Abstract
Aims
The aim of this study was to investigate whether prolonged and dispersed myocardial contraction duration assessed by tissue Doppler imaging (TDI) may serve as risk markers for cardiac events (documented arrhythmia, syncope, and cardiac arrest) in patients with long QT syndrome (LQTS).
Methods and results
Seventy-three patients with genetically confirmed LQTS (nine double- and 33 single-mutation carriers with previous cardiac events and 31 single-mutation carriers without events) were studied. Myocardial contraction duration was prolonged in each group of LQTS patients compared with 20 healthy controls (P < 0.001). Contraction duration was longer in single-mutation carriers with previous cardiac events compared with those without (0.46 ± 0.06 vs. 0.40 ± 0.06 s, P = 0.001). Prolonged contraction duration could better identify cardiac events compared with corrected QT (QTc) interval in single-mutation carriers [area under curve by receiver-operating characteristic analysis 0.77 [95% confidence interval (95% CI) 0.65–0.89] vs. 0.66 (95% CI 0.52–0.79)]. Dispersion of contraction was more pronounced in single-mutation carriers with cardiac events compared with those without (0.048 ± 0.018 vs. 0.031 ± 0.019 s, P = 0.001).
Conclusion
Dispersion of myocardial contraction assessed by TDI was increased in LQTS patients. Prolonged contraction duration was superior to QTc for risk assessment. These new methods can easily be implemented in clinical routine and may improve clinical management of LQTS patients.
Keywords: Long QT syndrome, Echocardiography, Ventricular arrhythmia, Myocardial contraction, Dispersion
Introduction
The long QT syndrome (LQTS) is a genetic disorder characterized by prolonged ventricular repolarization that predisposes to life-threatening arrhythmias.1 The pathophysiology behind the arrhythmias in LQTS is not precisely defined. Possible mechanisms include early after-depolarizations (EADs) and dispersion of myocardial repolarization.
Fifty years after its initial description,2–4 approaches for risk stratification are insufficiently defined. Risk stratification today is based on history of syncope, genotype, gender, and corrected QT (QTc) interval.1,5 Prolonged QTc is a marker of prolonged action potential duration which is associated with a prolonged left ventricular (LV) contraction.6–8 Electrocardiogram (ECG) has limited abilities to detect regional differences in LV electrical pattern. A relationship between motion abnormalities of the LV assessed by echocardiography and syncope or cardiac arrest in LQTS patients was first indicated by Schwartz and colleagues.9,10 Tissue Doppler imaging (TDI) has been established as a clinical method for the quantification of regional myocardial function.11 The diagnostic value of this new modality has not been previously described in LQTS patients.
The objective of this study was to determine whether myocardial velocities, time-intervals, or strains by TDI could be a tool for identifying high-risk individuals in LQTS patients. High risk was defined as patients with a previous cardiac event, i.e. documented arrhythmia, syncope, or cardiac arrest. Our hypothesis was that prolonged action potentials will cause a prolonged myocardial contraction that can be assessed by TDI. Furthermore, we hypothesized that myocardial mechanical dispersion can be assessed as heterogeneity in the regional myocardial contraction duration by TDI.
Methods
Long QT syndrome patients
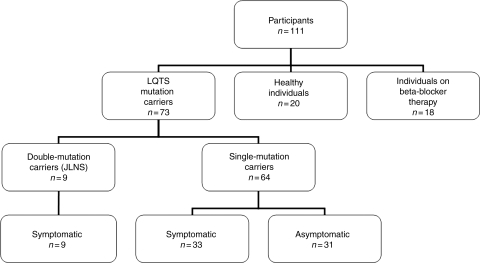
Seventy-three patients with molecularly defined LQTS were included in this study (Figure 1). None of the LQTS patients had structural heart disease of other origin or were ventricularly paced. All 73 LQTS mutation carriers were genotyped: 44 LQT1, 18 LQT2, 1 LQT3, 1 LQT5, and nine double-mutation carriers. Double-mutation carriers were considered a separate category and were analysed separately.
Figure 1.
Algorithm of study participants. The groups of healthy individuals and individuals on beta-blocker therapy represent the control groups.
