Abstract
Heart failure is a global health problem, appearing most commonly in patients with previous myocardial infarction (MI). Cardiac remodelling, particularly fibrosis, seen in both the infarcted and non-infarcted myocardium is recognized to be a major determinant of the development of impaired ventricular function, leading to a poor prognosis. Elucidating cellular and molecular mechanisms responsible for the accumulation of extracellular matrix is essential for designing cardioprotective and reparative strategies that could regress fibrosis after infarction. Multiple factors contribute to left ventricular remodelling at different stages post-MI. This review will discuss the role of oxidative stress and locally produced angiotensin II in the pathogenesis of myocardial repair/remodelling after MI.
Keywords: Myocardial infarction, Cardiac remodelling, Oxidative stress, Angiotensin II
1. Introduction
Following myocardial infarction (MI), cardiac structural remodelling is associated with an inflammatory reaction, followed by scar formation at the site of infarction as well as changes in the non-infarcted myocardium, including interstitial fibrosis and vascular remodelling. Fibrous tissue that forms at the site of cardiomyocyte loss preserves structural integrity and is integral to the heart’s recovery, whereas structural remodelling of viable myocardium impairs tissue behaviour. Substances involved in cardiac repair/remodelling are of considerable interest and an important clinical issue, given that the repairing response can be subjected to pharmacological intervention. Multiple factors may, in fact, contribute to left ventricular remodelling at different stages post-MI. There is growing recognition and experimental evidence that oxidative stress mediated by reactive oxygen species (ROS) and locally produced angiotensin II (AngII) play a role in the pathogenesis of myocardial repair/remodelling after MI. Taking into account that there is a plethora of factors that contribute to remodelling and repair in the heart after MI, this review will address these cellular and molecular events related to cardiac repair/remodelling following MI, in particular discussing roles of locally produced ROS and AngII in promoting cardiac remodelling.
2. Cardiac repair/remodelling following infarction
2.1. Infarct site
A highly regulated process of cardiac repair/remodelling follows the necrotic loss of cardiomyocytes after MI. It begins with the activation of latent matrix metalloproteinases (MMPs), which degrade the existing extracellular matrix and coronary vasculature.1 This proteolytic activity declines by the end of week 1 post-MI and is coincident with the increased expression of MMP inhibitors, termed tissue inhibitors of MMPs or TIMPs.2 Circulating inflammatory cells that include neutrophils and monocytes/macrophages arrive at the infarct site soon after MI. They respectively contribute to the proteolytic digestion and phagocytosis of the infarcted tissue. These inflammatory cells home to the site of MI drawn by adhesion molecules and chemoattractant cytokines (or chemokines) expressed by the endothelial cells of the coronary vasculature that borders on the infarct site. Their migration into the infarct site is facilitated by MMP proteolytic activity. This inflammatory response peaks at weeks 1 and 2 post-MI and then tapers off as inflammatory cells disappear from the infarct site within 3–4 weeks following MI, a consequence of their programmed cell death.
The fibrogenic component, which substitutes for lost parachymal cells, follows the initial phase of collagen degradation. It begins with the activation of transforming growth factor (TGF)-β1, the key mediator of fibrogenesis. Increased synthesis of fibrillar type III and type I collagens is preceded by an increased expression of their mRNA transcripts.3 Collagen fibres are morphologically evident at week 1 post-MI, while an organized assembly of these fibres in the form of scar tissue becomes evident at week 2.4 This assembly continues to accumulate over 8 weeks.
2.2. Remote site
In addition to the infarcted myocardium, interstitial fibrosis is developed in the non-infarcted myocardium. Fibrous tissue formation, evidenced by hydroxyproline assay and histochemistry, is observed at week 3 at remote sites in hearts with extensive MI.3 Perivascular fibrosis of intramyocardial coronary arteries is also seen at these sites. Thus, following large MI, extensive cardiac remodelling is developed not only in the infarcted myocardium, but also in remote sites.
3. Cells involved in cardiac remodelling by fibrous tissue
3.1. Infarct site
Cells responsible for fibrous tissue formation at the site of MI consist principally of phenotypically transformed fibroblast-like cells having distinctive morphological features and phenotypic characteristics. Their expression of α-smooth muscle actin (SMA) microfilaments, which give them contractility, earns them the name myofibroblast (myoFb).5,6 MyoFbs are found at the infarct site soon after the arrival of inflammatory cells. Cells that account for the appearance of myoFbs are uncertain. They may include: interstitial fibroblasts; adventitial fibroblasts; pericytes; a population of circulating fibroblasts known as fibrocytes; circulating monocytes; or circulating bone marrow-derived progenitor cells that transdifferentiate at the infarct site. Specific factors that facilitate this differentiation process have been identified. It is presumed that TGF-β1, elaborated by macrophages, governs the appearance of the myoFb phenotype.7 Evidence supporting this hypothesis can be seen in cases of ischaemia–reperfusion, wherein TGF-β1 plays a central role in the oxygen-dependent differentiation of cardiac fibroblasts to myoFbs triggered by perceived hyperoxia.8 After its appearance, MyoFbs rapidly proliferate and accumulate in the infarcted myocardium and are responsible for the formation of the scar via their expression of type I and III fibrillar collagens at both mRNA and protein levels.3,9 Gabbiani et al.10 demonstrated the contractile behaviour of myoFbs in scar tissue and is confirmed by other findings.11 MyoFbs and their α-SMA microfilaments are joined to one another through gap junctions. This creates a contractile scar tissue assembly.
MyoFbs have a diverse portfolio of metabolic activities. Studies have shown that these cells express renin, angiotensin-converting enzyme (ACE), and angiotensin receptors at the infarct site.12,13 MyoFbs obtained from the 4-week-old infarct scar and that are studied in culture under serum-deprived conditions that eliminate circulating renin, ACE, and AngI are found to express angiotensinogen, cathepsin D, ACE, and AngII receptors.14 Given the presence of α-SMA in myoFbs, which is also present in vascular smooth muscle cells, it is not surprising that AngII, endothelin-1, and vasopressin promote scar tissue contraction.14,15
3.2. Remote site
Interstitial fibroblasts are responsible for normal collagen turnover. In the infarcted heart, interstitial/perivascular fibrosis is developed at remote sites in the later stage of MI.3,16 Cells contributing to fibrosis in the non-infarcted myocardium are primarily interstitial fibroblasts. MyoFbs, however, do not appear at remote sites.
