Abstract
Hypertension and heart failure are worldwide health problems of ever-increasing proportions. A failure of the heart, during either systolic and/or diastolic phases of the cardiac cycle, has its origins rooted in an adverse structural, biochemical, and molecular remodelling of myocardium that involves its cellular constituents, extracellular matrix, and intramural coronary vasculature. Herein we focus on the pathogenic role of a dyshomeostasis of several macro- (i.e. Ca2+ and Mg2+) and micronutrients (i.e. Zn2+, Se2+, and vitamin D) in contributing to adverse remodelling of the myocardium and its failure as a pulsatile muscular pump. An improved understanding of how these macro- and micronutrients account for the causes and consequences of adverse myocardial remodelling carries with it the potential of identifying new biomarkers predictive of risk, onset and progression, and response to intervention(s), which could be monitored non-invasively and serially over time. Moreover, such incremental knowledge will serve as the underpinning to the development of novel strategies aimed at preventing and/or regressing the ongoing adverse remodelling of myocardium. The time is at hand to recognize the importance of macro- and micronutrient dyshomeostasis in the evaluation and management of hypertension and heart failure.
Keywords: Calcium, Magnesium, Zinc, Selenium, Vitamin D, Myocardial remodelling
1. Introduction
An adverse structural remodelling of myocardium, involving cellular constituents of its muscular, intramural coronary vascular, and interstitial compartments, contributes to the heart’s failure as a muscular pump during either systolic or diastolic phases of the cardiac cycle. The elucidation of molecular mechanisms involved in the pathogenesis of such remodelling, including those contributing to its progressive nature, are of considerable importance and the subject of ongoing research. Herein, we provide our perspective as to the role of macro- and micronutrient dyshomeostasis in promoting such adverse remodelling.
Macronutrients are chemical elements essential to life in large quantities. Calcium and magnesium are macronutrients (or macrominerals) available in milligram quantities and must be obtained from the environment. Micronutrients are present in microgram quantities. They too are essential, must be derived from external sources, and are integral components of enzymes or coenzymes involved in chemical reactions. Reduced circulating levels of such micronutrients as Zn2+ and Se2+, together with macronutrients, expressed as ionized hypocalcaemia and hypomagnesaemia, are found in patients with either hypertension or congestive heart failure (CHF), irrespective of race, ethnicity, or the aetiological origins of the failing heart.1–8 Increased excretory losses of Ca2+, Mg2+, and Zn2+ accompany pharmacological agents commonly used in the management of hypertension or CHF, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and loop diuretics.9 Symptomatic heart failure with reduced effort tolerance will constrain such patients to a housebound lifestyle deprived of sunlight. Thus, hypovitaminosis D is a common finding in these patients.7,8,10–12 This is especially the case in people with dark skin, where melanin is a natural sunscreen mandating longer exposure to the UVB component of sunlight for the skin to begin the process leading to vitamin D steroidogenesis.13 Hence, a deficiency of multiple macro- and micronutrients is an important accompaniment of hypertension and CHF. Each has the potential to adversely influence the structure of the failing myocardium.
Herein, we focus on a dyshomeostasis of Ca2+, Mg2+, Zn2+, Se2+, and vitamin D and their contribution to a remodelling of myocardial structure. Importantly, these nutrients are closely linked to one another and, therefore, no single entity would appear more important than another. Intracellular Ca2+ overloading, for example, is coupled to increased intracellular Zn2+ entry, while Mg2+ is a physiological antagonist of cellular and mitochondrial Ca2+ entry. The significance of these divalent cations and vitamin D is underscored by their pathophysiological roles in the appearance of oxidative stress in diverse tissues, and to the overall activity of antioxidant defenses found at these sites.
2. Polynutrient dyshomeostasis in hypertension and congestive heart failure
The importance of oxidative stress in the remodelling of the myocardium, where reactive oxygen (ROS) and nitrogen species (RNS) overwhelm antioxidant defenses and contribute to its progressive nature, has come to light in recent years. Moreover, the altered redox state appears concurrently in such diverse tissues as skin, muscle, peripheral blood mononuclear cells (PBMCs), and blood, which underscores its systemic nature. Collectively, these findings call into question the potential role of macro- and micronutrients involving diverse tissues, including the heart. One such overriding response in the pathogenesis of oxidative stress in multiple tissues relates to intracellular Ca2+ overloading. This includes elevated cytosolic free [Ca2+]i and mitochondrial Ca2+, where mitochondria are a major storage site for Ca2+ and the most redox-active organelle.14 An activation of NADPH oxidase and elaboration of superoxide with intracellular Ca2+ overloading is mediated by such calcitropic hormones as parathyroid hormone (PTH), angiotensin II, endothelin-1, and catecholamines. The importance of endogenous antioxidant defenses also deserves to be considered. This includes mitochondrial peroxiredoxin and such metalloenzymes as superoxide dismutase (SOD) and glutathione peroxidase, whose activities depend on Zn and Se, respectively.
Both hypertension and CHF represent progressive systemic illnesses15 whose major features include: (i) the presence of oxidative stress that overwhelms antioxidant defenses provided by Cu/Zn-SOD and Se-glutathione peroxidase, in diverse tissues including the heart; (ii) an immunostimulatory state, where a dyshomeostasis of intracellular Ca2+and Mg2+ contribute to endothelial and immune cell activation to produce adhesion molecules, chemokines, and proinflammatory cytokines that begets a vasculopathy of coronary, renal, and mesenteric arterioles; and (iii) a wasting of soft tissues, where Zn-dependent inhibition of ubiquitin-proteasome-mediated protein degradation of skeletal muscle is compromised, and ongoing PTH-mediated resorption of bone, eventuate in reduced lean body mass and the wasting syndrome termed cardiac cachexia.
An ongoing structural remodelling of myocardium accompanies this systemic illness. This includes a concentration-dependent oxidative stress-induced loss of cardiomyocytes, initially via apoptotic and ultimately via necrotic death pathways. Its extracellular matrix (ECM), that includes a fibrillar collagen scaffolding, is also involved. In both post-mortem and explanted failing human hearts harvested at the time of cardiac transplantation, ECM remodelling has been described in morphological terms as an adverse accumulation of fibrous tissue presenting as a perivascular/interstitial fibrosis, and as microscopic scarring replacing necrotic cardiomyocytes, and where cardiomyocytes, surrounded by fibrous tissue, become atrophic.16,17 A degradation of the collagenous scaffolding, induced by its proteolytic degradation and mediated by Zn-dependent matrix metalloproteinases, is an important pathogenic feature of the dilated thin-walled myocardium and is associated with muscle fibre slippage.18–20
Thus, factors contributing to the causes and consequences of the systemic illness that accompanies hypertension and CHF are simultaneously operative in promoting the heart’s ongoing structural remodelling. It is from this molecular perspective the present report has been structured. It will focus on the role of several macro- and micronutrients and their contribution to the adverse structural remodelling of myocardium found in the failing heart associated with hypertensive heart disease and the clinical syndrome of CHF.