Long QT syndrome single-mutation carriers
Sixty-four patients were single-mutation carriers of an LQTS-associated mutation. Of the single-mutation carriers, 33 (52%) had a history of documented arrhythmia, syncope, or cardiac arrest, here defined as ‘symptomatic’. All symptomatic and three asymptomatic single-mutation carriers received therapy with beta-blockers (n = 36). In addition to beta-blocker therapy, three single-mutation carriers were treated with an ICD and three with atrial pacemaker.
In addition, six single-mutation carriers were studied with ECG and echocardiography but deemed ineligible for inclusion. One of these six was an LQT1 patient who received chemotherapy owing to malignant disease and developed cardiomyopathy with reduced LV function. Five asymptomatic mutation carriers <15 years of age were ineligible for inclusion. We could not exclude future cardiac events in these young individuals and their status as asymptomatic in this study would therefore be inaccurate.
Long QT syndrome double-mutation carriers, Jervell and Lange-Nielsen syndrome
Nine patients were double-mutation carriers of an LQTS-associated mutation and had clinically Jervell and Lange-Nielsen syndrome (JLNS) with additional deafness. All JLNS patients had experienced repeated cardiac events and were treated with beta-blockers. In addition, one JLNS patient was treated with left sympathetic denervation, two with an ICD, and three with atrial pacemaker.
Control groups
Healthy individuals
Twenty healthy individuals were age- and sex-matched and recruited from hospital staff. All had normal clinical examination, ECG, QTc, and echocardiography.
Individuals on beta-blocker therapy
Since 64% of the LQTS patients were on beta-blocker therapy, we included 18 individuals on beta-blocker therapy for comparison. They were treated with beta-blockers for suspected angina pectoris from the referral institution ahead of an elective coronary angiography. They were included in our beta-blocker control group after findings of a normal coronary angiography at our hospital. In addition, normal findings were required for the clinical examination, ECG, QTc, and echocardiography. Beta-blocker treatment was discontinued in all these patients after the examination at our hospital.
Written informed consent was given by all participants. The study was approved by the Regional Committee for Medical Research Ethics.
Electrocardiogram
Twelve-lead ECG was obtained in all participants, either before or after echocardiography. The QT interval was corrected for heart rate using Bazett’s formula.12
Echocardiographic studies
The echocardiographic studies were performed using Vivid 7 (GE Healthcare, Horten, Norway) and analysed with commercially available software (EchoPAC®, GE). By conventional 2D echocardiography, we assessed LV ejection fraction ad modum Simpson. Systolic time interval was assessed by Doppler flow velocity measurement as the time from start of R on ECG to aortic valve closure.
Tissue Doppler imaging
Two-dimensional TDI recording of the LV was obtained from the basal and mid-segments from apical four-chamber, two-chamber, and long-axis views. Three cycles were analysed and the image frame rate obtained in this study was 124 ± 29 frames/s. The following parameters from TDI were assessed:
Peak ejection velocity.
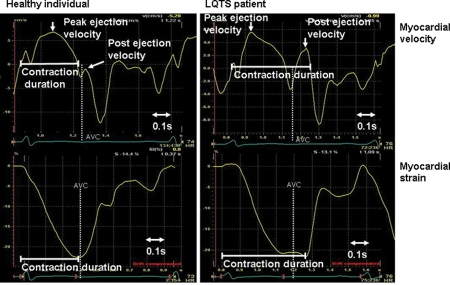
Peak myocardial velocity after aortic valve closure—post-ejection velocity (PEV). PEV was defined as the peak of the upstroke of the biphasic spike after ejection. In individuals without post-systolic shortening, this spike can occur below the zero line (Figure 2).
Maximum PEV in absolute value, negative or positive, was measured and the anatomical location was noted in each participant.
Myocardial contraction duration was measured in the basal septal segment and defined as the time from start of R on ECG to end of PEV (zero-crossing) if positive PEV was present. If no positive PEV was present, contraction duration was defined as the time from start of R on ECG to zero-crossing of the decreasing velocity slope in end-systole (Figure 2).
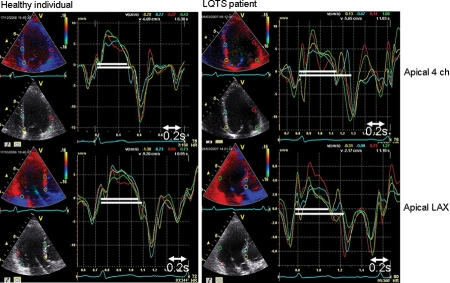
Contraction duration of the basal segment of the six LV wall positions was measured and the standard deviation of these values calculated as a parameter of mechanical dispersion of contraction (Figure 3).
