Abstract
Aims
Hypertensive and other effects of excess glucocorticoids might be in part mediated by the suppression of endothelial nitric oxide synthase (eNOS) expression. We studied the transcriptional and biochemical mechanisms that mediate or modulate the suppression of eNOS expression by glucocorticoids.
Methods and results
We found that a mere three-fold increase in the concentration of the natural glucocorticoid cortisol (from 30 to 100 nmol/L) significantly decreased the expression level of eNOS in human endothelial cells. Deletion analysis of the eNOS promoter indicated that the segment within −119 bp upstream from the transcription start site was significantly involved in the effect of cortisol. Site-directed mutagenesis and chromatin immunoprecipitation analyses demonstrated the presence of a suppressive glucocorticoid response element (GRE) at −111 to −105 bp. 11β-hydroxysteroid dehydrogenases (11β-HSD) catalyse the interconversion of active and inactive glucocorticoids. The suppression of 11β-HSD2 using small interfering RNA markedly exacerbated the inhibition of eNOS by cortisol. The suppression of 11β-HSD1 abolished the inhibition of eNOS expression by cortisol.
Conclusion
We identified the first GRE in the eNOS promoter region and demonstrated that endogenous 11β-HSD1 and 11β-HSD2 play significant and distinct roles in modulating the effect of glucocorticoids on eNOS expression.
Keywords: Hypertension, Promoter, Gene expression, Endothelium, RNA interference
1. Introduction
Systemic excess of glucocorticoids is known to cause hypertension and other abnormalities.1 The effect of glucocorticoids may be in part mediated by the suppression of endothelial nitric oxide synthase (eNOS),2 the product of which, nitric oxide (NO), plays an important role in cardiovascular and renal functions.3 Acute administration of glucocorticoids, however, may have beneficial effects on the cardiovascular system in part through non-transcriptional activation of eNOS.4
Dexamethasone, a synthetic glucocorticoid, as well as cortisol, has been shown to suppress the expression of eNOS in several types of vascular endothelial cells.2,5,6 Both transcriptional inhibition and a reduction in mRNA stability may contribute to this effect.2 Glucocorticoids typically bind to glucocorticoid receptors. Activated glucocorticoid receptors are translocated from the cytosol to the nucleus, where they bind to glucocorticoid response elements (GREs) and regulate gene expression.7,8 However, no GREs have been identified in the eNOS promoter.
The action of glucocorticoids might also be modulated by local metabolism. Local tissue metabolism of glucocorticoids is mainly regulated by 11β-hydroxysteroid dehydrogenases (11β-HSD).9–13 11β-HSD has two main isoforms. 11β-HSD1 acts predominantly as a reductase in vivo in many tissues, regenerating biologically active glucocorticoids (mainly cortisol in human and corticosterone in rodents) from their inactive forms (cortisone in human and 11-dehydrocorticosterone in rodents). 11β-HSD2, on the contrary, is a dehydrogenase that inactivates glucocorticoids.
11β-HSDs and local metabolism of glucocorticoids have emerged recently as an important mechanism in the regulation of cardiovascular and metabolic functions. Abnormally low levels of 11β-HSD2 or high levels of 11β-HSD1 in monogenic diseases or gene-targeting models can cause local glucocorticoid excess and have detrimental consequences including the development of hypertension and insulin resistance.9–13 There is also suggestive, although not yet conclusive, evidence indicating a possible role for 11β-HSDs in the development of common forms of hypertension.14–21 It would be highly significant to understand the role of 11β-HSDs in the regulation of eNOS, given the importance of both 11β-HSDs and NO in cardiovascular and renal functions. The role of the two kinetically distinct isoforms of 11β-HSD in the effect of glucocorticoids on eNOS expression, however, remains unknown, in part due to the lack of specific inhibitors for each isoform.
The goal of this study was to obtain new insights into the molecular and biochemical mechanisms that mediated or modulated the important effect of glucocorticoids on eNOS expression. We used promoter and mutagenesis analysis to identify the cis elements involved in the transcriptional inhibition of eNOS by glucocorticoids. Furthermore, we utilized RNA interference approaches to demonstrate important but distinct roles for 11β-HSD1 and 11β-HSD2 in the regulation of eNOS expression.
2. Methods
2.1. Chronic monitoring of arterial blood pressure in rats and renal medullary interstitial infusion
The experiments were performed as described previously22,23 in male Sprague–Dawley rats. The investigation conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The protocol was approved by the Institutional Animal Care and Use Committee.
2.2. Cell culture
Human umbilical vein endothelial cells (HUVEC) and dermal microvascular endothelial cells (HMEC-d) were obtained from Cambrex (East Rutherford, NJ, USA) and cultured as described,24 except that the glucocorticoid content in the culture medium was adjusted.
2.3. Promoter and deletion segment constructs
Human genomic DNA was isolated from HUVEC and used as the template for polymerase chain reaction (PCR) amplification of eNOS promoter segments. The eNOS promoter segments from −2091, −848, −430, −168, or −119 to 22 bp, relative to the transcription start site, were cloned into the XhoI–HindIII site of pGL4.81[hRlucCP/Neo] (Promega, Madison, WI, USA). This process generated constructs in which a promoter segment was linked to the Renilla luciferase reporter gene. The insertions were verified by DNA sequencing.
2.4. Site-directed mutagenesis
Site-directed mutagenesis was performed with the QuickChange II XL Site-Directed Mutagenesis Kit (Stratagene), following the protocol suggested by the company. Mutations were introduced at −516 to −513 or −110 to −107 bp and verified by DNA sequencing.