3. Macro- and micronutrients and myocardial remodelling
3.1. Calcium dyshomeostasis
Intracellular Ca2+ overloading, including cytosolic and mitochondrial, occurs as a pathophysiological response integral to the induction of oxidative stress and the subsequent appearance of cell injury. Such a scenario occurs with ischaemia/reperfusion (I/R) injury, catecholamine-induced cardiomyocyte necrosis, the secondary hyperparathyroidism (SHPT) that accompanies (see Table 1) either aldosteronism, chronic renal failure, or high dietary Na+, and the cardiomyopathy that appears in association with Duchenne muscular dystrophy. An altered redox state, where ROS and RNS overwhelm endogenous antioxidant defenses, leads to a concentration-dependent loss of cardiomyocytes. Apoptotic cell death is not accompanied by an inflammatory cell response and fibroblast-related repair and, therefore, a replacement fibrosis, or scarring, does not appear. At higher [Ca2+]i concentrations, ROS and RNS lead to necrotic cell death, which is followed by invading inflammatory cells and fibroblasts, and consequent replacement fibrosis. Microscopic scarring, in this case, appears at these sites and is left as a footprint of prior necrosis. In the setting where a circulating substance is involved in promoting intracellular Ca2+ overloading with oxidative stress and cardiomyocyte necrosis, such as accompanies elevations in plasma PTH or catecholamines, myocardial scarring is present in both the right and left heart.
Table 1.
Factors contributing to the appearance of secondary hyperparathyroidism in patients with hypertension or congestive heart failure
| Hypovitaminosis D |
| Reduced dietary Ca2+ |
| Increased dietary Na+ |
| Loop diuretic |
| Chronic renal failure |
| Aldosteronism |
| Hypoalbuminaemia |
| Hypocalcaemia |
| Hypomagnesaemia |
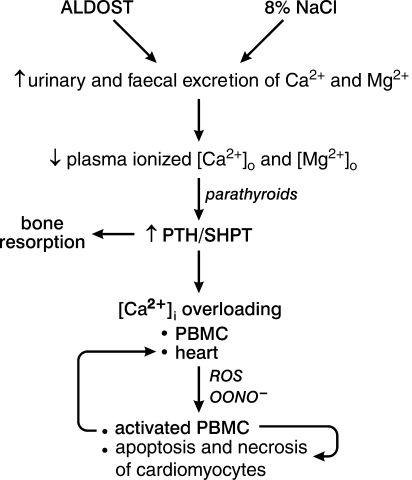
In aldosteronism, an integral feature of CHF and some forms of hypertension, intracellular Ca2+ overloading of diverse tissues occurs invariably and is PTH-mediated. As shown in Figure 1, elevations in circulating PTH occur in response to ionized hypocalcaemia and hypomagnesaemia caused by the heightened urinary and faecal excretion of Ca2+ and Mg2+ that accompanies aldosterone/1% NaCl treatment (ALDOST).21–27 SHPT is invoked during ALDOST to restore extracellular Ca2+ and Mg2+ homeostasis through bone resorption,28 and increased Ca2+ resorption from the kidney and gastrointestinal tract. The important role of PTH-mediated intracellular Ca2+ overloading is further evidenced by the hypertension, left ventricular hypertrophy, and adverse structural remodelling of myocardium, as well as myocardial and valvular calcification, arrhythmia and abnormal conduction, and altered vasomotor reactivity with vascular remodelling found in primary hyperparathyroidism.29,30 A high-Na+ diet (8%), which suppresses plasma aldosterone levels, is calciuric in rats and man, and like ALDOST it also leads to SHPT with PTH-mediated bone resorption and intracellular Ca2+ overloading (Figure 1).27,31,32 Low-renin hypertension is also accompanied by ionized hypocalcaemia, increased plasma PTH with elevations in platelet [Ca2+]i, and a favourable reduction in elevated blood pressure to dietary Ca2+ supplement or Ca2+ channel blocker.1,2,33–35
Figure 1.
In aldosteronism (ALDOST), where plasma aldosterone levels are inappropriately elevated relative to dietary Na+ intake, marked excretory losses of Ca2+ and Mg2+ lead to a fall in their plasma ionized concentrations. Reduced [Ca2+]o and [Mg2+]o are, respectively, major and minor stimuli to the parathyroid glands’ secretion of parathyroid hormone (PTH), with secondary hyperparathyroidism (SHPT) accounting for bone resorption in an attempt to restore the homeostasis of these divalent cations. In what is coined as a calcium paradox, elevations in plasma PTH promote intracellular Ca2+ overloading and induction of oxidative stress. Reactive oxygen species (ROS) and peroxynitrite (OONO−) contribute to intracellular signalling that, in a concentration-dependent manner, eventuates in cell activation (e.g. peripheral blood mononuclear cells, PBMC) and the expression of apoptotic and necrotic cell death pathways in cardiomyocytes. Urinary and faecal losses of Zn are likewise increased during ALDOST (data not shown). A high (8%) Na+ diet, which suppresses plasma aldosterone levels, also leads to SHPT because of increased excretory losses of Ca2+.
Oxidative stress is induced in diverse tissues during SHPT and is expressed by increased plasma 8-isoprostane, activation of NADPH oxidase with increased superoxide production, increased tissue levels of 3-nitrotyrosine, a stable product of peroxynitrite, formed by the reaction between short-lived superoxide and nitric oxide, and activation of redox-sensitive nuclear transcription factor (NF)-κB with a proinflammatory gene cascade it encodes.36–40 PTH receptors are found in various tissues, including heart, skeletal muscle, and immune cells. In the case of lymphocytes and monocytes, a proinflammatory phenotype accompanies the PTH-mediated intracellular Ca2+ overloading, and their activation leads to an invasion of the perivascular space of intramyocardial coronary and renal vasculature and mesenteric circulation. The increase in biologically active cytosolic free [Ca2+]i is coupled with their increased production of H2O2 and altered transcriptome.41,42 Upregulated gene expression in these cells includes antioxidant defenses, adhesion molecules, and proinflammatory chemokines and cytokines. This vasculitis, together with enhanced fibroblast collagen synthesis,43 leads to perivascular fibrosis. If sustained, the fibrous tissue response extends into the contiguous interstitial space resulting in interstitial fibrosis. Interventions which interfere with this pathophysiological scenario attenuates the appearance of microscopic scarring, and perivascular/interstitial fibrosis of the right and left atria and ventricles.44 These cardioprotective measures include: cotreatment with spironolactone, an aldosterone receptor antagonist, that prevents the increased urinary and faecal losses of Ca2+ and Mg2+ 21,39,45; parathyroidectomy performed prior to initiating ALDOST;46,47 cotreatment with either a calcium channel blocker or an exogenous antioxidant, or with an inhibitor of NADPH oxidase or an SOD mimetic.39,48–51
Thus, Ca2+ dyshomeostasis, together with PTH-mediated intracellular Ca2+ overloading and induction of oxidative stress, is integral to the adverse structural remodelling of myocardium that includes cardiomyocyte necrosis with scarring and appearance of an immunostimulatory state leading to vasculitis, and ultimately, a perivascular fibrosis of the intramural coronary circulation extending into the contiguous interstitial space. Iterations in Ca2+ balance, however, rarely occur in isolation. Mg2+ is a natural antagonist to intracellular Ca2+ entry through L-type Ca2+ channels and mitochondrial permeability transition pore.52 The contribution of hypomagnesaemia to cardiac remodelling has been studied in rodents using a Mg2+-deficient diet.