In diastole, we measured the time from start of R to onset of E′ and peak E′.
Contraction duration was measured in strain traces as the time from start of R on ECG to peak negative strain (Figure 2).
Figure 2.
Myocardial contraction duration by tissue Doppler imaging. Myocardial velocities (upper panels) and strains (lower panels) from a healthy individual (left) and a long QT syndrome patient (right).
Figure 3.
Myocardial mechanical dispersion by tissue Doppler imaging. The left panels show velocity curves from four different segments in a healthy individual in four-chamber (upper left) and apical long-axis views (lower left). The right panels show velocity curves from a symptomatic long QT syndrome patient. White markers show the shortest and the longest contraction durations in each person. The difference in contraction duration is 0.03 s in the healthy individual and 0.12 s in the long QT syndrome patient and is consistent with the mechanical dispersion of contraction.
Efforts were made to ensure good image quality in each patient. TDI parameters could be assessed in 98% of the myocardial segments in LQTS mutation carriers and in 94% of the subjects in the other groups. The primary analysis was done unblinded by a single observer and repeated in a blinded fashion 4 months later (K.H.H.). All analyses were repeated by an independent observer (T.E.), blinded to patient identity and other data. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Statistical analyses
Data were presented as mean ± standard deviation. Comparisons of means were analysed by ANOVA with the Bonferroni post hoc correction for multiple comparisons (SPSS 15.0). Receiver-operating characteristic (ROC) curves were constructed to determine the sensitivity and specificity of the parameters—QTc, myocardial contraction duration, and PEV—to identify cardiac events (documented arrhythmia, syncope, or cardiac arrest) in LQTS single-mutation carriers. For contraction duration and PEV, the optimal cut-offs were defined as the value of the ROC curve which was closest to the upper left corner (Figure 4). The reliability of the cut-off values was validated using bootstrap resampling (n = 1000),13 and 95% confidence intervals (95% CIs) based on bootstrap percentiles were presented. The statistical software package R (version 2.5.1) was used for bootstrap analysis. For QTc, we used the established cut-off value of 0.46 s.14 For the area under the ROC curve, 95% CIs were presented. Reproducibility was expressed as intraclass correlation coefficient for single measures. For all statistical analyses, P-values were two-sided, with results less than 0.05 considered significant.
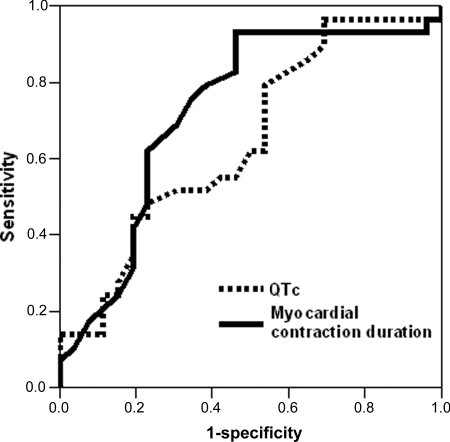
Figure 4.
Receiver-operating characteristic curves of cardiac events (documented arrhythmia, syncope, or aborted cardiac arrest) in 64 single long QT syndrome mutation carriers (33 symptomatic and 31 asymptomatic). Myocardial contraction duration shows higher sensitivity and specificity for cardiac events compared with corrected QT interval. Area under curve 0.77 (95% CI 0.65–0.89) vs. 0.66 (95% CI 0.52–0.79).
Results
All groups of LQTS mutation carriers were comparable with healthy individuals with respect to age and heart rate (Table 1). The QTc was prolonged in each of the LQTS groups compared with the control groups. EF and peak ejection velocity, as markers of systolic function, were normal in all LQTS groups, in healthy individuals and in individuals on beta-blocker therapy (Table 1).
Table 1.