4. Role of oxidative stress in cardiac repair/remodelling following myocardial infarction
4.1. Formation and metabolism of reactive oxygen species
Superoxide (O2−), hydroxyl (OH−), and peroxynitrite (ONOO−) are simple molecules characterized by the presence of unpaired electrons. ROS can be produced intracellularly through electron leakage from the mitochondria during oxidative phosphorylation and through the activation of several cellular enzymes, including NADPH oxidase, xanthine oxidase, and nitric oxide synthase.17–19 O2− can rapidly react with nitric oxide (NO) to form ONOO− or convert to H2O2 to form OH−.17
ROS in low concentrations serve as signalling molecules.20 However, these agents elicit harmful effects when produced in excess.17 Toxicity associated with the excessive production of these compounds is prevented by antioxidant defence systems that maintain a healthy cellular environment. Living cells have both enzymatic and non-enzymatic defence mechanisms to balance the multitude of oxidative challenges presented to them. The enzymatic subgroup includes superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSHPx).21,22 Dismutation of O2− by SOD results in the generation of H2O2, which catalase further metabolizes into water and oxygen. The non-enzymatic group includes a variety of biological molecules, such as vitamins E and C.23 In the normal myocardium, as in other tissues, antioxidants protect cells by maintaining O2− and H2O2 at low levels.
4.2. Oxidative stress in the infarcted heart
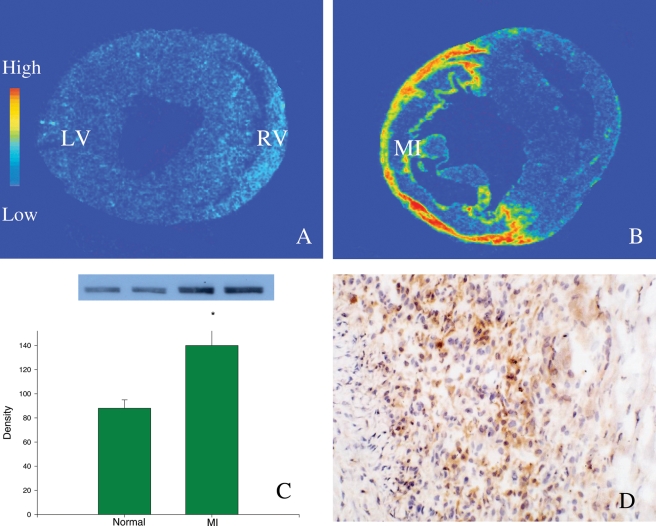
Oxidative stress occurs when ROS production is enhanced and/or antioxidant reserve is suppressed. Following acute MI, oxidative stress is developed in both infarcted and non-infarcted myocardium. NADPH oxidase is a major source of O2− in the heart.24 After MI, NADPH oxidase expression (gp22phox and gp91phox subunits) is significantly increased in the infarcted myocardium,25,26 with neutrophils and macrophages as the primary cells expressing the enzyme (Figure 1). Furthermore, macrophage-derived inducible nitric oxide synthase, a major source of NO in the repairing tissue, is significantly increased in the infarcted myocardium,27 leading to enhanced NO production. As a result, ROS production is elevated in the infarcted myocardium and contributes to the development of oxidative stress in the infarcted heart.
Figure 1.
NADPH oxidase (gp91phox) expression in the infarcted rat heart. Detected by in situ hybridization, low levels of gp91phox mRNA are present in both left and right ventricles (LV, RV) of the normal heart (A). At 1 week post-myocardial infarction, cardiac gp91phox mRNA levels are largely increased, particularly at the site of myocardial infarction (B). Detected by western blot, gp91phox protein levels are significantly increased in the infarcted myocardium compared with the normal myocardium (C). Immunohistochemistry reveals that cells expressing gp91phox in the infarcted myocardium are primarily inflammatory cells (D).
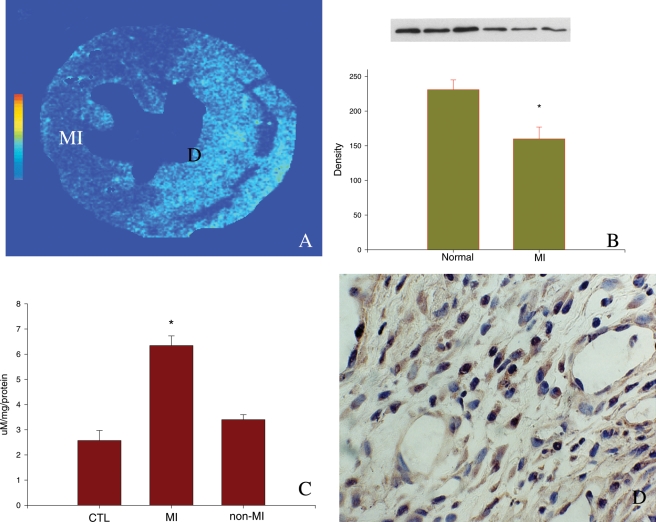
Impaired antioxidant capacity also plays a role in oxidative stress in the infarcted heart. Singal and colleague26 have shown evidence of the progressive decrease of SOD, catalase, and GSHPx activity as well as vitamin E levels in the infarcted rat heart, first in the infarcted myocardium followed by the non-infarcted myocardium.28 Our study has shown reduced SOD gene and protein expression in the infarcted myocardium (Figure 2). Cardiac glutathione levels are also decreased in patients with acute MI.29 Moreover, the expression of malondialdehyde (MDA) and 3-nitrotyrosine, markers of oxidative stress, are significantly increased in the infarcted myocardium, indicating the elevated occurrence of cardiac oxidative stress following MI (Figure 2).26
Figure 2.