3.2. Magnesium dyshomeostasis
Hypomagnesaemia occurs in patients with diabetes, metabolic syndrome, alcoholism, HIV, those receiving Mg-wasting drugs, and critically ill cancer patients.53 Hypomagnesaemia has been reported in 63% of intensive care patients and up to 45% of patients with acute myocardial infarction and is associated with increased mortality.54 Hereditary hypomagnesaemia can cause a progressive dilated cardiomyopathy and heart failure.55
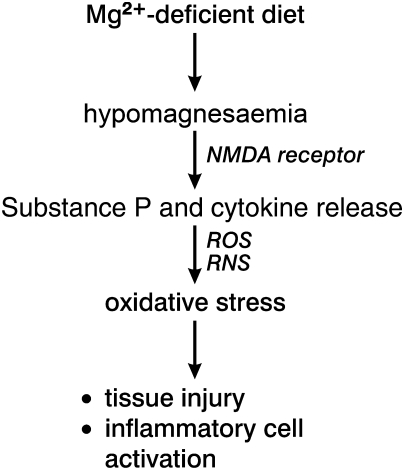
Severe dietary deficiency of Mg2+ (MgD) in animal models causes myocardial necrosis, neuromuscular hyperexcitability, arrhythmias, increased oxidative stress, and enhanced myocardial susceptibility to I/R stress.56 Circulating levels of proinflammatory neuropeptides, substance P (SP), and calcitonin gene-related peptide are increased in MgD due to their release from sensory-motor neurons (Figure 2). These neuropeptides may trigger inflammatory/oxidative events which promote cardiomyopathy.57 Increases in PGE2, circulating histamine, and hypersensitivity to applied catecholamine stress also occur. Importantly, elevations of plasma SP preceded that of nitric oxide (NO) in severe MgD rats and this was concurrent with an indicator of systemic oxidative stress, red blood cell glutathione (GSH) loss.58 L-NAME treatment attenuated this GSH depletion, suggesting a prooxidant role for NO during MgD. The N-methyl-d-aspartate (NMDA) receptor complex is an important mediator of neuropeptide release and this receptor is blocked by Mg2+ in a voltage-dependent manner. Pretreatment of MgD rats with MK-801, an NMDA receptor blocker, prevented SP loss from dorsal root ganglia59 and myocardial ICAM expression was decreased along with CD11b-positive inflammatory cells.59 SP also alters the functional state of endothelial cells, mast cells, macrophages, polymorphonuclear leukocytes (PMNs), and T-lymphocytes. MgD resulted in the elevation of T-cell-derived IFN-γ which was blocked by an SP receptor (NK-1) antagonist and MgD increased the number of circulating PMNs, which displayed significant increases (up to 10-fold) in basal superoxide production indicating systemic oxidative stress; SP receptor blockade also attenuated endogenous PMN activation to reduce superoxide generation.60,61
Figure 2.
Dietary Mg2+ deficiency is accompanied by hypomagnesaemia and release of substance P and cytokines, with ROS and RNS generation leading to oxidative stress, subsequent tissue injury, and inflammatory cell activation.
The gut is also rich in neuropeptides, and during MgD severe mucosal inflammation occurs with pronounced PMN infiltration along with enhanced gut permeability that may release endotoxin or lipopolysaccharide (LPS) into circulation.62 LPS alone induces systemic elevations of TNF-α, IL-1α, and IL-6, which mediate chronic cardiac dysfunction, and it can also stimulate TNF-α production by adult rat cardiomyocytes by activating LPS receptors (CD14),63 which are upregulated in MgD cardiac tissue.64 Thus, the substantial increases in plasma TNF-α, IL-1, and IL-6 in MgD rats are due, in part, to SP-mediated gut permeability that increases circulating LPS. SP receptor blockade in MgD rats significantly lowers plasma TNF-α levels in plasma and cardiac tissue,57 implicating a combined SP and LPS-mediated proinflammatory cascade. TNF-α may be elevated in chronic heart failure along with IL-6. Cardiac-specific overexpression of TNF-α resulted in a heart failure phenotype in mice that exhibited left ventricular dysfunction and cardiac remodelling.65 TNF-α is a major contributor to the cardiomyopathy of MgD since it was markedly elevated in both the plasma and myocardial lesions after only 3 weeks of MgD.66
Thus, both a blockade of SP release from neural tissue and inhibition of the SP receptor significantly reduce the proinflammatory state in the hearts of MgD animals. The prooxidant elevations of free radicals in the I/R rat heart were also inhibited by pretreatment with SP receptor blockers. Additional studies with antioxidant drugs also showed cardioprotection, since treatment of MgD rats with sustained-release pellets containing alpha-tocopherol, probucol, d-propranolol (non-beta-blocking form), and epicaptopril (the SH-donor stereoisomer of captopril) significantly reduced focal myocardial lesions.
In summary, SP can produce free radicals directly (superoxide and NO) and indirectly (by stimulating cytokine release). Blockade at each level of this neurogenic inflammatory cascade (the SP receptor, the NMDA receptor, and antioxidant treatment) prevented these prooxidant effects in MgD animals. Overall, these studies of cardiomyopathy due to MgD reveal striking parallels with multiple clinical disorders where hypomagnesaemia is present. Translation of therapies that are effective in these experimental MgD models to clinical applications represent a challenge for future studies.
3.3. Zinc dyshomeostasis
Zinc is an essential micronutrient integral to the activity of various metalloenzymes that include angiotensin-converting enzyme and matrix metalloproteinases.67,68 Hypozincaemia accompanies bodily injury including acute myocardial infarction.69–71 Zinc deficiency with hypozincaemia, coupled with an associated impairment in Zn-dependent metalloenzymes, has been reported in the elderly, in patients with hypertension, and in those with CHF having dilated cardiomyopathy.3–6,8,72–74
In the case of aldosteronism, a fall in plasma Zn is related to its increased urinary and faecal excretion and to its preferential translocation to sites of injury, including the heart, where it contributes to tissue repair and to antioxidant defenses provided by increased Cu/Zn-SOD activity.75–77 Further evidence in favour of a Zn deficiency during ALDOST includes: reduced plasma Cu/Zn-SOD activity; a fall in bone Zn that occurs in response to PTH-mediated bone resorption; thymic atrophy; and a failure to gain weight.75,76
The translocation of Zn to tissues, where it serves as an antioxidant, is intrinsically coupled to intracellular Ca2+ overloading that acts as a prooxidant. The relative preponderance of prooxidant:antioxidant determines the heart’s redox state and fate of cardiomyocytes. A Zn supplement in the setting of aldosteronism serves to attenuate scarring in keeping with reduced oxidative stress-induced cardiomyocyte necrosis. It does not prevent coronary vasculitis and subsequent perivascular fibrosis since the associated ionized hypocalcaemia and SHPT are not corrected by ZnSO4 cotreatment.78 However, Zn supplementation has proved efficacious in preventing a diabetic cardiomyopathy in streptozocin-treated rodents,79 and is cardioprotective in the Ca2+ overloading associated with I/R injury, each of which are not associated with SHPT.80
3.4. Selenium dyshomeostasis
Selenium is another essential micronutrient. Relevant to cardiac remodelling, the main selenoproteins are glutathione peroxidase (GSHPx) and thioredoxin reductase.81 A Se-deficient diet, as occurs with the consumption of vegetables grown in the Se-poor soil in the Keshan Province of China, is associated with the appearance of a DCM in local children known as Keshan’s disease, which is often reversible with Se supplementation.82 Se deficiency has reappeared in western medicine secondary to gastrointestinal disorders interfering with or contributing to the loss of dietary Se (e.g. Crohn’s disease), bariatric surgery for weight reduction, and long-term parenteral nutrition (TPN) devoid of Se. Several cases of life-threatening heart failure and DCM have been reported in patients on home TPN, or after marked weight loss following bariatric surgery.82 Reductions in serum Se, albeit of uncertain origins, have been found in African-American patients who reside in Memphis, Tennessee, and have a DCM. Tennessee soil is not known to be Se-deficient.8
Morphological features of this Se-deficient DCM include a widespread myocytolysis with replacement fibrosis scattered throughout the right and left ventricles and atria.83–85 In addition, diminished Se concentrations and reduced activities of GSHPx are found in blood, heart, liver, kidney, and skeletal muscle.83,86 Fuyu87 has suggested that Keshan’s disease is a mitochondrial cardiomyopathy with enlarged, swollen, and dysfunctional mitochondria having reduced oxidative phosphorylation and GSHPx activity, coupled with increased Ca2+ content. Furthermore, transgenic mice lacking functional mitochondrial thioredoxin reductase have been shown to develop a fatal form of cardiomyopathy.88 Further investigation into the role of Se deficiency in the appearance of a DCM is warranted.