2.5. Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) analysis was performed essentially as we described previously.25 HUVEC were treated with 0 or 100 nM cortisol for 2 h. After crosslinking, genomic DNA was isolated and sonicated. DNA fragments were immunoprecipitated with non-specific mouse IgG or specific antibodies. The antibodies for glucocorticoid receptor and Sp1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). DNA fragments prior to (input) or after immunoprecipitation were used as templates for PCR reactions. PCR primers that covered region around the predicted GRE at −111 to −105 bp were as follows: forward, 5′-GGCTTGTTCCTGTCCCATTGTGTAT-3′, reverse: 5′-TTAGGAAGAGGGAGGGGACCGAGAG-′3.
2.6. Transfection and reporter gene analysis
HUVEC at 60–70% confluency were transfected with reporter gene constructs using lipofectin (Invitrogen), following the protocol suggested for HUVEC. pGL2 containing the firefly luciferase gene was used to normalize the Renilla luciferase activity to control for transfection efficiency.
2.7. RNA interference
siRNAs were designed, synthesized, and transfected using Oligofectamine (Invitrogen) as described previously.24–26
2.8. Real-time polymerase chain reaction
Real-time PCR analysis was carried out using the Taqman 5′ nuclease method as described previously.23–27
2.9. Western blot
Western blot analysis was performed essentially as described previously.23–26,28–30 Primary antibodies for eNOS, 11β-HSD1, and 11β-HSD2 were obtained from BD Biosciences (San Jose, CA, USA), Cayman Chemical (Ann Arbor, MI, USA), and Alpha Diagnostic International (San Antonio, TX, USA), respectively.
2.10. Measurement of cyclic GMP
Cyclic GMP (cGMP) was measured using an EIA method following acetylation (Cayman Chemical).
2.11. Statistics
Data shown are mean ± SEM. Student’s t-test or analysis of variance followed by Holm–Sidak test was performed. P < 0.05 was considered significant.
An expanded Methods section is available in the Supplementary material online.
3. Results
3.1. Carbenoxolone, a putative 11β-hydroxysteroid dehydrogenase 2 inhibitor, suppressed endothelial nitric oxide synthase expression in vivo
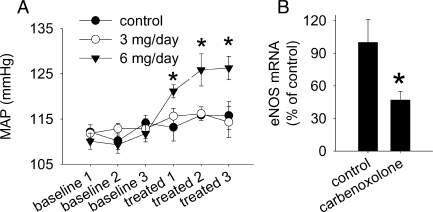
Renal medullary interstitial infusion of carbenoxolone, a putative 11β-HSD2 inhibitor, at a subpressor dose (Figure 1A) significantly reduced the level of eNOS mRNA in the renal outer medulla by 53% (Figure 1B). The subpressor dose was selected to avoid any confounding effects of hypertension on eNOS expression. Levels of eNOS mRNA in the renal cortex or inner medulla did not appear to be altered (data not shown).
Figure 1.
Renal medullary interstitial infusion of carbenoxolone at a subpressor dose reduced the expression level of endothelial nitric oxide synthase in the outer medulla in rats. (A) Renal interstitial infusion of carbenoxolone, a putative 11β-hydroxysteroid dehydrogenase 2 inhibitor, at 6 mg/day, but not 3 mg/day, caused hypertension (n = 5–8, *P < 0.05 vs. baseline). (B) Renal interstitial infusion of carbenoxolone at 3 mg/day reduced the level of endothelial nitric oxide synthase mRNA in the outer medulla (n = 5, *P < 0.05 vs. control). MAP, mean arterial pressure.
Carbenoxolone is not specific for 11β-HSD2 and could suppress eNOS through mechanisms independent of glucocorticoids. However, we have previously shown that similar administration of carbenoxolone in the renal medullary interstitium led to corticosterone excess specifically in the renal medulla.23 The in vivo suppression of eNOS expression by carbenoxolone motivated us to perform further studies on the relationship between glucocorticoids, eNOS, and 11β-HSDs.
3.2. Natural glucocorticoids suppressed endothelial nitric oxide synthase expression
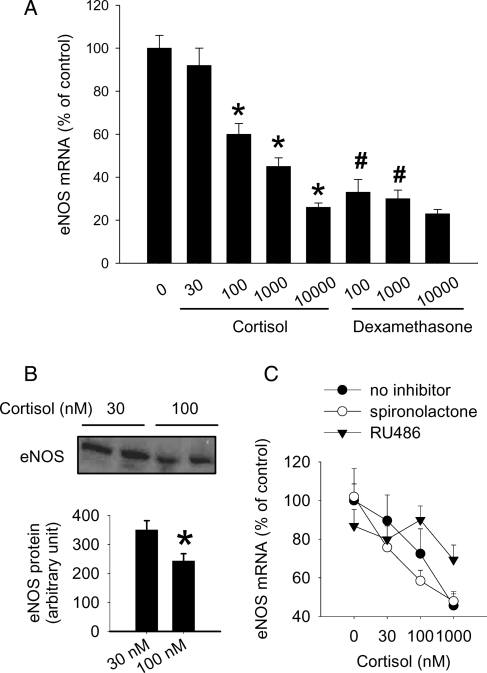
In cultured HUVEC, the natural glucocorticoid cortisol dose-dependently reduced eNOS mRNA levels (Figure 2A). A mere three-fold increase in cortisol concentration, from 30 to 100 nmol/L, was sufficient to significantly reduce eNOS mRNA levels by ∼40%, a reduction further reflected at the protein level (Figure 2B). Cortisol at 30 nmol/L did not significantly affect eNOS mRNA levels compared with 0 nmol/L and was used as the control in several subsequent experiments. The synthetic glucocorticoid dexamethasone also reduced eNOS mRNA levels as reported previously2 and appeared more potent than cortisol (Figure 2A).