Expression of superoxide dismutase, malondialdehyde, and 3-nitrotyrosine in the infarcted rat heart. At 1 week post-myocardial infarction, superoxide dismutase mRNA (A) and protein (B) levels are significantly reduced in the infarcted myocardium. Compared with normal heart, malondialdehyde levels are significantly increased in the infarcted myocardium (C). 3-Nitrotyrosine is highly expressed by inflammatory cells at the infarct site (D).
In remote sites, multiple sources contribute to oxidative stress. Increased mitochondrial production of ROS has been suggested in the non-infarcted myocardium.30 Increased ROS levels in remote sites also reflect the increased activity of intracellular oxidase complexes, such as NADPH oxidase, xanthine oxidase, and nitric oxide synthase.31 In addition, reduced SOD levels were observed in the failing heart with infarction.32 These observations indicate that the imbalance between ROS production and antioxidant defence capacity leads to oxidative stress in non-infarcted myocardium.
Experimental studies have demonstrated that oxidative stress can induce most, if not all, of the changes that are thought to contribute to myocardial remodelling, including pro-inflammatory response, cardiomyocyte apoptosis, fibrogenesis, cell proliferation, and hypertrophy. Herein, the potential relevance of oxidative stress on inflammatory/fibrogenic responses will be discussed.
4.3. Reactive oxygen species and cardiac inflammatory response following myocardial infarction
MI is associated with an inflammatory response, ultimately leading to healing and scar formation. Inflammatory response in the infarcted myocardium is related to the coordinated activation of a series of cytokine and adhesion molecule genes. A critical element in the regulation of these genes involves nuclear factor-kappa B (NF-κB), a redox-sensitive transcription factor. NF-κB maintains an inactive form bound to its inhibitory subunit I kappa B under normal conditions. When tissue is injured, NF-κB is activated by various local substances including ROS.33 Upon activation, NF-κB stimulates inflammatory and immune responses and cellular growth by increasing the expression of specific cellular genes. NF-κB activation has been demonstrated in various models of myocardial ischaemia.34,35 Activated NF-κB triggers gene expression of interstitial and vascular adhesion molecules, as well as monocyte chemoattractant protein-1, leading to leucocyte infiltration into the infarcted myocardium.
NF-κB also triggers gene expression of pro-inflammatory cytokines, such as tissue necrosis factor (TNF)-α and interleukins, initiating an inflammatory response.36 In rodent models of MI, TNF-α expression is significantly up-regulated in the infarcted myocardium as well as in the non-infarcted myocardium.37 It plays a key role in stimulating inflammatory protein synthesis as well as macrophage phagocytosis, cell growth, differentiation, and apoptosis.38
In the infarcted myocardium, elevated NADPH oxidase is spatially coincident with activated NF-κB and enhanced TNF-α expression in inflammatory cells.34 In addition to the NF-κB pathway, recent studies suggest that H2O2 can directly induce cardiac TNF-α production via the p38 MAPK pathway and, in turn, mediate myocardial inflammation.39 Furthermore, free radical scavenger treatment has been demonstrated to diminish inflammatory response and cardiac remodelling.40 The antioxidant, probucol, has been shown to attenuate cardiac inflammation and improve ventricular function.41 These findings indicate that ROS serve as a pro-inflammatory mediator in cardiac healing process following infarction and its role in cardiac inflammation involves several pathways.
4.4. Reactive oxygen species and progressive myocyte death following myocardial infarction
Following MI, leucocytes are recruited into the infarcted region and initiate cardiac repair. Phagocyte-derived oxidative stress occurs at the site of infarction. This is particularly evident at the border zone, the area adjacent to the non-infarcted myocardium. ROS can directly attack DNA, proteins, and cell membranes and therefore have the potential to injure myocytes and vascular cells in the neighbouring non-infarcted myocardium, causing additional cardiac damage and extending infarct size. Oxidative stress also activates multiple cellular pathways, leading to cardiac damage. In the murine myocardium following the initial insult caused by ischaemia–reperfusion, progressive myocyte death is noted in the border zone. Thus, myocardial remodelling following ischaemia/reperfusion includes secondary myocyte death followed by the loss of cardiac function over time.42
4.5. Reactive oxygen species and cardiac fibrogenic response following myocardial infarction
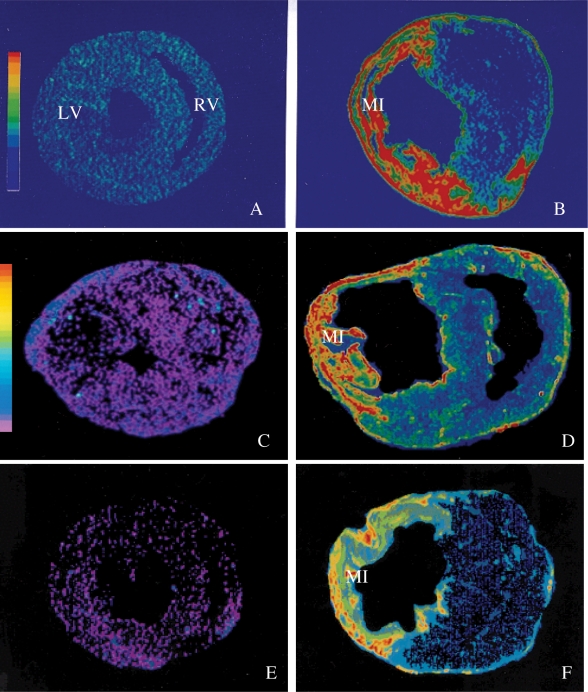
A growing bulk of evidence supports a causative role of oxidative stress in fibrogenesis in various tissues including liver, lung, arteries, nervous system, and heart.43,44 ROS is shown to up-regulate the expression of TGF-β and type I collagen in various tissues.45,46 Following MI, enhanced expressions of TGF-β and NADPH oxidase are spatially coincident at the site of the infarcted myocardium (Figures 1 and 3). Treatment with the antioxidant taurine reduces oxidative stress, suppresses TGF-β gene expression, and attenuates hepatic fibrosis.47 Furthermore, in vitro studies have indicated that ROS promotes fibroblast proliferation and type I collagen gene expression in cardiac fibroblasts.48 Chronic antioxidant treatment is shown to attenuate cardiac fibrosis.49–51
Figure 3.