3.5. Copper dyshomeostasis
Other micronutrients, such as Cu2+ and iron can also contribute to an adverse remodelling of myocardial structure. However, a discussion of the adverse consequences of iron deficiency or iron overload is beyond the scope of this brief review.
In both rodents or pigs, a deficiency of Cu2+ has been found to be accompanied by a remodelling of myocardium. This includes the appearance of a dilated, thin-walled cardiomyopathy and even its rupture.89 At an ultrastructural level, non-aligned myofibrils with disrupted Z bands are accompanied by increased volume density of mitochondria with disarranged cristae.90–92 A repletion of dietary Cu2+ is accompanied by a reversal of these iterations in structure. The role of Cu in regulating the activity of lysyl oxidase, integral to promoting the crosslinking of collagen and elastin, and Cu/Zn-superoxide dismutase, an antioxidant defense mechanism, is thought to contribute to the slippage of muscle fibres, a weakening of myocardium, and unbridled oxidative stress that includes structural–functional impairments of its mitochondria.
3.6. Vitamin D dyshomeostasis
Low vitamin D status affects myocardial structure and function and this relationship has clinical relevance.11,93,94 Vitamin D3 deficiency and reduced levels of the active vitamin D metabolite, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) have been associated with the aetiology and pathogenesis of CHF.11 More recently a study of 18 225 men enrolled in the Health Professionals Follow-Up Study reported that men with low vitamin D status had 2.5 times the risk of having a myocardial infarction.95 Zittermann et al.96 have recently reported that low serum calcitriol levels are independently associated with poor clinical outcome in patients with CHF awaiting cardiac transplantation. In these patients, whose lifestyle is often constrained to housebound because of symptomatic heart failure, marked reductions in plasma 25(OH)D levels are often present, together with elevations in serum PTH in keeping with SHPT.97,98 In the absence of sunlight therapy, addressing the optimal intake of vitamin D to correct the profound levels of vitamin D deficiency found in these patients becomes a major challenge.99
Prior to these clinical observations, studies from the Simpson lab demonstrated that vitamin D3 deficiency alters myocardial function, morphology, and ECM.100–102 Recently, it was shown that ablation of the vitamin D receptor (VDR) signalling system results in profound changes in heart structure103 and that the VDR knockout (VDRKO) mouse phenotype is characterized by cardiac hypertrophy and fibrosis with increased interstitial collagen deposition.104 Animal studies have revealed that 1,25(OH)2D3 affects two processes central to cardiomyocyte function. First, 1,25(OH)2D3 was shown to alter Ca2+ handling resulting in increased sensitivity of heart to contractile stimuli, and second it influences remodelling of heart and increases heart size and collagen content.11,93,103–105
Analysis of the promoter region of the collagen-I (COL1A1) gene shows sequence homology to a VDR responsive element.105,106 Moreover, matrix metalloproteinase-13 (MMP-13) has been shown to be transcriptionally upregulated by 1,25(OH)2D3 in osteoblasts.107 Thus, vitamin D status has been linked to the regulation of ECM formation, turnover, and integrity in target tissues, including heart. Fibrosis is a classic feature of cardiac hypertrophy characterized by the turnover of ECM and the accumulation of collagen, particularly, collagen type I.108 The collagen content of the heart is determined by a balance between the synthesis and degradation of collagen, which has been shown to be consistently increased in the models of heart disease.109 MMPs and tissue inhibitors of metalloproteinases (TIMPs) contribute to tissue remodelling in a number of disease states, including heart disease. ADAMTS-1, like MMP-2, possesses gelatinolytic activity, thus degrades type I collagen.110 A recent report showed greater accumulation of total collagen, fibrosis, and COL1A1 protein levels in the hypertrophic hearts of VDRKO mice.104 This observation was interpreted as being a result of VDR ablation affecting COL1A1 stability through an alteration of MMP, TIMP, and ADAMTS expression in heart and associated cells. It has been shown that MMPs have a predominant role in hydrolyzing ECM proteins and these enzymes have been proposed to mediate collagen degradation leading to left ventricular dilation, and ultimately to CHF.111 The complex nature of heart failure-related remodelling has been addressed in recent studies showing that caveolin-1 null-mutant mice have increased MMP-2 activity,112 and angiotensin-converting enzyme inhibitors reduce MMP-2 activity.113 Furthermore, left ventricular hypertrophy has been associated with increased MMP-2 activity, and its transition to heart failure with increased TIMP-2 levels and collagen deposition.114
In an effort to better understand VDR’s role in ventricular remodelling and fibrosis, microarray and real-time RT–PCR were used to identify possible ECM genes that are differentially expressed in VDRKO mice (unpublished observations). Subsequently, levels of the identified ECM gene products were analysed by immunoblotting. In these studies, it was found that hypertrophic VDRKO hearts had increased MMP-2 expression at both the transcriptional and translational levels. Increased TIMP-2 mRNA and protein expression, with a concomitant decrease in TIMP-1 and TIMP-3 gene expression in the hearts of female VDRKO mice relative to the WT mice, was also observed. Moreover, there was a significant increase in VDRKO/WT ratio for TIMP-2 protein expression in female mice when compared with the male VDRKO mice, indicative of a lesser degree of heart remodelling in females. This approach ultimately revealed that a member of ADAMTS subfamily, ADAMTS-1, is upregulated both at transcriptional and protein levels in VDRKO mice when compared with control wild type (WT) mice. Overall, it was observed that expression of ADAMTS, collagen, MMP, and TIMP forms are regulated in the cascade of events leading to ventricular remodelling in vivo in the VDRKO mouse.
Studies have shown a relationship between the robust increase in TIMPs and degree of left ventricular hypertrophy in patients with heart failure.115 It has been reported that TIMP-1 and TIMP-2 form a complex with MMP-1 and MMP-2, respectively.116 However, the modulation of TIMPs expression was reported to be independent of MMP expression.117 It has been shown that the ratios, specifically of TIMP-2/MMP-2, were significantly increased in the left ventricular remodelling, and suggest that upregulation of TIMP-2 expression might be independent of MMP-2 expression in ventricular remodelling, and ultimately cardiac hypertrophy. Studies suggest that the observed decrease in remodelling in female mice involves an increased relative expression of TIMP-2 in female VDRKO vs. WT mice, in contrast to the increase in male VDRKO TIMP-2 levels. The mechanism of regulation of TIMP-2 at present is unclear. However, a transcriptional action of vitamin D on TIMP-2 expression is supported by the presence of an AP-1 element in the promoter of its gene.118 Cardiomyocyte Ca2+ handling and contraction requires PKC activation, and thus PKC activity could modulate transcriptional regulation of such AP-1 elements.119
Increased MMP-2 activity is found in the fibrotic hearts of the VDRKO mice when compared with WT mice.104 The increase in MMP-2 activity, and its expression in the VDRKO mice, may be induced by the alterations in the myocardial environment that take place in response to hypertrophic stimuli, such as lack of cardiac VDR signalling and/or release of inflammatory mediators. An increase in MMP-2 protein expression, with concomitant increase of TIMP-2, is associated with the enhanced COL1A1 levels and fibrosis and cardiac hypertrophy in VDRKO mice. TIMPs bind to the active site of MMP, blocking their access for degradation of collagen substrates.119 In addition, ADAMTS-1 is partially inhibited by TIMP-2 and TIMP-3.120,121
Thus, vitamin D levels play an important role in maintaining myocardial viability and ECM integrity by regulating the dynamics (activity, production, and expression) between MMPs, ADAMTSs, and TIMPs in heart remodelling. These findings demonstrate a collective role for ADAMTS-1, COL1A1, MMP-2, and its endogenous inhibitors (TIMPs) in the cardiac hypertrophy and fibrosis that accompanies vitamin D deficiency101,102 and VDR ablation.104
4. Summary and conclusions
The heart’s failure as a muscular pump has its origins rooted in an adverse structural, biochemical, and molecular remodelling of myocardium that includes its cardiomyocytes, ECM, and intramural coronary vasculature. The ongoing nature of such remodelling contributes to the progressive nature of heart failure.