Figure 2.
Cortisol reduced the expression level of endothelial nitric oxide synthase in human endothelial cells. Human umbilical vein endothelial cells were treated with the indicated agents for 48 h. (A) Cortisol or dexamethasone, both in nM, dose-dependently reduced the mRNA expression level of endothelial nitric oxide synthase in human umbilical vein endothelial cells (n = 6–8; *P < 0.05 vs. control; #P < 0.05 vs. cortisol at the same concentration. (B) Cortisol (100 nmol/L) reduced the protein level of endothelial nitric oxide synthase (n = 6, *P < 0.05 vs. 30 nmol/L cortisol). (C) The effect of cortisol was attenuated by RU486, a glucocorticoid receptor antagonist, but not spironolactone (both at 1 µmol/L, n = 4–7, P < 0.05 for the effect of cortisol in the group ‘no inhibitor’ or ‘spironolactone’, P > 0.05 for the group ‘RU486’).
The cortisol-induced reduction of eNOS mRNA appeared to be mediated by glucocorticoid receptors since the glucocorticoid receptor antagonist RU486 (1 µmol/L), but not the mineralocorticoid receptor antagonist spironolactone (0.1 or 1 µmol/L), attenuated the dose-dependent effect of cortisol (Figure 2C).
3.3. Identification of cis elements involved in the suppression of endothelial nitric oxide synthase expression by cortisol
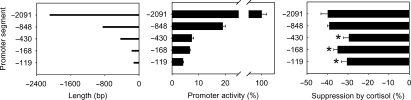
There were 22 predicted possible GREs within 2091 bp upstream from the transcription start site of human eNOS gene, according to Signal Scan (http://www-bimas.cit.nih.gov/molbio/signal/). None of them, however, were typical GREs. To identify promoter regions containing cis elements important for the suppression of eNOS expression by cortisol, we generated reporter gene constructs containing deletion segments of the eNOS promoter of five different lengths (Figure 3A). Basal promoter activities decreased substantially from the −2091 segment to the −848 segment (Figure 3B). Promoter activities further decreased as the segments shortened, but the shortest segment (−119 bp) still retained significant activities (Figure 3B).
Figure 3.
Effect of cortisol on the activity of human endothelial nitric oxide synthase promoter and its deletion segments. (A) Five endothelial nitric oxide synthase promoter segments of various lengths were cloned and linked to a luciferase reporter gene. (B) Basal activity of endothelial nitric oxide synthase promoter segments (n = 8). Promoter activities were normalized by co-transfected control plasmid. (C) Cortisol significantly reduced the activity of endothelial nitric oxide synthase promoter, and the effect of cortisol was significantly blunted in promoter segments shorter than 848 bp (n = 8; *P < 0.05 vs. the −2091 bp segment).
Cortisol suppressed the activities of the −2091 and the −848 segments by a similar extent of ∼40% (Figure 3C). The effects of cortisol on the −430, −168, and −119 segments were significantly less than the effect on the −848 segment. Nonetheless, the activity of the shortest segment of −119 bp was still significantly suppressed by cortisol by ∼30% (Figure 3C). The data suggested that there might be suppressive GREs between −848 and −430 bp and, more importantly, within the −119 bp segment.
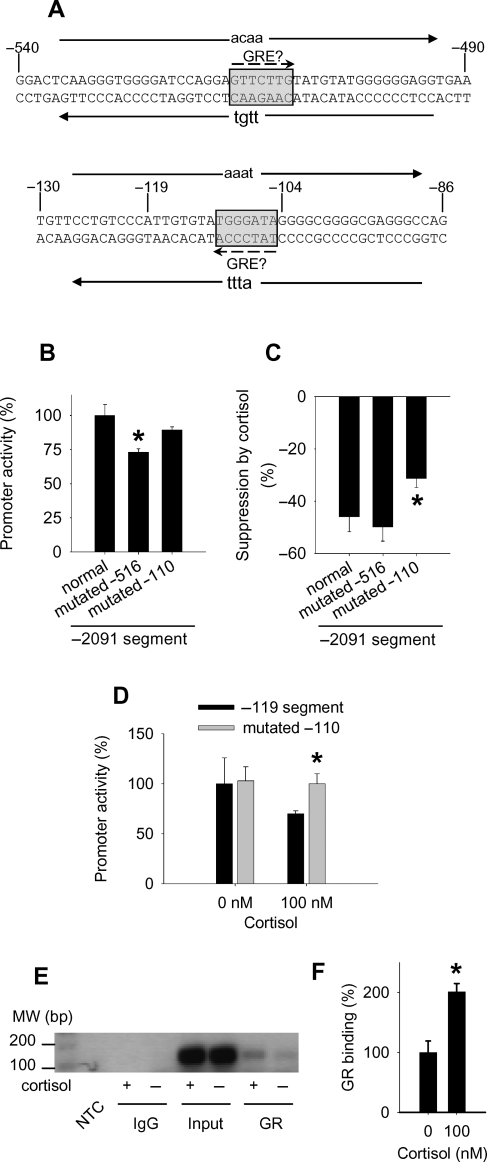
There are indeed a predicted GRE at −516 to −510 bp and another at −111 to −105 bp. Alignment analysis indicated that the sequence at −111 to −105 bp was conserved between human, rat, and mouse, although the sequence at −516 to −510 bp was not.