Transforming growth factor-β1, angiotensin-converting enzyme, and AT1 receptor expression in the infarcted heart. By in situ hybridization, normal myocardium contains low levels of Transforming growth factor-β1 (A). At week 1 post-myocardial infarction, Transforming growth factor-β1 gene expression is largely increased at the site of myocardial infarction (B). Detected by autoradiography, binding density of angiotensin-converting enzyme (D) and AT1 receptors (F) are largely increased in both the infarcted and non-infarcted myocardium compared with the normal heart (C and E, respectively).
After acute MI, progressive global left ventricular dilation occurs over the following months.52 MMPs favour this adverse remodelling. It was shown that inhibition of MMPs decreases the severity of remodelling in the infarcted heart.53 In vitro studies have shown that ROS activates MMPs in cardiac fibroblasts.54 Oxidative stress may, therefore, play a role in the pathogenesis of left ventricular dilation following infarction. However, in vivo studies on the regulatory role of ROS on MMPs and cardiac dilation are lacking and further studies are required on this concept.
The extensive body of literature in animal studies suggests that there is great potential benefit in therapies that can improve cardiac remodelling and function in humans. However, existing evidence for the role of oxidative stress in the pathogenesis of cardiac remodelling and ventricular dysfunction in humans is not compelling. Clinical trials of antioxidant therapy for heart failure are few in number and so far have failed to demonstrate convincing benefits. This might be due to several potential reasons.
First, ROS are derived from multi-sources in the failing heart. ROS can be produced intracellularly through electron leakage from mitochondria during oxidative phosphorylation and through the activation of several cellular enzymes, including NADPH oxidase, xanthine oxidase, and nitric oxide synthase. Treatment with a specific antioxidant, such as NADPH oxidase inhibitor, may therefore not suppress oxidative stress due to the redundant sources of ROS production.
Secondly, most currently recognized antioxidants that were used in animals including probucol, pyrrolidine dithiocarbamate, vitamin E or C, and N-acetyl cysteine are not either a strong antioxidant or a specific antioxidant with many other effects. Moreover, combination of antioxidants, dosing and timing of treatment, and duration of the process need to be further established in patients with heart failure.
5. Cardiac angiotensin II and myocardial repair/remodelling
In addition to oxidative stress, AngII is considered another local factor in mediating cardiac remodelling following MI. Circulating AngII has multiple well-known endocrine properties in the cardiovasculature. AngII, produced de novo within the heart independent of plasma angiotensinogen, plasma renin activity, and endothelial cell-bound ACE, also has various autocrine and paracrine properties on resident cells. These cells include cardiomyocytes, representing one-third of all cells found in the myocardium; and fibroblasts, endothelial and vascular smooth muscle cells, and macrophages, which represent the remaining two-thirds. Based on current evidence, AT1 receptor-ligand binding accounts for the majority of these respective endocrine and auto-/paracrine actions of AngII on blood vessels and cardiac tissue.
5.1. Cardiac angiotensin II production
Renin expression (mRNA and protein) is undetectable in the normal myocardium. Low ACE levels are present throughout the myocardium of the right and left atria and ventricles of the adult rat heart, as is also the case for AngII receptors. Following experimental MI in rats, renin, ACE, and AT1 receptor expression is significantly increased at the infarcted myocardium, coincident with inflammatory response and the initial accumulation of fibrillar collagen.12,13,55–57 Moreover, elevated AngII concentration is found at the infarct site.58 Renin is expressed by macrophages and myoFbs at the infarct site.13 ACE-positive cells at the infarct site involve endothelial cells of the neovasculature (constitutive ACE) and macrophages and myoFbs (recruitable ACE).57,59 AT1 receptors are primarily expressed by macrophages, myoFbs, and vascular smooth muscle cells in the infarcted myocardium.56 The expression of ACE and AT1 receptors in macrophages and myoFbs in the infarcted heart suggests that locally generated AngII plays a role in inflammatory and fibrogenic reactions in an autocrine manner.
Non-ischaemic models of cardiac repair have also been examined relative to ACE expression. They included: AngII infusion via an implanted mini-pump;60 administration of isoproterenol, a synthetic catecholamine;61 and chronic (>3 weeks) administration of aldosterone by a mini-pump in uninephrectomized rats on a high salt diet.62 At each site of non-ischaemic cardiac repair, and irrespective of its aetiological basis, the temporal and spatial appearance of high levels of ACE expression is coincident with fibrous tissue formation that resembles reparative responses observed with ischaemic necrosis following MI. Thus, irrespective of the aetiological basis of cardiac injury, a recruitable source of ACE appears at sites of cardiac repair and contributes to local AngII production and consequently cardiac repair.