Contributory to such pathological remodelling, irrespective of the aetiological origins of heart failure, is a dyshomeostasis of such macro- and micronutrients as Ca2+, Mg2+, Zn2+, Se2+, and vitamin D, which are predominantly derived from external sources and often inextricably dependent on one another (e.g. hypovitaminosis D begets SHPT with PTH-mediated [Ca2+]i overloading). Insufficient dietary intake, inadequate sunlight exposure, excessive excretory losses, and/or a preferential translocation of cations from the intravascular compartment to injured tissues, where they contribute to wound healing, lead to disturbances in their homeostasis. The resulting nutrient imbalance is the basis for a common pathophysiological response, i.e. the induction of oxidative stress. ROS and RNS overwhelm endogenous antioxidant defenses, and lead to untoward intracellular signalling that accounts for reduced cardiomyocyte survival, an immunostimulatory state with activated inflammatory cells contributing to a proinflammatory vascular phenotype, and the appearance of fibrous tissue (or fibrosis). The replacement of any one of these nutrients alone will not suffice and would not prove to be completely cardioprotective. Polynutrient supplements are therefore essential to correct the dyshomeostasis of these interconnected nutrients and to prevent adverse myocardial remodelling.
An improved understanding of how these macro- and micronutrients account for the causes and consequences of adverse myocardial remodelling carries with it the potential of identifying new biomarkers predictive of risk, onset and progression, and response to intervention(s), which can be monitored non-invasively and serially over time. Moreover, such incremental knowledge will serve as the underpinning to the development of novel strategies aimed at preventing and/or regressing the ongoing adverse remodelling of myocardium. The time is at hand and propitious to recognize the importance of macro- and micronutrient dyshomeostasis in the evaluation of hypertension and CHF.
Funding
This work was supported, in part, by National Institutes of Health/National Heart, Lung, and Blood Institute grants R01-HL073043 (K.T.W.), R01-HL062282, R01-HL065178 (W.B.W.), and R01-HL074894 (R.U.S.).
Conflict of interest: none declared.
References
- 1.Resnick LM, Laragh JH. Renin, calcium metabolism and the pathophysiologic basis of antihypertensive therapy. Am J Cardiol. 1985;56:68H–74H. doi: 10.1016/0002-9149(85)90547-8. [DOI] [PubMed] [Google Scholar]
- 2.McCarron DA, Pingree PA, Rubin RJ, Gaucher SM, Molitch M, Krutzik S. Enhanced parathyroid function in essential hypertension: a homeostatic response to a urinary calcium leak. Hypertension. 1980;2:162–168. doi: 10.1161/01.hyp.2.2.162. [DOI] [PubMed] [Google Scholar]
- 3.Oster O. Trace element concentrations (Cu, Zn, Fe) in sera from patients with dilated cardiomyopathy. Clin Chim Acta. 1993;214:209–218. doi: 10.1016/0009-8981(93)90112-h. [DOI] [PubMed] [Google Scholar]
- 4.Ripa S, Ripa R, Giustiniani S. Are failured cardiomyopathies a zinc-deficit related disease? A study on Zn and Cu in patients with chronic failured dilated and hypertrophic cardiomyopathies. Minerva Med. 1998;89:397–403. [PubMed] [Google Scholar]
- 5.Kosar F, Sahin I, Acikgoz N, Aksoy Y, Kucukbay Z, Cehreli S. Significance of serum trace element status in patients with rheumatic heart disease: a prospective study. Biol Trace Elem Res. 2005;107:1–10. doi: 10.1385/BTER:107:1:001. [DOI] [PubMed] [Google Scholar]
- 6.Topuzoglu G, Erbay AR, Karul AB, Yensel N. Concentrations of copper, zinc, and magnesium in sera from patients with idiopathic dilated cardiomyopathy. Biol Trace Elem Res. 2003;95:11–17. doi: 10.1385/bter:95:1:11. [DOI] [PubMed] [Google Scholar]
- 7.LaGuardia SP, Dockery BK, Bhattacharya SK, Nelson MD, Carbone LD, Weber KT. Secondary hyperparathyroidism and hypovitaminosis D in African-Americans with decompensated heart failure. Am J Med Sci. 2006;332:112–118. doi: 10.1097/00000441-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Arroyo M, LaGuardia SP, Bhattacharya SK, Nelson MD, Johnson PL, Carbone LD, et al. Micronutrients in African-Americans with decompensated and compensated heart failure. Transl Res. 2006;148:301–308. doi: 10.1016/j.trsl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Koren-Michowitz M, Dishy V, Zaidenstein R, Yona O, Berman S, Weissgarten J, et al. The effect of losartan and losartan/hydrochlorothiazide fixed-combination on magnesium, zinc, and nitric oxide metabolism in hypertensive patients: a prospective open-label study. Am J Hypertens. 2005;18:358–363. doi: 10.1016/j.amjhyper.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Lee AH, Mull RL, Keenan GF, Callegari PE, Dalinka MK, Eisen HJ, et al. Osteoporosis and bone morbidity in cardiac transplant recipients. Am J Med. 1994;96:35–41. doi: 10.1016/0002-9343(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 11.Shane E, Mancini D, Aaronson K, Silverberg SJ, Seibel MJ, Addesso V, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197–207. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 12.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 13.Alsafwah S, LaGuardia SP, Nelson MD, Battin DL, Newman KP, Carbone LD, et al. Hypovitaminosis D in African Americans residing in Memphis, Tennessee with and without heart failure. Am J Med Sci. 2008;335:292–297. doi: 10.1097/MAJ.0b013e318167b0bd. [DOI] [PubMed] [Google Scholar]
- 14.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 15.Weber KT. The proinflammatory heart failure phenotype: a case of integrative physiology. Am J Med Sci. 2005;330:219–226. doi: 10.1097/00000441-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Cotran RS, Kumar V, Robbins SL. Robbins' Pathologic Basis of Disease. 4th ed. Philadelphia: WB Saunders; 1989. [Google Scholar]
- 17.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 18.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 19.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 20.Creemers EE, Cleutjens JP, Smits JF, Daemen MJ. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure? Circ Res. 2001;89:201–210. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 21.Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005;111:871–878. doi: 10.1161/01.CIR.0000155621.10213.06. [DOI] [PubMed] [Google Scholar]
- 22.Horton R, Biglieri EG. Effect of aldosterone on the metabolism of magnesium. J Clin Endocrinol Metab. 1962;22:1187–1192. doi: 10.1210/jcem-22-12-1187. [DOI] [PubMed] [Google Scholar]
- 23.Rastegar A, Agus Z, Connor TB, Goldberg M. Renal handling of calcium and phosphate during mineralocorticoid ‘escape’ in man. Kidney Int. 1972;2:279–286. doi: 10.1038/ki.1972.107. [DOI] [PubMed] [Google Scholar]