We introduced mutations into each of the two predicted GREs in the −2091 segment of the eNOS promoter (Figure 4A). The mutations had no or mild effects on the basal activity of the promoter (Figure 4B). Compared with the normal promoter (the −2091 segment), mutations at −110 to −107 bp within the predicted GRE at −111 to −105 bp significantly blunted the suppression of the promoter activity by cortisol by ∼30% (Figure 4C). Mutations at −516 to −513 within the predicted GRE at −516 to −510 bp did not affect the suppression of the promoter activity by cortisol (Figure 4C).
Figure 4.
Identification of a glucocorticoid response element in the human endothelial nitric oxide synthase promoter. (A) Mutations at −516 to −513 and −110 to −107 bp were introduced into predicted glucocorticoid response elements (shaded boxes) at −516 to −510 and −111 to −105 bp, respectively. The locations and orientations of the primers used for site-directed mutagenesis are shown with solid arrows, with the mutations shown in lowercase letters. (B) Basal activity of the normal (the −2091 segment) and mutated endothelial nitric oxide synthase promoters (n = 4; *P < 0.05 vs. normal promoter. (C) The suppression of the endothelial nitric oxide synthase promoter (the −2091 segment) activity by cortisol was significantly blunted by mutations at −110 to −107 within the predicted glucocorticoid response element at −111 to −105 bp (n = 4; *P < 0.05 vs. normal promoter). (D) The suppression of the shortest −119 segment by cortisol was abolished by mutations at −110 to −107 bp (n = 4; *P < 0.05 vs. the normal −119 segment). (E) A chromatin immunoprecipitation analysis indicated that cortisol increased the binding of glucocorticoid receptors to the promoter region around the putative glucocorticoid response element at −111 to −105 bp. The result shown was a representative polymerase chain reaction using genomic DNA templates immunoprecipitated from human umbilical vein endothelial cells treated with or without cortisol (100 nM) for 2 h. NTC, no template control; IgG, non-specific mouse IgG; Input, DNA prior to immunoprecipitation; GR, DNA precipitated with a glucocorticoid receptor antibody. (F) Quantification of the chromatin immunoprecipitation results (n = 3; *P < 0.05 vs. 0 nM cortisol).
We then introduced similar mutations into the predicted GRE at −111 to −105 bp within the shortest −119 bp segment of the promoter. The mutations abolished the suppressive effect of cortisol on the −119 segment (Figure 4D). The mutations did not affect the basal activity of the −119 segment.
ChIP analysis using a glucocorticoid receptor antibody, but not non-specific mouse IgG, detected the binding of glucocorticoid receptors to the promoter region around the predicted GRE at −111 to −105 bp (Figure 4E). Importantly, cortisol significantly increased the binding of glucocorticoid receptors to this region of the promoter by ∼two-fold (Figure 4E and F). The basal binding might be caused by trace amounts of cortisol that remained in the cells after cortisol removal. Cortisol did not significantly affect the binding of Sp1, a positive transcriptional factor with a binding site in the proximity of the GRE, to this region (data not shown).
3.4. 11β-hydroxysteroid dehydrogenase 2 buffered the suppression of endothelial nitric oxide synthase by cortisol
The suppression of eNOS by glucocorticoids might be mediated in part by the GRE identified earlier. The next question was whether the effect could be modulated by 11β-HSD isoforms that metabolize glucocorticoids. Western blot analyses of 11β-HSD1 and 11β-HSD2 demonstrated that these genes were expressed in HUVEC, consistent with that reported by Brem et al.31 The protein expression levels of 11β-HSD isoforms, however, were not significantly altered by an increase of cortisol concentration from 30 to 100 nmol/L (data not shown).
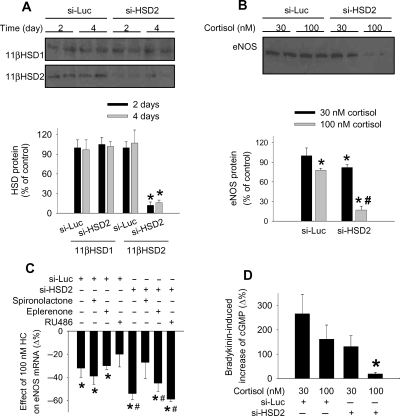
At a final concentration of 90 nmol/L, siRNA targeting human 11β-HSD2 (si-HSD2) substantially reduced the protein expression level of 11β-HSD2 in HUVEC by >85% after 48 h of transfection (Figure 5A). The expression level of 11β-HSD1 was not affected by si-HSD2. A control siRNA targeting luciferase (si-Luc) did not affect the expression of either 11β-HSD1 or 11β-HSD2 in HUVEC. The suppression of 11β-HSD2 by si-HSD2 persisted for at least another 48 h after a change of the culture medium (Figure 5A), which mimicked the time course of the cortisol treatment used in subsequent experiments.
Figure 5.