Clinical characteristics and echocardiographic results
| Healthy individuals (n = 20) | LQTS single-mutation carrier asymptomatic (n = 31) | LQTS single-mutation carrier symptomatic (n = 33) | LQTS double-mutation (JLNS) carrier symptomatic (n = 9) | Individuals on beta-blocker medication (n = 18) | P-value (ANOVA F-test) | |
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age (years) | 34 ± 11 | 41 ± 14 | 33 ± 15 | 27 ± 21 | 59 ± 10 | 0.04 |
| Women [n (%)] | 11 (55) | 21 (68) | 25 (76) | 8 (89) | 10 (56) | |
| RR (s) | 0.90 ± 0.16 | 0.93 ± 0.17 | 0.99 ± 0.19 | 0.94 ± 0.23 | 0.93 ± 0.19 | 0.47 |
| QTc | 0.39 ± 0.02 | 0.46 ± 0.03* | 0.48 ± 0.04* | 0.56 ± 0.05** | 0.41 ± 0.03 | <0.001 |
| Echocardiographic results | ||||||
| EF (%) | 67 ± 3 | 64 ± 6 | 64 ± 6 | 63 ± 5 | 66 ± 5 | 0.59 |
| CD by velocity (s) | 0.36 ± 0.04 | 0.40 ± 0.06* | 0.46 ± 0.06*,*** | 0.48 ± 0.06*,*** | 0.38 ± 0.04 | <0.001 |
| CD by strain (s) | 0.39 ± 0.03 | 0.45 ± 0.05* | 0.49 ± 0.05*,*** | 0.50 ± 0.07*,*** | 0.40 ± 0.03 | <0.001 |
| Standard deviation of CD by velocity (s) | 0.014 ± 0.013 | 0.031 ± 0.019* | 0.048 ± 0.018*,*** | 0.036 ± 0.021* | 0.024 ± 0.016 | <0.001 |
| Time to aortic valve closure (s) | 0.36 ± 0.01 | 0.39 ± 0.03* | 0.40 ± 0.03* | 0.42 ± 0.05* | 0.40 ± 0.03 | <0.001 |
| Peak ejection velocity (cm/s) | 6.2 ± 1.0 | 6.1 ± 1.1 | 5.9 ± 0.8 | 5.5 ± 0.9 | 5.5 ± 0.9 | 0.27 |
| PEV (cm/s) | −0.2 ± 0.7 | 1.1 ± 1.3* | 2.3 ± 1.3*,*** | 2.5 ± 1.3*,*** | 0.0 ± 0.8 | <0.001 |
| Onset E′ wave (s) | 0.40 ± 0.03 | 0.44 ± 0.04* | 0.47 ± 0.05* | 0.48 ± 0.07* | 0.46 ± 0.04 | <0.001 |
| E′ (cm/s) | 9.8 ± 2.3 | 8.8 ± 2.0 | 7.9 ± 2.0* | 7.8 ± 2.3 | 9.8 ± 2.7 | 0.02 |
| E deceleration time (s) | 0.15 ± 0.01 | 0.19 ± 0.03 | 0.19 ± 0.04 | 0.23 ± 0.08* | 0.20 ± 0.04 | 0.10 |
Mean ± SD. Right column shows P-values for ANOVA test. Flags for significance are obtained from the post hoc pair-wise comparison using the Bonferroni correction.
Results from individuals on beta-blocker medication are not included in the ANOVA analyses.
CD, contraction duration.
*P < 0.05 compared with healthy individuals.
**P < 0.001 compared with each of the other groups.
***P < 0.05 compared with asymptomatic LQTS single-mutation carriers.
Echocardiographic analysis by tissue Doppler imaging
Contraction duration was assessed in both myocardial velocity and strain traces with consistent results.
Long QT syndrome single-mutation carriers
Contraction duration was significantly longer in symptomatic LQTS single-mutation carriers compared with asymptomatic carriers and healthy individuals (P = 0.001) (Table 1). In contrast to healthy individuals, LQTS mutation carriers showed contraction durations that exceeded the time to aortic valve closure. Patients on beta-blocker therapy did not have prolonged contraction duration. Therefore, the prolonged contraction duration in LQTS mutation carriers could not be attributed to the use of beta-blocker medication. Mechanical dispersion, assessed as standard deviation of contraction duration, was significantly greater in LQTS single-mutation carriers compared with healthy individuals (P < 0.001) and compared with individuals on beta-blocker therapy (P < 0.001). In addition, mechanical dispersion was significantly greater in symptomatic compared with asymptomatic single-mutation carriers (P = 0.001). The correlation between contraction duration and QTc was significant (R = 0.43, P < 0.001).