- 24.Zikos D, Langman C, Gafter U, Delahaye B, Lau K. Chronic DOCA treatment increases Ca absorption: role of hypercalciuria and vitamin D. Am J Physiol. 1986;251:E279–E284. doi: 10.1152/ajpendo.1986.251.3.E279. [DOI] [PubMed] [Google Scholar]
- 25.Rossi E, Sani C, Perazzoli F, Casoli MC, Negro A, Dotti C. Alterations of calcium metabolism and of parathyroid function in primary aldosteronism, and their reversal by spironolactone or by surgical removal of aldosterone-producing adenomas. Am J Hypertens. 1995;8:884–893. doi: 10.1016/0895-7061(95)00182-O. [DOI] [PubMed] [Google Scholar]
- 26.Gehr MK, Goldberg M. Hypercalciuria of mineralocorticoid escape: clearance and micropuncture studies in the rat. Am J Physiol. 1986;251:F879–F888. doi: 10.1152/ajprenal.1986.251.5.F879. [DOI] [PubMed] [Google Scholar]
- 27.Cappuccio FP, Kalaitzidis R, Duneclift S, Eastwood JB. Unravelling the links between calcium excretion, salt intake, hypertension, kidney stones and bone metabolism. J Nephrol. 2000;13:169–177. [PubMed] [Google Scholar]
- 28.Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, Xing Z, et al. Loss of bone minerals and strength in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2004;287:H2023–H2026. doi: 10.1152/ajpheart.00477.2004. [DOI] [PubMed] [Google Scholar]
- 29.Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease–a review. Eur Heart J. 2004;25:1776–1787. doi: 10.1016/j.ehj.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Stefenelli T, Mayr H, Bergler-Klein J, Globits S, Woloszczuk W, Niederle B. Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am J Med. 1993;95:197–202. doi: 10.1016/0002-9343(93)90260-v. [DOI] [PubMed] [Google Scholar]
- 31.Goulding A, McIntosh J. Effects of NaCl on calcium balance, parathyroid function and hydroxyproline excretion in prednisolone-treated rats consuming low calcium diet. J Nutr. 1986;116:1037–1044. doi: 10.1093/jn/116.6.1037. [DOI] [PubMed] [Google Scholar]
- 32.Titze J, Rittweger J, Dietsch P, Krause H, Schwind KH, Engelke K, et al. Hypertension, sodium retention, calcium excretion and osteopenia in Dahl rats. J Hypertens. 2004;22:803–810. doi: 10.1097/00004872-200404000-00024. [DOI] [PubMed] [Google Scholar]
- 33.Resnick LM, Nicholson JP, Laragh JH. Calcium metabolism and the renin-aldosterone system in essential hypertension. J Cardiovasc Pharmacol. 1985;7(Suppl. 6):S187–S193. doi: 10.1097/00005344-198500076-00033. [DOI] [PubMed] [Google Scholar]
- 34.Resnick LM, Müller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med. 1986;105:649–654. doi: 10.7326/0003-4819-105-5-649. [DOI] [PubMed] [Google Scholar]
- 35.McCarron DA, Morris CD. Epidemiological evidence associating dietary calcium and calcium metabolism with blood pressure. Am J Nephrol. 1986;6(Suppl. 1):3–9. doi: 10.1159/000167206. [DOI] [PubMed] [Google Scholar]
- 36.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. Am J Hypertens. 2004;17:31–36. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Pu Q, Neves MF, Virdis A, Touyz RM, Schiffrin EL. Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodeling. Hypertension. 2003;42:49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. [DOI] [PubMed] [Google Scholar]
- 38.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart. Role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD., Jr Renal NF-κB activation and TNF-α upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1817–R1824. doi: 10.1152/ajpregu.00153.2006. [DOI] [PubMed] [Google Scholar]
- 41.Ahokas RA, Warrington KJ, Gerling IC, Sun Y, Wodi LA, Herring PA, et al. Aldosteronism and peripheral blood mononuclear cell activation. A neuroendocrine-immune interface. Circ Res. 2003;93:e124–e135. doi: 10.1161/01.RES.0000102404.81461.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerling IC, Sun Y, Ahokas RA, Wodi LA, Bhattacharya SK, Warrington KJ, et al. Aldosteronism: an immunostimulatory state precedes the proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H813–H821. doi: 10.1152/ajpheart.00113.2003. [DOI] [PubMed] [Google Scholar]
- 43.Chapman D, Eghbali M. Expression of fibrillar types I and III and basement membrane collagen type IV genes in myocardium of tight skin mouse. Cardiovasc Res. 1990;24:578–583. doi: 10.1093/cvr/24.7.578. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Ramires FJA, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 45.Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricle in experimental hypertension. Circ Res. 1990;67:1355–1364. doi: 10.1161/01.res.67.6.1355. [DOI] [PubMed] [Google Scholar]
- 46.Nickerson PA, Conran RM. Parathyroidectomy ameliorates vascular lesions induced by deoxycorticosterone in the rat. Am J Pathol. 1981;105:185–190. [PMC free article] [PubMed] [Google Scholar]
- 47.Vidal A, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC, Weber KT. The calcium paradox of aldosteronism and the role of the parathyroid glands. Am J Physiol Heart Circ Physiol. 2006;290:H286–H294. doi: 10.1152/ajpheart.00535.2005. [DOI] [PubMed] [Google Scholar]
- 48.Nickerson PA. A low dose of a calcium antagonist (nitrendipine) ameliorates cardiac and renal lesions induced by DOC in the rat. Exp Mol Pathol. 1984;41:309–320. doi: 10.1016/0014-4800(84)90018-2. [DOI] [PubMed] [Google Scholar]
- 49.Ahokas RA, Sun Y, Bhattacharya SK, Gerling IC, Weber KT. Aldosteronism and a proinflammatory vascular phenotype. Role of Mg2+, Ca2+ and H2O2 in peripheral blood mononuclear cells. Circulation. 2005;111:51–57. doi: 10.1161/01.CIR.0000151516.84238.37. [DOI] [PubMed] [Google Scholar]
- 50.Park YM, Park MY, Suh YL, Park JB. NAD(P)H oxidase inhibitor prevents blood pressure elevation and cardiovascular hypertrophy in aldosterone- infused rats. Biochem Biophys Res Commun. 2004;313:812–817. doi: 10.1016/j.bbrc.2003.11.173. [DOI] [PubMed] [Google Scholar]
- 51.Iglarz M, Touyz RM, Viel EC, Amiri F, Schiffrin EL. Involvement of oxidative stress in the profibrotic action of aldosterone. Interaction with the renin-angiotensin system. Am J Hypertens. 2004;17:597–603. [PubMed] [Google Scholar]
- 52.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 53.Deheinzelin D, Negri EM, Tucci MR, Salem MZ, da Cruz VM, Oliveira RM, et al. Hypomagnesemia in critically ill cancer patients: a prospective study of predictive factors. Braz J Med Biol Res. 2000;33:1443–1448. doi: 10.1590/s0100-879x2000001200007. [DOI] [PubMed] [Google Scholar]