5.2. Regulation of angiotensin II on cardiac repair/remodelling
A paradigm of tissue repair has been proposed in which ACE and local AngII are integral to the orderly and sequential nature of repair that eventuates in fibrosis.63 ACE is involved in a two-part de novo generation of AngII within the granulation tissue that forms at the infarct site. The first component to local AngII generation is provided by activated macrophages. In an autocrine manner, macrophage-derived AngII stimulates NADPH oxidase expression that induces ROS production, triggering inflammatory response in various tissues.26,64–66 MyoFbs next generate AngII whose autocrine induction of TGF-β1 regulates collagen turnover at sites of fibrous tissue formation.1 ACE expression is spatially and temporally concordant with the expression of TGF-β1 mRNA, type I collagen mRNAs, and AT1 and TGF-β receptors at these sites. Studies have demonstrated that cardiac AngII stimulates TGF-β1 expression, thus promoting cardiac fibrosis.67
ACE inhibitors and AT1 receptor blockers have been proven effective in modulating the process of remodelling and in reducing the occurrence of adverse events in heart failure. Chronic treatment of ACE inhibitor decreased cardiac fibrosis after MI in rats.68–70 Captopril and enalapril begun at or close to the onset of MI have each reduced infarct size, infarct expansion, and thinning, and hydroxyproline concentration at the infarct site.71–73 Losartan begun on day 1 after coronary artery ligation in a dose that reduced AT1 receptor binding by 50% reduces infarct scar area.74 Moreover, the expected rise in tissue Ang II concentration found at the infarct site 3 weeks after coronary artery ligation in rats is markedly attenuated by either delapril or TCV-116, an AT1Ra, introduced on post-operative day 1, so did left ventricular remodelling.58 It has also been demonstrated that the combination of AT1 receptor blockade and ACE inhibitor is more effective than individual treatment on ventricular remodelling and survival after MI in rats.69
Fibrous tissue formation at sites remote to MI is also influenced by these pharmacological interventions. Perindopril, given 1 week after MI, attenuates the endocardial fibrosis that appears in the non-necrotic segment of the rat left ventricle.75 Captopril, commenced at the time of coronary artery ligation, attenuates the expected fibrosis of the non-infarcted left and right ventricles of the rat76 and proliferation of fibroblasts and endothelial cells that appears at remote sites 1 and 2 weeks following MI.76 Losartan likewise prevents fibrosis at remote sites.74,77,78
These favourable tissue-protective effects of ACE inhibition or AT1 receptor antagonism are not confined to the infarcted heart. These interventions prevent the appearance of fibrosis in diverse organs with experimentally induced or naturally occurring tissue injury, where the circulating renin–ngiotensin system is not activated. These include: pericardial fibrosis post-pericardiotomy;4 tubulointerstitial fibrosis associated with unilateral urethral obstruction79–81 or renal injury following irradiation;82 cardiovascular and glomerulosclerosis that appear in stroke-prone spontaneously hypertensive rats;83–86 or interstitial pulmonary fibrosis that follows irradiation.87 Attenuation of fibrous tissue formation by these interventions in diverse organs with various forms of injury supports the importance of local AngII in promoting fibrosis.
Aldosterone production in the heart as well as aldosterone plasma levels are increased after MI and in congestive heart failure, correlating with the severity of disease. Aldosterone promotes sodium and water retention, sympathoadrenergic activation, endothelial dysfunction, and cardiovascular fibrosis and hypertrophy. Studies have demonstrated that aldosterone receptor blockade markedly reduces mortality among patients with heart failure. Reduction of excessive extracellular matrix turnover leading to decreased fibrosis appears to be the most important effect of mineralocorticoid receptor antagonism in heart failure. Other mechanisms such as regression of hypertrophy, improvement of endothelial function, reduction of superoxide formation, and enhanced renal sodium excretion may contribute. Recent data showed that in rats with left ventricular dysfunction after extensive MI, eplerenone on top of ACE inhibition more effectively improved cardiac remodelling and molecular alterations than ACE inhibition alone.88,89
6. Summary
Following MI, cardiac structural remodelling is associated with an inflammatory reaction, followed by scar formation at the site of infarction as well as interstitial fibrosis and vascular remodelling in the non-infarcted myocardium. Factors that regulate cardiac repair/remodelling include ROS and AngII produced in the infarcted heart. ROS mediate cardiac repair via activating NF-κB with its pro-inflammatory cascade, promoting fibrogenic cytokine production. Local AngII regulates cardiac remodelling by stimulating NADPH oxidase, which, in turn, enhances ROS production and promotes TGF-β synthesis in an autocrine manner. Blockade of ACE and/or AT1 receptors are effective in attenuating tissue fibrosis in animals and patients. Suppression of cardiac oxidative stress improves cardiac remodelling and ventricular dysfunction in animals with MI. However, the potential regulation of oxidative stress in the pathogenesis of cardiac remodelling and ventricular dysfunction requires further studies in patients with MI and heart failure.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health (R01HL067888 to Y.S., R01HL077668 to Y.S.).
References
- 1.Cleutjens JPM, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 2.Sun Y, Zhang JQ, Zhang J, Lamparter S. Cardiac remodeling by fibrous tissue after infarction in rats. J Lab Clin Med. 2000;135:316–323. doi: 10.1067/mlc.2000.105971. [DOI] [PubMed] [Google Scholar]