A marked PEV was observed in LQTS mutation carriers and was significantly greater than PEV found in healthy individuals (Figure 2, Table 1). In addition, the maximum PEV in symptomatic LQTS single-mutation carriers was greater than that in asymptomatic LQTS mutation carriers (P = 0.001). The maximum PEV was most often localized in the posterior part of the LV septum (n = 34), but was also found in the anterior part of the septum (n = 17), in the anterior wall (n = 12), in the lateral wall (n = 9), and in the posterior wall (n = 1) of the LV.
The onset of the E′ was delayed in symptomatic LQTS single-mutation carriers compared with asymptomatic mutation carriers and control subjects. Age-corrected E′-wave velocities were lower in symptomatic than in asymptomatic mutation carriers [7.9 ± 1.9 vs. 8.9 ± 1.9 cm/s; P = 0.02 (Univariate Analysis of Variance)].
Subgroup analysis of LQT1 and LQT2 patients (data not presented) did not show significant differences in the echocardiographic parameters.
We wanted to explore the incremental value provided by echocardiography in LQTS mutation carriers. JLNS patients (double mutation) were considered a separate category and not necessarily representative of the more common patients with a single mutation. Therefore, we constructed ROC curves for LQTS single-mutation carriers exclusively (n = 64). By ROC analysis, contraction duration identified single-mutation carriers with a history of cardiac events with better specificity and sensitivity than QTc did (Figure 4). QTc ≥0.46 s showed a sensitivity of 70% (95% CI 67–88) and a specificity of 50% (95% CI 40–61) to identify single-mutation carriers with a history of events. Contraction duration identified single-mutation carriers with a history of events with a sensitivity of 79% (95% CI 68–87) and a specificity of 74% (95% CI 62–83) with an optimal cut off value of 0.44 s (95% CI by bootstrapping 0.41–0.46). Importantly, contraction duration was prolonged in all six symptomatic single-mutation carriers that exhibited QTc shorter than 0.46 s. Optimal cut-off value for PEV was 1.65 cm/s (95% CI by bootstrapping 1.24–2.23) and demonstrated a sensitivity of 70% (95% CI 58–79) and a specificity of 68% (95% CI 55–78) for a history of cardiac events in single-mutation carriers by ROC analysis.
Long QT syndrome double-mutation carriers
QTc was markedly prolonged in double-mutation carriers compared with all other groups (P < 0.001) (Table 1). Contraction duration was prolonged and PEV augmented compared with healthy individuals and asymptomatic single-mutation carriers. Mechanical dispersion was longer than in healthy individuals, but not significantly different from single-mutation carriers.
The intra- and interobserver variabilities of PEV measurements demonstrated an intraclass correlation coefficient of 0.92 (95% CI 0.84–0.96) and 0.79 (95% CI 0.68–0.86), respectively, and for contraction duration measurements of 0.97 (95% CI 0.92–0.99) and 0.92 (95% CI 0.72–0.98), respectively.
Discussion
The present study demonstrated that analyses of tissue Doppler velocities added important information in patients with LQTS. Prolonged contraction duration showed better specificity and sensitivity than QTc as a marker of cardiac events and therefore provided added value in risk assessment in LQTS mutation carriers. The contraction duration showed greater heterogeneity in LQTS mutation carriers than in controls, reflecting dispersion of myocardial contraction, presumably caused by electrical dispersion of repolarization. LQTS mutation carriers showed a marked increase in PEV, indicating myocardial shortening after aortic valve closure. These findings were associated with a history of cardiac events.
Possible mechanisms
In the normal heart, several mechanisms regulate myocyte repolarization. As the QT interval on the surface ECG represents the summation of action potentials in ventricular myocytes, QT prolongation implies action potential prolongation in at least some portions of the ventricle. A prolongation of the action potential duration is associated with an increase in the tension developed by the ventricular muscle, leading to a prolonged contraction.6–8 In this study, the prolonged contraction in LQTS mutation carriers was assessed by TDI.