- 54.Rubeiz GJ, Thill-Baharozian M, Hardie D, Carlson RW. Association of hypomagnesemia and mortality in acutely ill medical patients. Crit Care Med. 1993;21:203–209. doi: 10.1097/00003246-199302000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Riggs JE, Klingberg WG, Flink EB, Schochet SS, Jr, Balian AA, Jenkins JJ., III Cardioskeletal mitochondrial myopathy associated with chronic magnesium deficiency. Neurology. 1992;42:128–130. doi: 10.1212/wnl.42.1.128. [DOI] [PubMed] [Google Scholar]
- 56.Kramer JH, Phillips TM, Weglicki WB. Magnesium-deficiency-enhanced post-ischemic myocardial injury is reduced by substance P receptor blockade. J Mol Cell Cardiol. 1997;29:97–110. doi: 10.1006/jmcc.1996.0255. [DOI] [PubMed] [Google Scholar]
- 57.Weglicki WB, Mak IT, Phillips PM. Blockade of cardiac inflammation in Mg2+ deficiency by substance P receptor inhibition. Circ Res. 1994;74:1009–1013. doi: 10.1161/01.res.74.5.1009. [DOI] [PubMed] [Google Scholar]
- 58.Mak IT, Komarov AM, Wagner TL, Stafford RE, Dickens BF, Weglicki WB. Enhanced NO production during Mg deficiency and its role in mediating red blood cell glutathione loss. Am J Physiol. 1996;271:C385–C390. doi: 10.1152/ajpcell.1996.271.1.C385. [DOI] [PubMed] [Google Scholar]
- 59.Tejero-Taldo MI, Chmielinska JJ, Gonzalez G, Mak IT, Weglicki WB. N-methyl-D-aspartate receptor blockade inhibits cardiac inflammation in the Mg2+-deficient rat. J Pharmacol Exp Ther. 2004;311:8–13. doi: 10.1124/jpet.104.070003. [DOI] [PubMed] [Google Scholar]
- 60.Mak IT, Dickens BF, Komarov AM, Wagner TL, Phillips TM, Weglicki WB. Activation of the neutrophil and loss of plasma glutathione during Mg-deficiency–modulation by nitric oxide synthase inhibition. Mol Cell Biochem. 1997;176:35–39. [PubMed] [Google Scholar]
- 61.Mak IT, Kramer JH, Weglicki WB. Suppression of neutrophil and endothelial activation by substance P receptor blockade in the Mg-deficient rat. Magnes Res. 2003;16:91–97. [PubMed] [Google Scholar]
- 62.Scanlan BJ, Tuft B, Elfrey JE, Smith A, Zhao A, Morimoto M, et al. Intestinal inflammation caused by magnesium deficiency alters basal and oxidative stress-induced intestinal function. Mol Cell Biochem. 2007;306:59–69. doi: 10.1007/s11010-007-9554-y. [DOI] [PubMed] [Google Scholar]
- 63.Comstock KL, Krown KA, Page MT, Martin D, Ho P, Pedraza M, et al. LPS-induced TNF-α release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 1998;30:2761–2775. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- 64.Chmielinska JJ, Tejero-Taldo MI, Mak IT, Weglicki WB. Intestinal and cardiac inflammatory response shows enhanced endotoxin receptor (CD14) expression in magnesium deficiency. Mol Cell Biochem. 2005;278:53–57. doi: 10.1007/s11010-005-2733-9. [DOI] [PubMed] [Google Scholar]
- 65.Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, et al. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor α can be modulated by anti-tumor necrosis factor α therapy. Proc Natl Acad Sci USA. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weglicki WB, Mak IT, Kramer JH, Dickens BF, Cassidy MM, Stafford RE, et al. Role of free radicals and substance P in magnesium deficiency. Cardiovasc Res. 1996;31:677–682. [PubMed] [Google Scholar]
- 67.Hambidge M. Human zinc deficiency. J Nutr. 2000;130(Suppl. 5S):1344S–1349S. doi: 10.1093/jn/130.5.1344S. [DOI] [PubMed] [Google Scholar]
- 68.Maret W, Krezel A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol Med. 2007;13:371–375. doi: 10.2119/2007-00036.Maret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan IK, Chua KS, Toh AK. Serum magnesium, copper, and zinc concentrations in acute myocardial infarction. J Clin Lab Anal. 1992;6:324–328. doi: 10.1002/jcla.1860060513. [DOI] [PubMed] [Google Scholar]
- 70.Arnaud J, Faure H, Bourlard P, Denis B, Favier AE. Longitudinal changes in serum zinc concentration and distribution after acute myocardial infarction. Clin Chim Acta. 1994;230:147–156. doi: 10.1016/0009-8981(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 71.Lekakis J, Kalofoutis A. Zinc concentrations in serum as related to myocardial infarction. Clin Chem. 1980;26:1660–1661. [PubMed] [Google Scholar]
- 72.Prasad AS, Fitzgerald JT, Hess JW, Kaplan J, Pelen F, Dardenne M. Zinc deficiency in elderly patients. Nutrition. 1993;9:218–224. [PubMed] [Google Scholar]
- 73.Tubek S. Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol Trace Elem Res. 2007;117:39–51. doi: 10.1007/BF02698082. [DOI] [PubMed] [Google Scholar]
- 74.Kosar F, Sahin I, Taskapan C, Kucukbay Z, Gullu H, Taskapan H, et al. Trace element status (Se, Zn, Cu) in heart failure. Anadolu Kardiyol Derg. 2006;6:216–220. [PubMed] [Google Scholar]
- 75.Thomas M, Vidal A, Bhattacharya SK, Ahokas RA, Sun Y, Gerling IC, et al. Zinc dyshomeostasis in rats with aldosteronism. Response to spironolactone. Am J Physiol Heart Circ Physiol. 2007;293:H2361–H2366. doi: 10.1152/ajpheart.00200.2007. [DOI] [PubMed] [Google Scholar]
- 76.Selektor Y, Parker RB, Sun Y, Zhao W, Bhattacharya SK, Weber KT. Tissue 65zinc translocation in a rat model of chronic aldosteronism. J Cardiovasc Pharmacol. 2008;51:359–364. doi: 10.1097/FJC.0b013e318165b96e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia Zozaya JL, Padilla Viloria M. [Alterations of calcium, magnesium, and zinc in essential hypertension: their relation to the renin-angiotensin- aldosterone system] [Spanish] Invest Clin. 1997;38(Suppl. 2):27–40. [PubMed] [Google Scholar]
- 78.Gandhi MS, Deshmukh PA, Kamalov G, Zhao T, Zhao W, Whaley JT, et al. Causes and consequences of zinc dyshomeostasis in rats with chronic aldosteronism. J Cardiovasc Pharmacol. 2008;52:245–252. doi: 10.1097/FJC.0b013e3181833eb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, et al. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113:544–554. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- 80.Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther. 2007;321:517–525. doi: 10.1124/jpet.107.119644. [DOI] [PubMed] [Google Scholar]
- 81.Alexander J. Selenium. Novartis Found Symp. 2007;282:143–149. [PubMed] [Google Scholar]
- 82.Boldery R, Fielding G, Rafter T, Pascoe AL, Scalia GM. Nutritional deficiency of selenium secondary to weight loss (bariatric) surgery associated with life-threatening cardiomyopathy. Heart Lung Circ. 2007;16:123–126. doi: 10.1016/j.hlc.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 83.Fleming CR, Lie JT, McCall JT, O'Brien JF, Baillie EE, Thistle JL. Selenium deficiency and fatal cardiomyopathy in a patient on home parenteral nutrition. Gastroenterology. 1982;83:689–693. [PubMed] [Google Scholar]
- 84.Nezelof C, Bouvier R, Dijoud F. Multifocal myocardial necrosis: a distinctive cardiac lesion in cystic fibrosis, lipomatous pancreatic atrophy, and Keshan disease. Pediatr Pathol Mol Med. 2002;21:343–352. doi: 10.1080/02770930290056550. [DOI] [PubMed] [Google Scholar]