- 3.Cleutjens JPM, Verluyten MJA, Smits JFM, Daemen MJAP. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, Cleutjens JPM, Diaz-Arias AA, Weber KT. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc Res. 1994;28:1423–1432. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- 5.Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 6.Desmouliére A, Gabbiani G. The role of the myofibroblast in wound healing and fibrocontractive diseases. In: Clark RAF,, editor. The Molecular and Cellular Biology of Wound Repair. 2nd ed. New York: Plenum Press; 1996. pp. 391–423. [Google Scholar]
- 7.Desmouliére A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, et al. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1) Circ Res. 2003;92:264–271. doi: 10.1161/01.res.0000056770.30922.e6. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Zhang JQ, Zhang J, Ramires FJA. Angiotensin II, transforming growth factor-beta1 and repair in the infarcted heart. J Mol Cell Cardiol. 1998;30:1559–1569. doi: 10.1006/jmcc.1998.0721. [DOI] [PubMed] [Google Scholar]
- 10.Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1973;135:719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appleton I, Tomlinson A, Chander CL, Willoughby DA. Effect of endothelin-1 on croton oil-induced granulation tissue in the rat. A pharmacologic and immunohistochemical study. Lab Invest. 1992;67:703–710. [PubMed] [Google Scholar]
- 12.Passier RC, Smits JF, Verluyten MJ, Daemen MJ. Expression and localization of renin and angiotensinogen in rat heart after myocardial infarction. Am J Physiol. 1996;271:H1040–H1048. doi: 10.1152/ajpheart.1996.271.3.H1040. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Zhang J, Zhang JQ, Weber KT. Renin expression at sites of repair in the infarcted rat heart. J Mol Cell Cardiol. 2001;33:995–1003. doi: 10.1006/jmcc.2001.1365. [DOI] [PubMed] [Google Scholar]
- 14.Katwa LC, Campbell SE, Tyagi SC, Lee SJ, Cicila GT, Weber KT. Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol. 1997;29:1375–1386. doi: 10.1006/jmcc.1997.0376. [DOI] [PubMed] [Google Scholar]
- 15.Goto T, Yanaga F, Ohtsuki I. Studies on the endothelin-1-induced contraction of rat granulation tissue pouch mediated by myofibroblasts. Biochim Biophys Acta. 1998;1405:55–66. doi: 10.1016/s0167-4889(98)00105-0. [DOI] [PubMed] [Google Scholar]
- 16.Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, et al. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.cir.89.5.2273. [DOI] [PubMed] [Google Scholar]
- 17.Sorescu D, Griendling K. Reactive oxygen species, mitochondria, and NADPH oxidases in the development and progression of heart failure. CHF. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 18.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–52. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 19.Finkel T. Signal transduction by reactive oxygen species in non-phagocytic cells. J Leukoc Biol. 1999;65:337–340. doi: 10.1002/jlb.65.3.337. [DOI] [PubMed] [Google Scholar]
- 20.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig heart. Am J Physiol. 1994;266:H1280–H1285. doi: 10.1152/ajpheart.1994.266.4.H1280. [DOI] [PubMed] [Google Scholar]
- 21.Young IS, Woodside JV. Antioxidants in hearth and disease. J Clin Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaziri ND, Lin C, Farmand F, Sindhu K. Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int. 2003;63:186–194. doi: 10.1046/j.1523-1755.2003.00711.x. [DOI] [PubMed] [Google Scholar]
- 23.Gasparetto C, Malinverno A, Gulacciati D, Gritti D, Prosperini PG, Specchia G, et al. Antioxidant vitamins reduce oxidative stress and ventricular remodeling in patients with acute myocardial infarction. Int J Immunopathol Pharmacol. 2005;18:487–496. doi: 10.1177/039463200501800308. [DOI] [PubMed] [Google Scholar]
- 24.Mohazzab-H KM, Kaminski PM, Wolin MS. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997;15:614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- 25.Fukui T, Yoshiyama M, Hanatani A, Omura T, Yoshikawa J, Abe Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun. 2001;281:1200–1206. doi: 10.1006/bbrc.2001.4493. [DOI] [PubMed] [Google Scholar]
- 26.Lu L, Quinn MT, Sun Y. Oxidative stress in the infarcted heart: role of de novo angiotensin II production. Biochem Biophys Res Commun. 2004;325:943–951. doi: 10.1016/j.bbrc.2004.10.106. [DOI] [PubMed] [Google Scholar]
- 27.Takimoto Y, Aoyama T, Keyamura R. Differential expression of three types of nitric oxide synthase in both infarcted and non-infarcted left ventricles after myocardial infarction in the rat. Int J Cardiol. 2000;176:135–145. doi: 10.1016/s0167-5273(00)00394-6. [DOI] [PubMed] [Google Scholar]
- 28.Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–2420. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- 29.Usal A, Acarturk E, Yuregir GT, Unlukurt I, Demirci C, Kurt HI, et al. Decreased glutathione levels in acute myocardial infarction. Jpn Heart J. 1996;37:177–182. doi: 10.1536/ihj.37.177. [DOI] [PubMed] [Google Scholar]
- 30.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 31.Grieve DJ, Byrne JA, Cave AC, Shah AM. Role of oxidative stress in cardiac remodeling after myocardial infarction. Heart Lung Circ. 2004;13:132–138. doi: 10.1016/j.hlc.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Khaper N, Kaur K, Li T, Farahmand F, Singal PK. Antioxidant enzyme gene expression in congestive heart failure following myocardial infarction. Mol Cell Biochem. 2003;251:9–15. [PubMed] [Google Scholar]
- 33.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Chen SS, Zhang JQ, Ramires FJ, Sun Y. Activation of nuclear factor-kappaB and its proinflammatory mediator cascade in the infarcted rat heart. Biochem Biophys Res Commun. 2004;321:879–885. doi: 10.1016/j.bbrc.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 35.Nichols TC. NF-kappaB and reperfusion injury. Drug News Perspect. 2004;17:99–104. doi: 10.1358/dnp.2004.17.2.829042. [DOI] [PubMed] [Google Scholar]