Suppression of 11β-hydroxysteroid dehydrogenase 2 markedly exacerbated the effect of cortisol on endothelial nitric oxide synthase. (A) siRNA targeting human 11β-hydroxysteroid dehydrogenase 2 significantly reduced the protein level of 11β-hydroxysteroid dehydrogenase 2, but not 11β-hydroxysteroid dehydrogenase 1, in human umbilical vein endothelial cells after 2 days of transfection (2 days). The effect persisted for at least another 2 days after a change of culture medium (4 days) (n = 6, *P < 0.05 vs. cells transfected with a control siRNA targeting luciferase. (B) The reducing effect of cortisol on the protein level of endothelial nitric oxide synthase was significantly exacerbated in human umbilical vein endothelial cells pre-treated with siRNA targeting human 11β-hydroxysteroid dehydrogenase 2. The cells were transfected with siRNA targeting luciferase or siRNA targeting human 11β-hydroxysteroid dehydrogenase 2 in the presence of 30 nmol/L cortisol for 48 h and then treated with 30 or 100 nmol/L cortisol for another 48 h (n = 6–7; *P < 0.05 vs. human umbilical vein endothelial cells treated with siRNA targeting luciferase and 30 nmol/L cortisol; #P < 0.05 vs. human umbilical vein endothelial cells treated with siRNA targeting luciferase and 100 nmol/L cortisol). (C) Effect of mineralocorticoid or glucocorticoid receptor antagonists on the exacerbated suppression of endothelial nitric oxide synthase. The treatment protocol was similar to (B), except that spironolactone (1 µmol/L), eplerenone (5 µmol/L), or RU486 (1 µmol/L) was included in the second 48 h treatment period. The data are expressed as per cent changes relative to cells treated with 30 nM cortisol (n = 4–6; *P < 0.05 vs. 30 nmol/L cortisol; #P < 0.05 vs. corresponding siRNA targeting luciferase). (D) Suppression of endothelial nitric oxide synthase expression was concomitant with a reduction in bradykinin-induced cGMP production. The treatment protocol was similar to (B), except that one-half of the cells were treated with bradykinin (100 nM) for 3 min prior to the preparation of cell lysate for cGMP analysis. The data are expressed as per cent changes caused by bradykinin (n = 6–7; *P < 0.05 vs. all other groups).
Remarkably, the cortisol-induced reduction of eNOS expression in HUVEC was substantially exacerbated when 11β-HSD2 was suppressed by si-HSD2 (Figure 5B). The protein level of eNOS in HUVEC that were treated with si-HSD2 followed by 100 nmol/L cortisol was decreased to <20% of the control.
The exacerbated suppression of eNOS caused by si-HSD2 occurred at the mRNA level in addition to the protein level (Figure 5C). The exacerbated suppression was attenuated by the mineralocorticoid receptor antagonist spironolactone, but not by RU486 (Figure 5C). However, eplerenone (5 µmol/L), another mineralocorticoid receptor antagonist, did not significantly attenuate the effect of 100 nM of cortisol in cells pre-treated with either si-Luc or si-HSD2. Moreover, aldosterone did not significantly alter eNOS mRNA levels in HUVEC when used at 1 or 100 nM concentrations (83 ± 16 and 98 ± 10%, respectively, of control, n = 5, NS vs. control).
We examined whether the changes in eNOS expression led to any changes in the NO-producing capability of HUVEC. We used bradykinin-induced increase of the cellular cGMP level as an index of eNOS-dependent, biologically active NO production. The average basal level of cGMP in HUVEC cultured in the presence of 30 nM cortisol was found to be 0.18 pmol/mg protein, similar to what had been reported previously.32 As shown in Figure 5D, treatment with 100 nM bradykinin for 3 min increased cGMP by ∼2.5-folds in control cells (30 nM cortisol). Following the treatment with si-HSD2 and 100 nM cortisol, the bradykinin-induced increase of cGMP was nearly abolished. Treatment with si-HSD2 or 100 nM cortisol alone tended to attenuate bradykinin-induced increases of cGMP, but the effect did not reach statistical significance. Cortisol at the concentrations we used did not have significant effects on mRNA levels of guanylate cyclase 1B3 or bradykinin receptor B1 or B2.
3.5. 11β-hydroxysteroid dehydrogenase 1 permitted or enhanced the suppression of endothelial nitric oxide synthase expression by cortisol
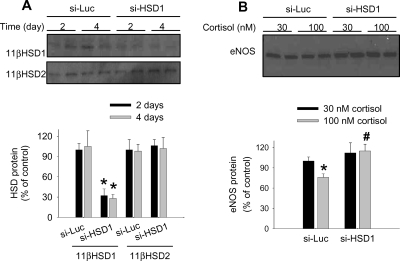
The siRNA targeting human 11β-HSD1 (si-HSD1), although not as potent as si-HSD2, significantly suppressed the protein expression of 11β-HSD1 in HUVEC by ∼70%, without affecting the expression of 11β-HSD2 (Figure 6A). The effect of si-HSD1 again persisted for at least another 48 h after a change of the culture medium. Importantly, pre-treatment with si-HSD1 abolished the reducing effect of 100 nmol/L cortisol on eNOS expression (Figure 6B).
Figure 6.
Suppression of 11β-hydroxysteroid dehydrogenase 1 abolished the effect of cortisol on endothelial nitric oxide synthase. (A) siRNA targeting human 11β-hydroxysteroid dehydrogenase 1 significantly reduced the protein level of 11β-hydroxysteroid dehydrogenase 1, but not 11β-hydroxysteroid dehydrogenase 2, in human umbilical vein endothelial cells after 2 days of transfection (2 days). The effect persisted for at least another 2 days after a change of culture medium (4 days) [n = 6; *P < 0.05 vs. cells transfected with a control siRNA targeting luciferase (si-Luc)]. (B) The reducing effect of cortisol on the protein level of endothelial nitric oxide synthase was abolished in human umbilical vein endothelial cells pre-treated with siRNA targeting human 11β-hydroxysteroid dehydrogenase 1. The time course of treatment was similar to that described in Figure 5 (n = 6–7; *P < 0.05 vs. human umbilical vein endothelial cells treated with siRNA targeting luciferase and 30 nmol/L cortisol; #P < 0.05 vs. human umbilical vein endothelial cells treated with siRNA targeting luciferase and 100 nmol/L cortisol).
