Skudlarek MD, Orgebin-Crist MC. Rat sperm plasma membrane mannosidase: localization and evidence for proteolytic processing during epididymal maturation. Dev Biol. 1995;167:584–595. doi: 10.1006/dbio.1995.1050. [DOI] [PubMed] [Google Scholar]
- Turner TT. Necessity’s potion: inorganic ions and small organic molecules in the epididymal lumen. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 131–150. [Google Scholar]
- Turner TT, Riley TA. p53 independent, region-specific epithelial apoptosis is induced in the rat epididymis by deprivation of luminal factors. Mol Reprod Dev. 1999;53:188–197. doi: 10.1002/(SICI)1098-2795(199906)53:2<188::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Turner TT, Riley TA, Mruk DD, Cheng CY. Obstruction of the vas deferens alters protein secretion by the rat caput epididymidal epithelium in vivo. J Androl. 1999;20:289–297. [PubMed] [Google Scholar]
- Turner TT, Bomgardner D, Jacobs JP, Nguyen QA. Association of segmentation of the epididymal interstitium with segmented tubule function in rats and mice. Reproduction. 2003;125:871–878. doi: 10.1530/rep.0.1250871. [DOI] [PubMed] [Google Scholar]
- Turner TT, Bang HJ, Attipoe SA, Johnston DS, Tomsig JL. Sonic hedgehog pathway inhibition alters epididymal function as assessed by the development of sperm motility. J Androl. 2006;27:225–232. doi: 10.2164/jandrol.05114. [DOI] [PubMed] [Google Scholar]
- Turner TT, Johnston DS, Jelinsky SA, Tomsig JL, Finger JN. Segment boundaries of the adult rat epididymis limit interstitial signaling by potential paracrine factors and segments lose differential gene expression after efferent duct ligation. Asian J Androl. 2007;9:565–573. doi: 10.1111/j.1745-7262.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- Veri JP, Hermo L, Robaire B. Immunocytochemical localization of the Yf subunit of glutathione S-transferase P shows regional variation in the staining of epithelial cells of the testis, efferent ducts, and epididymis of the male rat. J Androl. 1993;14:23–44. [PubMed] [Google Scholar]
- von Horsten HH, Johnson SS, SanFrancisco SK, Hastert MC, Whelly SM, Cornwall GA. Oligomerization and transglutaminase cross-linking of the cystatin CRES in the mouse epididymal lumen: potential mechanism of extracellular quality control. J Biol Chem. 2007;282:32912–32923. doi: 10.1074/jbc.M703956200. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Kamiguchi Y, Mikamo K, Suzuki F, Yanagimachi H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am J Anat. 1985;172:317–330. doi: 10.1002/aja.1001720406. [DOI] [PubMed] [Google Scholar]
- Yang L, Fox SA, Kirby JL, Troan BV, Hinton BT. Putative regulation of expression of members of the Ets variant 4 transcription factor family and their downstream targets in the rat epididymis. Biol Reprod. 2006;74:714–720. doi: 10.1095/biolreprod.105.044354. [DOI] [PubMed] [Google Scholar]
- Yenugu S, Hamil KG, Grossman G, Petrusz P, French FS, Hall SH. Identification, cloning and functional characterization of novel sperm associated antigen 11 (SPAG11) isoforms in the rat. Reprod Biol Endocrinol. 2006;4:23. doi: 10.1186/1477-7827-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C, Cooper TG. Acquisition and development of sperm motility upon maturation in the epididymis. In: Robaire B, Hinton BT, editors. The Epididymis: From Molecules to Clinical Practice. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 417–434. [Google Scholar]
- Yeung CH, Cooper TG, Bergmann M, Schulze H. Organization of tubules in the human caput epididymidis and the ultrastructure of their epithelia. Am J Anat. 1991;191:261–279. doi: 10.1002/aja.1001910306. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG, Oberpenning F, Schulze H, Nieschlag E. Changes in movement characteristics of human spermatozoa along the length of the epididymis. Biol Reprod. 1993;49:274–280. doi: 10.1095/biolreprod49.2.274. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Perez-Sanchez F, Soler C, Poser D, Kliesch S, Cooper TG. Maturation of human spermatozoa (from selected epididymides of prostatic carcinoma patients) with respect to their morphology and ability to undergo the acrosome reaction. Hum Reprod Update. 1997;3:205–213. doi: 10.1093/humupd/3.3.205. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Breton S, Setiawan I, Xu Y, Lang F, Cooper TG. Increased luminal pH in the epididymis of infertile c-ros knockout mice and the expression of sodium-hydrogen exchangers and vacuolar proton pump H+-ATPase. Mol Reprod Dev. 2004;68:159–168. doi: 10.1002/mrd.20067. [DOI] [PubMed] [Google Scholar]
- Yu X, Suzuki K, Wang Y, Gupta A, Jin R, Orgebin-Crist MC, Matusik R. The role of forkhead box A2 to restrict androgen-regulated gene expression of lipocalin 5 in the mouse epididymis. Mol Endocrinol. 2006;20:2418–2431. doi: 10.1210/me.2006-0008. [DOI] [PubMed] [Google Scholar]
- Yuan H, Liu A, Zhang L, Zhou H, Wang Y, Zhang H, Wang G, Zeng R, Zhang Y, Chen Z. Proteomic profiling of regionalized proteins in rat epididymis indicates consistency between specialized distribution and protein functions. J Proteome Res. 2006;5:299–307. doi: 10.1021/pr050324s. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Pakarainen T, Zhu F, Poutanen M, Huhtaniemi I. Molecular characterization of postnatal development of testicular steroidogenesis in luteinizing hormone receptor knockout mice. Endocrinology. 2004;145:1453–1463. doi: 10.1210/en.2003-1049. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Liu Q, Li YM, Hall SH, French FS, Zhang YL. Genome-wide profiling of segmental-regulated transcriptomes in human epididymis using oligo microarray. Mol Cell Endocrinol. 2006;250:169–177. doi: 10.1016/j.mce.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Liaw L, Hogan BL. Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development. 1998;125:1103–1112. doi: 10.1242/dev.125.6.1103. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Chen YX, Liu XM, Xu Z, Qi X. Mutation in Bmp7 exacerbates the phenotype of Bmp8a mutants in spermatogenesis and epididymis. Dev Biol. 2001;240:212–222. doi: 10.1006/dbio.2001.0448. [DOI] [PubMed] [Google Scholar]
- Zhu GZ, Myles DG, Primakoff P. Testase 1 (ADAM 24) a plasma membrane-anchored sperm protease implicated in sperm function during epididymal maturation or fertilization. J Cell Sci. 2001;114:1787–1794. doi: 10.1242/jcs.114.9.1787. [DOI] [PubMed] [Google Scholar]