- 36.Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol. 2000;15:1075–1083. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs M, Staufenberger S, Gergs U, Meuter K, Brandstatter K, Hafner M, et al. Tumor necrosis factor-alpha at acute myocardial infarction in rats and effects on cardiac fibroblasts. J Mol Cell Cardiol. 1999;31:1949–1959. doi: 10.1006/jmcc.1999.1007. [DOI] [PubMed] [Google Scholar]
- 38.Ceconi C, Curello S, Bachetti T, Corti A, Ferrari R Tumor necrosis factor in congestive heart failure: a mechanism of disease for the new millennium? Prog Cardiovasc Dis. 1998;41:25–30. doi: 10.1016/s0033-0620(98)80028-5. [DOI] [PubMed] [Google Scholar]
- 39.Meldrum DR, Dinarello CA, Cleveland JC, Jr, Cain BS, Shames BD, Meng X, et al. Hydrogen peroxide induces tumor necrosis factor alpha-mediated cardiac injury by a P38 mitogen-activated protein kinase-dependent mechanism. Surgery. 1998;124:291–296. doi: 10.1067/msy.1998.90570. [DOI] [PubMed] [Google Scholar]
- 40.Sia YT, Lapointe N, Parker T, Tsoporis JN, Deschepper CF, Calderone A, et al. Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation. 2002;105:2549–2555. doi: 10.1161/01.cir.0000016721.84535.00. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura R, Egashira K, Machida Y, Hayashidani S, Takeya M, Utsumi H, et al. Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure: roles of oxidative stress and inflammation. Circulation. 2002;106:362–367. doi: 10.1161/01.cir.0000021430.04195.51. [DOI] [PubMed] [Google Scholar]
- 42.Ojha N, Roy S, Radtke J, Simonetti O, Gnyawali S, Zweier JL, et al. Characterization of the structural and functional changes in the myocardium following focal ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;294:H2435–H2443. doi: 10.1152/ajpheart.01190.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukamoto H. Oxidative stress, antioxidants, and alcoholic liver fibrogenesis. Alcohol. 1993;10:465–467. doi: 10.1016/0741-8329(93)90066-w. [DOI] [PubMed] [Google Scholar]
- 44.Mastruzzo C, Crimi N, Vancheri C. Role of oxidative stress in pulmonary fibrosis. Monaldi Arch Chest Dis. 2002;57:173–176. [PubMed] [Google Scholar]
- 45.Iglesias-De La Cruz MC, Ruiz-Torres P, Alcamí J, Díez-Marqués L, Ortega-Velázquez R, Chen S, et al. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int. 2001;59:87–95. doi: 10.1046/j.1523-1755.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 46.Novitskiy G, Potter JJ, Wang L, Mezey E. Influences of reactive oxygen species and nitric oxide on hepatic fibrogenesis. Liver Int. 2006;26:1248–1257. doi: 10.1111/j.1478-3231.2006.01364.x. [DOI] [PubMed] [Google Scholar]
- 47.Miyazaki T, Karube M, Matsuzaki Y, Ikegami T, Doy M, Tanaka N, et al. Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis. J Hepatol. 2005;43:117–125. doi: 10.1016/j.jhep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 48.Murrell AC, Francis JO, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1993;265:659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL. Imporved post-myocardial infarction survival with probucol in rats: effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Col Cardiol. 2002;39:148–156. doi: 10.1016/s0735-1097(01)01709-0. [DOI] [PubMed] [Google Scholar]
- 50.Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Müller M, et al. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation. 2004;110:2175–2179. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- 51.Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, et al. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2004;109:544–549. doi: 10.1161/01.CIR.0000109701.77059.E9. [DOI] [PubMed] [Google Scholar]
- 52.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 53.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, et al. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 54.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 55.Sun Y, Weber KT. Angiotensin-converting enzyme and wound healing in diverse tissues of the rat. J Lab Clin Med. 1996;127:94–101. doi: 10.1016/s0022-2143(96)90170-5. [DOI] [PubMed] [Google Scholar]
- 56.Sun Y, Weber KT. Cells expressing angiotensin II receptors in fibrous tissue of rat heart. Cardiovasc Res. 1996;31:518–525. [PubMed] [Google Scholar]
- 57.Sun Y, Weber KT. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J Mol Cell Cardiol. 1996;28:851–8588. doi: 10.1006/jmcc.1996.0080. [DOI] [PubMed] [Google Scholar]
- 58.Yamagishi H, Kim S, Nishikimi T, Takeuchi K, Takeda T. Contribution of cardiac renin-angiotensin system to ventricular remodelling in myocardial-infarcted rats. J Mol Cell Cardiol. 1993;25:1369–1380. doi: 10.1006/jmcc.1993.1149. [DOI] [PubMed] [Google Scholar]
- 59.Falkenhahn M, Franke F, Bohle RM, Zhu YC, Stauss HM, Bachmann S, et al. Cellular distribution of angiotensin-converting enzyme after myocardial infarction. Hypertension. 1995;25:219–226. doi: 10.1161/01.hyp.25.2.219. [DOI] [PubMed] [Google Scholar]
- 60.Ratajska A, Campbell SE, Sun Y, Weber KT. Angiotenin II associated cardiac myocyte necrosis: role of adrenal catecholamines. Cardiovasc Res. 1994;28:684–690. doi: 10.1093/cvr/28.5.684. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y, Weber KT. Angiotensin-converting enzyme and wound healing in diverse tissues of the rat. J Lab Clin Med. 1996;127:94–101. doi: 10.1016/s0022-2143(96)90170-5. [DOI] [PubMed] [Google Scholar]
- 62.Sun Y, Ratajska A, Zhou G, Weber KT. Angiotensin converting enzyme and myocardial fibrosis in the rat receiving angiotensin II or aldosterone. J Lab Clin Med. 1993;122:395–403. [PubMed] [Google Scholar]
- 63.Weber KT. Extracellular matrix remodeling in heart failure. A role for de novo angiotensin II generation. Circulation. 1997;96:4065–4082. doi: 10.1161/01.cir.96.11.4065. [DOI] [PubMed] [Google Scholar]
- 64.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–866. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 66.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 67.Weber KT. Fibrosis, a common pathway to organ failure: angiotensin II and tissue repair. Semin Nephrol. 1997;17:467–491. [PubMed] [Google Scholar]