- 85.Li GS, Wang F, Kang D, Li C. Keshan disease: an endemic cardiomyopathy in China. Hum Pathol. 1985;16:602–609. doi: 10.1016/s0046-8177(85)80110-6. [DOI] [PubMed] [Google Scholar]
- 86.Quercia RA, Korn S, O'Neill D, Dougherty JE, Ludwig M, Schweizer R, et al. Selenium deficiency and fatal cardiomyopathy in a patient receiving long-term home parenteral nutrition. Clin Pharm. 1984;3:531–535. [PubMed] [Google Scholar]
- 87.Fuyu Y. Keshan disease and mitochondrial cardiomyopathy. Sci China C Life Sci. 2006;49:513–518. doi: 10.1007/s11427-006-2041-y. [DOI] [PubMed] [Google Scholar]
- 88.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klevay LM. Cardiovascular disease from copper deficiency–a history. J Nutr. 2000;130:489S–492S. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Wang L, Schuschke DA, Zhou Z, Saari JT, Kang YJ. Marginal dietary copper restriction induces cardiomyopathy in rats. J Nutr. 2005;135:2130–2136. doi: 10.1093/jn/135.9.2130. [DOI] [PubMed] [Google Scholar]
- 91.Wildman RE, Medeiros DM, Hamlin RL, Stills H, Jones DA, Bonagura JD. Aspects of cardiomyopathy in copper-deficient pigs. Electrocardiography, echocardiography, and ultrastructural findings. Biol Trace Elem Res. 1996;55:55–70. doi: 10.1007/BF02784168. [DOI] [PubMed] [Google Scholar]
- 92.Davidson J, Medeiros DM, Hamlin RL. Cardiac ultrastructural and electrophysiological abnormalities in postweanling copper-restricted and copper-repleted rats in the absence of hypertrophy. J Nutr. 1992;122:1566–1575. doi: 10.1093/jn/122.7.1566. [DOI] [PubMed] [Google Scholar]
- 93.Reinhart GA. Vitamin D analogs: novel therapeutic agents for cardiovascular disease? Curr Opin Investig Drugs. 2004;5:947–951. [PubMed] [Google Scholar]
- 94.Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev. 2006;11:25–33. doi: 10.1007/s10741-006-9190-8. [DOI] [PubMed] [Google Scholar]
- 95.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zittermann A, Schleithoff SS, Gotting C, Dronow O, Fuchs U, Kuhn J, et al. Poor outcome in end-stage heart failure patients with low circulating calcitriol levels. Eur J Heart Fail. 2008;10:321–327. doi: 10.1016/j.ejheart.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 97.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 98.Zittermann A, Fischer J, Schleithoff SS, Tenderich G, Fuchs U, Koerfer R. Patients with congestive heart failure and healthy controls differ in vitamin D-associated lifestyle factors. Int J Vitam Nutr Res. 2007;77:280–288. doi: 10.1024/0300-9831.77.4.280. [DOI] [PubMed] [Google Scholar]
- 99.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 100.O'Connell TD, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of myocardial growth and c-myc levels in the rat heart. Biochem Biophys Res Commun. 1995;213:59–65. doi: 10.1006/bbrc.1995.2098. [DOI] [PubMed] [Google Scholar]
- 101.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258:E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 102.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79:1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–524. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 105.Kream BE, Rowe D, Smith MD, Maher V, Majeska R. Hormonal regulation of collagen synthesis in a clonal rat osteosarcoma cell line. Endocrinology. 1986;119:1922–1928. doi: 10.1210/endo-119-5-1922. [DOI] [PubMed] [Google Scholar]
- 106.Pavlin D, Bedalov A, Kronenberg MS, Kream BE, Rowe DW, Smith CL, et al. Analysis of regulatory regions in the COL1A1 gene responsible for 1,25-dihydroxyvitamin D3-mediated transcriptional repression in osteoblastic cells. J Cell Biochem. 1994;56:490–501. doi: 10.1002/jcb.240560409. [DOI] [PubMed] [Google Scholar]
- 107.Uchida M, Shima M, Chikazu D, Fujieda A, Obara K, Suzuki H, et al. Transcriptional induction of matrix metalloproteinase-13 (collagenase-3) by 1α,25-dihydroxyvitamin D3 in mouse osteoblastic MC3T3-E1 cells. J Bone Miner Res. 2001;16:221–230. doi: 10.1359/jbmr.2001.16.2.221. [DOI] [PubMed] [Google Scholar]
- 108.Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- 109.Graham HK, Trafford AW. Spatial disruption and enhanced degradation of collagen with the transition from compensated ventricular hypertrophy to symptomatic congestive heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1364–H1372. doi: 10.1152/ajpheart.00355.2006. [DOI] [PubMed] [Google Scholar]
- 110.Lind T, Birch MA, McKie N. Purification of an insect derived recombinant human ADAMTS-1 reveals novel gelatin (type I collagen) degrading activities. Mol Cell Biochem. 2006;281:95–102. doi: 10.1007/s11010-006-0637-y. [DOI] [PubMed] [Google Scholar]
- 111.Spinale FG, Coker ML, Krombach SR, Mukherjee R, Hallak H, Houck WV, et al. Matrix metalloproteinase inhibition during the development of congestive heart failure: effects on left ventricular dimensions and function. Circ Res. 1999;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 112.Chow AK, Cena J, El-Yazbi AF, Crawford BD, Holt A, Cho WJ, et al. Caveolin-1 inhibits matrix metalloproteinase-2 activity in the heart. J Mol Cell Cardiol. 2007;42:896–901. doi: 10.1016/j.yjmcc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 113.Brower GL, Levick SP, Janicki JS. Inhibition of matrix metalloproteinase activity by ACE inhibitors prevents left ventricular remodeling in a rat model of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H3057–H3064. doi: 10.1152/ajpheart.00447.2006. [DOI] [PubMed] [Google Scholar]
- 114.Tozzi R, Palladini G, Fallarini S, Nano R, Gatti C, Presotto C, et al. Matrix metalloprotease activity is enhanced in the compensated but not in the decompensated phase of pressure overload hypertrophy. Am J Hypertens. 2007;20:663–669. doi: 10.1016/j.amjhyper.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 115.Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 116.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 117.Werb Z, Alexander CM. Proteinases and matrix degradation. In: Kelly WN, Harris ED Jr, Ruddy S, Sledge CB, editors. Textbook of Rheumatology. 4th ed. Philadelphia: WB Saunders; 1993. pp. 248–268. [Google Scholar]
- 118.Hammani K, Blakis A, Morsette D, Bowcock AM, Schmutte C, Henriet P, et al. Structure and characterization of the human tissue inhibitor of metalloproteinases-2 gene. J Biol Chem. 1996;271:25498–25505. doi: 10.1074/jbc.271.41.25498. [DOI] [PubMed] [Google Scholar]
- 119.Rutschow S, Li J, Schultheiss HP, Pauschinger M. Myocardial proteases and matrix remodeling in inflammatory heart disease. Cardiovasc Res. 2006;69:646–656. doi: 10.1016/j.cardiores.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 120.Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, et al. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- 121.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]