Action potential prolongation can lead to the development of early EADs, which are oscillations in the membrane potential before repolarization is complete. EADs may result in a second contraction and lead to ectopic beats if occurring in a substantial part of the myocardium.15 Triggered upstrokes from EADs are a likely initiating mechanism for torsade de pointes ventricular tachycardia.16–18 The prolonged contraction duration and the augmented PEV in LQTS mutation carriers could represent the mechanical equivalent of an electrical EAD as proposed by De Ferrari et al.10 This is consistent with the finding that symptomatic LQTS mutation carriers had greater PEV than asymptomatic mutation carriers. EADs may be present at subthreshold levels in LQTS mutation carriers also in basal conditions, without leading to arrhythmias but causing contraction prolongation and PEV.
In the majority of our LQTS mutation carriers, we found the greatest magnitude of the PEV in basal and mid-LV septal regions. Previous reports have demonstrated that action potentials of the mid-myocardial cells (M-cells) prolong more than epicardial or endocardial in response to a lowering of heart rate or to action potential-prolonging agents.19 A prolonged action potential leads to a higher risk of EAD.15 M-cells have been identified in the interventricular septum and in the anterior and lateral walls.20 The localization of the reported M-cells matched the regions of the greatest PEV in our LQTS patients. Therefore, we speculated that PEV and prolonged contraction assessed by TDI could be a result of the prolonged action potentials in these M-cells that are known to have the longest action potential duration.
A possible mechanism of arrhythmia in LQTS patients is increased dispersion of repolarization.21 Dispersion of repolarization may be localized between two different ventricular regions and as a transmural phenomenon.22,23 The measurement of QTc and QT dispersion as indicators of ventricular repolarization prolongation and dispersion have been widely used during the last two decades.24 However, identification of the end of the T-wave is difficult and measurements of dispersion by ECG have modest reproducibility.25 The TDI method has the ability to measure detailed time intervals in different regions of the LV. Our study clearly demonstrated that the dispersion of mechanical contraction can be assessed by TDI, as a regional difference in contraction duration throughout the LV. Furthermore, that mechanical dispersion was more pronounced in symptomatic compared with asymptomatic LQTS patients.
Double-mutation carriers, JLNS patients, represent the most severe clinical phenotype and were therefore analysed separately in this study. The presence of two mutations (JLNS) is associated with higher risk of cardiac events and more widespread current loss.26 In our study, these facts were supported by a more prolonged contraction duration in a greater number of cardiac segments in JLNS patients compared with single-mutation carriers. Standard deviation of contraction duration was therefore lower in the JLNS patients. Pronounced prolongation of contraction duration may indicate that EADs are the most likely mechanism for arrhythmia in JLNS patients.
The contraction duration in LQTS mutation carriers exceeded the time to aortic valve closure. This indicated myocardial contraction after aortic valve closure, which may be a result of an inhomogeneous end of contraction not sufficient to maintain the opening of the aortic valve. If the dispersion of contraction reflects the electrical dispersion of repolarization, a major arrhythmogenic factor present in LQTS mutation carriers can be shown by echocardiography.
A prolonged myocardial contraction will lead to inhomogeneous and delayed onset of the E′ wave. Furthermore, an inhomogeneous onset of diastolic lengthening will cause reduced E′ amplitude and prolonged duration of E′. These assumptions were confirmed in our study that showed delayed onset of E′, reduced amplitude of the E′, and prolonged E deceleration time. These findings imply an impairment of diastolic function in a number of symptomatic LQTS mutation carriers.
Previous studies
Echocardiography has traditionally been used to exclude structural heart disease in LQTS patients. However, Nador et al. presented an echocardiographic study which showed specific ventricular wall abnormalities in 42 LQTS patients.9 Using M-mode technique, they demonstrated the occurrence of a slow contraction in the late myocardial thickening phase. Further they demonstrated a dip in the later part of contraction followed by a second anterior movement of the endocardium producing a double-peak image. Interestingly, their findings were associated with a greater probability of syncope or cardiac arrest. These findings are confirmed in our study. We showed that prolonged contraction duration and increased PEV, which give a similar double-peak pattern, are associated with cardiac events. Another study has shown mechanical abnormality by M-mode technique in LQTS patients.27 Their findings were attributed to mechanical dispersion possibly caused by electrical dispersion of repolarization. A recent case report indicated mechanical dysfunction in a patient with extreme QT prolongation.28 A recent study showed myocardial velocity abnormalities in 10 LQTS patients.29 Our study confirms these findings using a new modality of echocardiography. We believe that TDI measurements may be an easier and more objective way of quantifying abnormal regional motion than M-mode echocardiography. The study by Nador et al. was followed by a study giving calcium channel blockers to 10 LQTS patients.10 Verapamil abolished the wall motion abnormality, suggesting that symptomatic LQTS patients may have an abnormal increase in the intracellular calcium concentration before relaxation has completed, possibly linked to an EAD, and that the contraction abnormality may be the mechanical equivalent of an EAD.