- 68.Seeland U, Kouchi I, Zolk O, Itter G, Linz W, Böhm M. Effect of ramipril and furosemide treatment on interstitial remodeling in post-infarction heart failure rat hearts. J Mol Cell Cardiol. 2002;34:151–163. doi: 10.1006/jmcc.2001.1497. [DOI] [PubMed] [Google Scholar]
- 69.Yu CM, Tipoe GL, Wing-Hon Lai K, Lau CP. Effects of combination of angiotensin-converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarction. J Am Coll Cardiol. 2001;38:1207–1215. doi: 10.1016/s0735-1097(01)01518-2. [DOI] [PubMed] [Google Scholar]
- 70.Patten RD, Aronovitz MJ, Einstein M, Lambert M, Pandian NG, Mendelsohn ME, et al. Effects of angiotensin II receptor blockade versus angiotensin-converting-enzyme inhibition on ventricular remodelling following myocardial infarction in the mouse. Clin Sci. 2003;104:109–118. doi: 10.1042/CS20020219. [DOI] [PubMed] [Google Scholar]
- 71.Jugdutt BI, Khan MI, Jugdutt SJ, Blinston GE. Effect of enalapril on ventricular remodeling and function during healing after anterior myocardial infarction in the dog. Circulation. 1995;91:802–812. doi: 10.1161/01.cir.91.3.802. [DOI] [PubMed] [Google Scholar]
- 72.Jugdutt BI, Schwarz-Michorowski BL, Khan MI. Effect of long-term captopril therapy on left ventricular remodeling and function during healing of canine myocardial infarction. J Am Coll Cardiol. 1992;19:713–721. doi: 10.1016/s0735-1097(10)80298-0. [DOI] [PubMed] [Google Scholar]
- 73.Jugdutt BI. Effect of captopril and enalapril on left ventricular geometry, function and collagen during healing after anterior and inferior myocardial infarction in a dog model. J Am Coll Cardiol. 1995;25:1718–1725. doi: 10.1016/0735-1097(95)00040-b. [DOI] [PubMed] [Google Scholar]
- 74.Frimm Cde C, Sun Y, Weber KT. Angiotensin II receptor blockade and myocardial fibrosis of the infarcted rat heart. J Lab Clin Med. 1997;129:439–446. doi: 10.1016/s0022-2143(97)90077-9. [DOI] [PubMed] [Google Scholar]
- 75.Michel JB, Lattion AL, Salzmann JL, Cerol ML, Philippe M, Camilleri JP, et al. Hormonal and cardiac effects of converting enzyme inhibition in rat myocardial infarction. Circ Res. 1988;62:641–650. doi: 10.1161/01.res.62.4.641. [DOI] [PubMed] [Google Scholar]
- 76.van Krimpen C, Smits JFM, Cleutjens JPM, Debets JJ, Schoemaker RG, Struyker-Boudier HA, et al. DNA synthesis in the non-infarcted cardiac interstitium after left coronary artery ligation in the rat heart: effects of captopril. J Mol Cell Cardiol. 1991;23:1245–1253. doi: 10.1016/0022-2828(91)90082-w. [DOI] [PubMed] [Google Scholar]
- 77.Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, et al. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.cir.89.5.2273. [DOI] [PubMed] [Google Scholar]
- 78.Smits JFM, van Krimpen C, Schoemaker RG, Cleutjens JPM, Daemen MJAP. Angiotensin II receptor blockade after myocardial infarction in rats: effects on hemodynamics, myocardial DNA synthesis, and interstitial collagen content. J Cardiovasc Pharmacol. 1992;20:772–778. [PubMed] [Google Scholar]
- 79.Morrissey JJ, Ishidoya S, McCracken R, Klahr S. The effect of ACE inhibitors on the expression of matrix genes and the role of p53 and p21 (WAF1) in experimental renal fibrosis. Kidney Int. 1996;49:S83–S87. [PubMed] [Google Scholar]
- 80.Ishidoya S, Morrissey J, McCracken R, Reyes A, Klahr S. Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int. 1995;47:1285–1294. doi: 10.1038/ki.1995.183. [DOI] [PubMed] [Google Scholar]
- 81.Kaneto H, Morrissey J, McCracken R, Reyes A, Klahr S. Enalapril reduces collagen type IV synthesis and expansion of the interstitium in the obstructed rat kidney. Kidney Int. 1994;45:1637–1647. doi: 10.1038/ki.1994.215. [DOI] [PubMed] [Google Scholar]
- 82.Juncos LI, Carrasco Due¤as S, Cornejo JC, Broglia CA, Cejas H. Long-term enalapril and hydrochlorothiazide in radiation nephritis. Nephron. 1993;64:249–255. doi: 10.1159/000187322. [DOI] [PubMed] [Google Scholar]
- 83.Nakamura T, Honma H, Ikeda Y, Kuroyanagi R, Takano H, Obata J, et al. Renal protective effects of angiotensin II receptor I antagonist CV-11974 in spontaneously hypertensive stroke-prone rats (SHR-sp) Blood Press. 1994;3:61–66. [PubMed] [Google Scholar]
- 84.Nakamura T, Obata J, Kuroyanagi R, Kimura H, Ikeda Y, Takano H, et al. Involvement of angiotensin II in glomerulosclerosis of stroke-prone spontaneously hypertensive rats. Kidney Int. 1996;49:S109–S112. [PubMed] [Google Scholar]
- 85.Kim S, Ohta K, Hamaguchi A, Omura T, Yukimura T, Miura K, et al. Contribution of renal angiotensin II type I receptor to gene expressions in hypertension-induced renal injury. Kidney Int. 1994;46:1346–1358. doi: 10.1038/ki.1994.404. [DOI] [PubMed] [Google Scholar]
- 86.Kim S, Ohta K, Hamaguchi A, Omura T, Yukimura T, Miura K, et al. Angiotensin II type I receptor antagonist inhibits the gene expression of transforming growth factor-á1 and extracellular matrix in cardiac and vascular tissues of hypertensive rats. J Pharmacol Exp Ther. 1995;273:509–515. [PubMed] [Google Scholar]
- 87.Ward WF, Molteni A, Ts’ao C, Kim YT, Hinz JM. Radiation pneumotoxicity in rats: modification by inhibitors of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 1992;22:623–625. doi: 10.1016/0360-3016(92)90890-t. [DOI] [PubMed] [Google Scholar]
- 88.Fraccarollo D, Galuppo P, Bauersachs J. Mineralocorticoid receptor antagonism and cardiac remodeling in ischemic heart failure. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:287–294. doi: 10.2174/1568016043356219. [DOI] [PubMed] [Google Scholar]
- 89.Cittadini A, Monti MG, Isgaard J, Casaburi C, Strömer H, Di Gianni A, et al. Aldosterone receptor blockade improves left ventricular remodeling and increases ventricular fibrillation threshold in experimental heart failure. Cardiovasc Res. 2003;58:555–564. doi: 10.1016/s0008-6363(03)00251-7. [DOI] [PubMed] [Google Scholar]