3.6. Roles of 11β-hydroxysteroid dehydrogenase isoforms in human dermal microvascular endothelial cells were similar to those in human umbilical vein endothelial cells
We examined whether the 11β-HSD isoforms played a role in endothelial cells of a different origin similar to their role in HUVEC. In HMEC-d pre-treated with si-Luc, incubation with 100 nM cortisol for 48 h significantly reduced eNOS protein levels to 77 ± 3% (n = 4, P < 0.05 vs. 30 nM). In cells pre-treated with si-HSD2, eNOS protein levels were reduced to 70 ± 8 and 33 ± 2% in the presence of 30 and 100 nM cortisol, respectively (n = 4, P < 0.05 vs. si-Luc, and P < 0.05 for 30 vs. 100 nM). Pre-treatment with si-HSD1 tended to increase eNOS protein levels and abolished the significant difference between 30 and 100 nM cortisol (120 ± 13 and 106 ± 6%, respectively). These results were, in general, similar to those obtained from HUVEC shown in Figures 5B and 6B.
4. Discussion
This study provided novel insights into the molecular and biochemical mechanisms that regulate the suppression of eNOS by glucocorticoids. We identified a GRE in the eNOS promoter region and demonstrated that endogenous 11β-HSD1 and 11β-HSD2 played significant and distinct roles in modulating the effect of glucocorticoids on eNOS.
The concentrations of cortisol used in these experiments were within the physiological range of the plasma level of cortisol, suggesting that the findings of this study might be physiologically relevant. Plasma levels of cortisol in normal human subjects are commonly known to be at the level of several 100 nmol/L and to fluctuate by several folds in a normal circadian rhythm.33 We have determined that plasma levels of corticosterone in male Sprague–Dawley rats were in the same range.23
It appears that the effect of cortisol on eNOS is largely mediated at the transcriptional level, as suggested by the promoter analysis. A typical GRE contains a palindromic sequence with the two binding sites separated by three nucleotides. The presence of GREs in eNOS promoters has not been reported. Indeed, bioinformatic analysis cannot identify typical GREs in the human eNOS promoter. However, GREs can exist and function in various fashions.7,8 The GRE at −111 to −105 bp of the eNOS promoter could be a GRE half-site similar to those reported by a number of studies.7 It is possible that the binding of GR to the GRE at −111 to −105 bp interferes with the activity of the adjacent positive regulatory domains34,35 and, thereby, suppresses eNOS expression. Although we did not detect significant changes in the binding of Sp1, there are binding sites for several other positive transcriptional factors in this region. Moreover, negative GREs may reduce the binding of positive transcriptional factors with binding sites that are not in the proximity of the GRE.8 How the GRE at −111 to −105 bp suppresses eNOS transcription may be an interesting subject for future studies.
Mutations introduced into the GRE at −111 to −105 bp significantly blunted, but did not completely abolish, the suppression of the −2091 segment of the eNOS promoter by cortisol. It suggests that additional, atypical GREs might exist in other regions of the eNOS promoter.
The effect of glucocorticoids on eNOS expression appears to be significantly modulated by endogenous 11β-HSDs. 11β-HSD2 represents a potent, physiological mechanism that buffers the suppressing effect of natural glucocorticoids on eNOS expression. 11β-HSD1, on the contrary, appears to play a permissive or enhancing role for the cortisol-induced suppression of eNOS. The novel link between 11β-HSDs and NO has important physiological and pathophysiological implications. It is widely accepted that 11β-HSD2 in the distal nephron of the kidney is critical for preventing the activation of mineralocorticoid receptors by glucocorticoids.11,36 The finding in this study suggests that 11β-HSD isoforms might also play an important role in controlling effects of cortisol in the vasculature. The finding also suggests that the suppression of NO might be involved in the development of pathological consequences of 11β-HSD2 deficiency or 11β-HSD1 overexpression, such as hypertension. Furthermore, specific manipulations of each 11β-HSD isoform might be a valuable way to correct abnormalities in the level of NO in settings compatible with 11β-HSD manipulations.
The notion that 11β-HSDs may be important for vascular function is fairly new. It is consistent with the study by Souness et al.,37 in which 11β-HSD antisenses were shown to affect the constrictive response of rat aortic rings to phenylephrine. Interestingly, 11β-HSD1 antisense was shown to enhance the constrictive response, similar to 11β-HSD2 antisense.37 The enhanced constrictive response with 11β-HSD1 antisense might be due to factors other than changes in endothelial NO or to the 11β-HSD1 kinetics under the specific cellular and metabolic conditions of the aortic ring preparation. Aortas from 11β-HSD2, but not 11β-HSD1, knockout mice exhibit enhanced vasoconstrictive and reduced vasodilatory responses, suggesting impairments of the NO system in 11β-HSD2 null mice.38 However, questions have been raised as to whether the changes observed in these aortas might be secondary to long-term effects of the knockout, such as hypertension.39
Both glucocorticoid and mineralocorticoid receptors have been implicated in the suppression of eNOS expression. The role of glucocorticoid receptors has been supported by the ability of glucocorticoid receptor antagonists to attenuate glucocorticoid-induced eNOS suppression.2 Mineralocorticoid receptor antagonists have been shown to increase eNOS expression in vivo,40,41 although it is not entirely clear whether the observed effect reflected direct or indirect suppression of eNOS by activated mineralocorticoid receptors. The interpretation of these studies as well as the receptor antagonist data in this study may be complicated by the non-specific nature of the antagonists. The discovery of the GRE in this study supports a role for the glucocorticoid receptors. However, further experiments with the knockdown of each receptor or cells from receptor null mice are needed to fully delineate the role of glucocorticoid and mineralocorticoid receptors in the regulation of eNOS expression.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
National Institutes of Health (HL077263 to M.L.).