Post-systolic shortening after aortic valve closure, which is similar but not identical to PEV, has been demonstrated in ischaemic myocardium30,31 and may also occur in healthy individuals.32 PEV in ischaemic heart disease is of greater magnitude compared with PEV in healthy individuals, and a coexisting reduction in ejection velocity is obligate.32 PEV can even be negative if post-systolic shortening is missing in healthy individuals.32,33 The LQTS mutation carriers had normal systolic ejection velocities and ejection fraction. Thus, the elevated PEV in LQTS mutation carriers was not considered to be a result of earlier cardiac arrests with concomitant myocardial ischaemia.
Clinical implications
QTc is the most rational parameter for screening patients with unexplained syncope or cardiac arrest. In known LQTS patients, risk stratification is based on the occurrence of previous syncope, genotype, gender, and QTc.1 The recent development from family cascade genetic screening has brought us numerous LQTS mutation carriers with normal QTc, who would have been undiagnosed before the genetic era. These asymptomatic mutation carriers demonstrate neither clinical symptoms nor prolonged QTc. Nevertheless, they have increased risk of ventricular arrhythmia and sudden cardiac death. It is a clinical challenge to decide whether these asymptomatic mutation carriers should receive prophylactic treatment or not. Assessment of myocardial contraction duration may add important information in risk stratification in LQTS mutation carriers when QTc is normal or mildly prolonged. Our data showed that myocardial contraction duration had higher sensitivity and specificity for a history of cardiac events than QTc. Importantly, in all symptomatic mutations carriers that QTc failed to identify, contraction duration by echocardiography was prolonged (≥0.44 s).
Our study did not provide data that actually demonstrated that asymptomatic LQTS mutation carriers with prolonged contraction duration and elevated PEV were more likely to develop arrhythmias than those without. This requires a prospective study in which asymptomatic untreated patients are followed for an adequate period of time, which is difficult for ethical reasons. Nevertheless, we propose that TDI should become part of the routine clinical evaluation for LQTS mutation carriers. The myocardial contraction duration is relatively simple to obtain and has excellent reproducibility for inter and intra-observations.
Limitations
Prolonged contraction duration by strain in this study was highly significant as a risk parameter for cardiac events. However, the results assessed by myocardial velocities were significantly associated with cardiac events and therefore we preferred velocity to strain measurements since the reproducibility of this method is superior.34 Circumferential and radial strains were not analysed in this study.
A limitation of TDI is marked angle dependency, which makes it sensitive to mal-alignment between the principal direction of myocardial shortening and the Doppler beam. Owing to less angle dependency, time interval assessment provided by TDI is a better reproducible parameter than measurements of velocity amplitudes.
The association between myocardial mechanical and electrical dispersion in LQTS mutation carriers should be studied in experimental studies.
Conclusion
This study showed that TDI can be of important value in risk stratification of LQTS mutation carriers. Mechanical dispersion of myocardial contraction assessed by TDI was increased in LQTS patients. Prolonged myocardial contraction duration and augmented PEV were better related to cardiac events compared with QTc. Therefore, we propose that these novel parameters might be assessed in the management of LQTS patients.
Funding
This work was supported by Inger and John Fredriksen’s Foundation and the South-Eastern Norway Regional Health Authority. Funding to pay the Open Access publication charges for this article was provided by Prof. Jan P. Amlie, University of Oslo.
Conflict of interest: none declared.
Acknowledgements
We thank Are Hugo Pripp and Odd O. Aalen for statistical assistance. We thank the funders and the patients and control subjects who participated.