Conflict of interest: none declared.
Supplementary Material
References
- 1.Raff H, Findling JW. A physiologic approach to diagnosis of the Cushing syndrome. Ann Intern Med. 2003;138:980–991. doi: 10.7326/0003-4819-138-12-200306170-00010. [DOI] [PubMed] [Google Scholar]
- 2.Wallerath T, Witte K, Schafer SC, Schwarz PM, Prellwitz W, Wohlfart P, et al. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc Natl Acad Sci USA. 1999;96:13357–13362. doi: 10.1073/pnas.96.23.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang M, Knox FG. Production and functional roles of nitric oxide in the proximal tubule. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1117–R1124. doi: 10.1152/ajpregu.2000.278.5.R1117. [DOI] [PubMed] [Google Scholar]
- 4.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schafer SC, Wallerath T, Closs EI, Schmidt C, Schwarz PM, Forstermann U, et al. Dexamethasone suppresses eNOS and CAT-1 and induces oxidative stress in mouse resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H436–H444. doi: 10.1152/ajpheart.00587.2004. [DOI] [PubMed] [Google Scholar]
- 6.Rogers KM, Bonar CA, Estrella JL, Yang S. Inhibitory effect of glucocorticoid on coronary artery endothelial function. Am J Physiol Heart Circ Physiol. 2002;283:H1922–H1928. doi: 10.1152/ajpheart.00364.2002. [DOI] [PubMed] [Google Scholar]
- 7.Schoneveld OJ, Gaemers IC, Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. 2004;1680:114–128. doi: 10.1016/j.bbaexp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10:2807–2816. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- 9.Draper N, Stewart PM. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol. 2005;186:251–271. doi: 10.1677/joe.1.06019. [DOI] [PubMed] [Google Scholar]
- 10.Quinkler M, Stewart PM. Hypertension and the cortisol–cortisone shuttle. J Clin Endocrinol Metab. 2003;88:2384–2392. doi: 10.1210/jc.2003-030138. [DOI] [PubMed] [Google Scholar]
- 11.Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 12.Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet. 1987;2:821–824. doi: 10.1016/s0140-6736(87)91014-2. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal AK, Giacchetti G, Lavery G, Nikkila H, Palermo M, Ricketts M, et al. CA-repeat polymorphism in intron 1 of HSD11B2: effects on gene expression and salt sensitivity. Hypertension. 2000;36:187–194. doi: 10.1161/01.hyp.36.2.187. [DOI] [PubMed] [Google Scholar]
- 15.Bocchi B, Kenouch S, Lamarre-Cliche M, Muffat-Joly M, Capron MH, Fiet J, et al. Impaired 11-beta hydroxysteroid dehydrogenase type 2 activity in sweat gland ducts in human essential hypertension. Hypertension. 2004;43:803–808. doi: 10.1161/01.HYP.0000121362.64182.ad. [DOI] [PubMed] [Google Scholar]
- 16.Franks PW, Knowler WC, Nair S, Koska J, Lee YH, Lindsay RS, et al. Interaction between an 11betaHSD1 gene variant and birth era modifies the risk of hypertension in Pima Indians. Hypertension. 2004;44:681–688. doi: 10.1161/01.HYP.0000144294.28985.d5. [DOI] [PubMed] [Google Scholar]
- 17.Liang M, Yuan B, Rute E, Greene AS, Olivier M, Cowley AW., Jr Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics. 2003;12:229–237. doi: 10.1152/physiolgenomics.00089.2002. [DOI] [PubMed] [Google Scholar]
- 18.Watson B, Jr, Bergman SM, Myracle A, Callen DF, Acton RT, Warnock DG. Genetic association of 11 beta-hydroxysteroid dehydrogenase type 2 (HSD11B2) flanking microsatellites with essential hypertension in blacks. Hypertension. 1996;28:478–482. doi: 10.1161/01.hyp.28.3.478. [DOI] [PubMed] [Google Scholar]
- 19.Brand E, Kato N, Chatelain N, Krozowski ZS, Jeunemaitre X, Corvol P, et al. Structural analysis and evaluation of the 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) gene in human essential hypertension. J Hypertens. 1998;16:1627–1633. doi: 10.1097/00004872-199816110-00009. [DOI] [PubMed] [Google Scholar]
- 20.Melander O, Orho-Melander M, Bengtsson K, Lindblad U, Rastam L, Groop L, et al. Association between a variant in the 11 beta-hydroxysteroid dehydrogenase type 2 gene and primary hypertension. J Hum Hypertens. 2000;14:819–823. doi: 10.1038/sj.jhh.1001116. [DOI] [PubMed] [Google Scholar]
- 21.White PC, Agarwal AK, Li A, Nikkila H, Pratt JH, Caulfield M, et al. Possible association but no linkage of the HSD11B2 gene encoding the kidney isozyme of 11beta-hydroxysteroid dehydrogenase to hypertension in Black people. Clin Endocrinol (Oxf) 2001;55:249–252. doi: 10.1046/j.1365-2265.2001.01314.x. [DOI] [PubMed] [Google Scholar]
- 22.Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006;47:692–698. doi: 10.1161/01.HYP.0000203161.02046.8d. [DOI] [PubMed] [Google Scholar]
- 23.Usa K, Singh RJ, Netzel BC, Liu Y, Raff H, Liang M. Renal interstitial corticosterone and 11-dehydrocorticosterone in conscious rats. Am J Physiol Renal Physiol. 2007;293:F186–F192. doi: 10.1152/ajprenal.00484.2006. [DOI] [PubMed] [Google Scholar]
- 24.Liang M, Pietrusz JL. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol. 2007;27:77–83. doi: 10.1161/01.ATV.0000251006.54632.bb. [DOI] [PubMed] [Google Scholar]
- 25.Tian Z, Greene AS, Usa K, Matus IR, Bauwens J, Pietrusz JL, et al. Renal regional proteomes in young Dahl salt-sensitive rats. Hypertension. 2008;51:899–904. doi: 10.1161/HYPERTENSIONAHA.107.109173. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Park F, Pietrusz JL, Jia G, Singh RJ, Netzel BC, et al. Suppression of 11{beta}-hydroxysteroid dehydrogenase type 1 with RNA interference substantially attenuates 3T3-L1 adipogenesis. Physiol Genomics. 2008;32:343–351. doi: 10.1152/physiolgenomics.00067.2007. [DOI] [PubMed] [Google Scholar]
- 27.Knoll K, Pietrusz JL, Liang M. Tissue-specific transcriptome responses in rats with early streptozotocin-induced diabetes. Physiol Genomics. 2005;21:222–229. doi: 10.1152/physiolgenomics.00231.2004. [DOI] [PubMed] [Google Scholar]
- 28.Liang M, Knox FG. Nitric oxide activates PKCa and inhibits Na+-K+-ATPase in opossum kidney cells. Am J Physiol Renal Physiol. 1999;277:F859–F865. doi: 10.1152/ajprenal.1999.277.6.F859. [DOI] [PubMed] [Google Scholar]
- 29.Liang M, Croatt TJ, Nath KA. Mechanisms underlying the induction of heme oxygenase-1 by nitric oxide in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2000;279:F728–F735. doi: 10.1152/ajprenal.2000.279.4.F728. [DOI] [PubMed] [Google Scholar]
- 30.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res. 2008;18:404–411. doi: 10.1101/gr.6587008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brem AS, Bina RB, King TC, Morris DJ. Localization of 2 11beta-OH steroid dehydrogenase isoforms in aortic endothelial cells. Hypertension. 1998;31:459–462. doi: 10.1161/01.hyp.31.1.459. [DOI] [PubMed] [Google Scholar]
- 32.Gooch KJ, Frangos JA. Flow- and bradykinin-induced nitric oxide production by endothelial cells is independent of membrane potential. Am J Physiol. 1996;270:C546–C551. doi: 10.1152/ajpcell.1996.270.2.C546. [DOI] [PubMed] [Google Scholar]
- 33.Gorges R, Knappe G, Gerl H, Ventz M, Stahl F. Diagnosis of Cushing’s syndrome: re-evaluation of midnight plasma cortisol vs urinary free cortisol and low-dose dexamethasone suppression test in a large patient group. J Endocrinol Invest. 1999;22:241–249. doi: 10.1007/BF03343551. [DOI] [PubMed] [Google Scholar]
- 34.Zhang R, Min W, Sessa WC. Functional analysis of the human endothelial nitric oxide synthase promoter. Sp1 and GATA factors are necessary for basal transcription in endothelial cells. J Biol Chem. 1995;270:15320–15326. doi: 10.1074/jbc.270.25.15320. [DOI] [PubMed] [Google Scholar]
- 35.Karantzoulis-Fegaras F, Antoniou H, Lai SL, Kulkarni G, D’Abreo C, Wong GK, et al. Characterization of the human endothelial nitric-oxide synthase promoter. J Biol Chem. 1999;274:3076–3093. doi: 10.1074/jbc.274.5.3076. [DOI] [PubMed] [Google Scholar]
- 36.Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, et al. Localization of 11 beta-hydroxysteroid dehydrogenase—tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;2:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- 37.Souness GW, Brem AS, Morris DJ. 11 beta-hydroxysteroid dehydrogenase antisense affects vascular contractile response and glucocorticoid metabolism. Steroids. 2002;67:195–201. doi: 10.1016/s0039-128x(01)00148-9. [DOI] [PubMed] [Google Scholar]
- 38.Hadoke PW, Christy C, Kotelevtsev YV, Williams BC, Kenyon CJ, Seckl JR, et al. Endothelial cell dysfunction in mice after transgenic knockout of type 2, but not type 1, 11beta-hydroxysteroid dehydrogenase. Circulation. 2001;104:2832–2837. doi: 10.1161/hc4801.100077. [DOI] [PubMed] [Google Scholar]
- 39.Christy C, Hadoke PW, Paterson JM, Mullins JJ, Seckl JR, Walker BR. 11beta-hydroxysteroid dehydrogenase type 2 in mouse aorta: localization and influence on response to glucocorticoids. Hypertension. 2003;42:580–587. doi: 10.1161/01.HYP.0000088855.06598.5B. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi N, Yoshida K, Nakano S, Ohno T, Honda T, Tsubokou Y, et al. Cardioprotective mechanisms of eplerenone on cardiac performance and remodeling in failing rat hearts. Hypertension. 2006;47:671–679. doi: 10.1161/01.HYP.0000203148.42892.7a. [DOI] [PubMed] [Google Scholar]
- 41.Quaschning T, Ruschitzka F, Shaw S, Luscher TF. Aldosterone receptor antagonism normalizes vascular function in liquorice-induced hypertension. Hypertension. 2001;37:801–805. doi: 10.1161/01.hyp.37.2.801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.