References
- 1.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 2.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 3.Romano C, Gemme G, Pongiglione R. Rare cardiac arrhythmias of the pediatric age. II. Syncopal attacks due to paroxysmal ventricular fibrillation. Presentation of 1st case in Italian pediatric literature. Clin Pediatr (Bologna) 1963;45:656–683. [PubMed] [Google Scholar]
- 4.Ward OC. A new familial cardiac syndrome in children. J Ir Med Assoc. 1964;54:103–106. [PubMed] [Google Scholar]
- 5.Hofman N, Wilde AA, Kaab S, van Langen I, Tanck MW, Mannens MM, Hinterseer M, Beckmann BM, Tan HL. Diagnostic criteria for congenital long QT syndrome in the era of molecular genetics: do we need a scoring system? Eur Heart J. 2007;28:575–580. doi: 10.1093/eurheartj/ehl355. [DOI] [PubMed] [Google Scholar]
- 6.Morad M, Trautwein W. The effect of the duration of the action potential on contraction in the mammalian heart muscle. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299:66–82. doi: 10.1007/BF00362542. [DOI] [PubMed] [Google Scholar]
- 7.Arreola J, Dirksen RT, Shieh RC, Williford DJ, Sheu SS. Ca2+ current and Ca2+ transients under action potential clamp in guinea pig ventricular myocytes. Am J Physiol. 1991;261:C393–C397. doi: 10.1152/ajpcell.1991.261.2.C393. [DOI] [PubMed] [Google Scholar]
- 8.Morad M, Orkand RK. Excitation–concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971;219:167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nador F, Beria G, De Ferrari GM, Stramba-Badiale M, Locati EH, Lotto A, Schwartz PJ. Unsuspected echocardiographic abnormality in the long QT syndrome. Diagnostic, prognostic, and pathogenetic implications. Circulation. 1991;84:1530–1542. doi: 10.1161/01.cir.84.4.1530. [DOI] [PubMed] [Google Scholar]
- 10.De Ferrari GM, Nador F, Beria G, Sala S, Lotto A, Schwartz PJ. Effect of calcium channel block on the wall motion abnormality of the idiopathic long QT syndrome. Circulation. 1994;89:2126–2132. doi: 10.1161/01.cir.89.5.2126. [DOI] [PubMed] [Google Scholar]
- 11.Edvardsen T, Urheim S, Skulstad H, Steine K, Ihlen H, Smiseth OA. Quantification of left ventricular systolic function by tissue Doppler echocardiography: added value of measuring pre- and postejection velocities in ischemic myocardium. Circulation. 2002;105:2071–2077. doi: 10.1161/01.cir.0000014614.63980.ba. [DOI] [PubMed] [Google Scholar]
- 12.Bazett HC. An analysis of the time-relations of electrocardiography. Heart. 1920;7:353–370. [Google Scholar]
- 13.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals and other methods of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 14.Goldman MJ. Principles of Clinical Electrocardiography. 8th ed. Lange Medical; 1973. [Google Scholar]
- 15.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 16.el-Sherif N, Zeiler RH, Craelius W, Gough WB, Henkin R. QTU prolongation and polymorphic ventricular tachyarrhythmias due to bradycardia-dependent early afterdepolarizations. Afterdepolarizations and ventricular arrhythmias. Circ Res. 1988;63:286–305. doi: 10.1161/01.res.63.2.286. [DOI] [PubMed] [Google Scholar]
- 17.Clusin WT. Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit Rev Clin Lab Sci. 2003;40:337–375. doi: 10.1080/713609356. [DOI] [PubMed] [Google Scholar]
- 18.Kannankeril PJ, Roden DM. Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 2007;22:39–43. doi: 10.1097/HCO.0b013e32801129eb. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu W, Antzelevitch C. Effects of a K(+) channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome. Circulation. 2000;102:706–712. doi: 10.1161/01.cir.102.6.706. [DOI] [PubMed] [Google Scholar]
- 20.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921–1927. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 21.Kuo CS, Atarashi H, Reddy CP, Surawicz B. Dispersion of ventricular repolarization and arrhythmia: study of two consecutive ventricular premature complexes. Circulation. 1985;72:370–376. doi: 10.1161/01.cir.72.2.370. [DOI] [PubMed] [Google Scholar]
- 22.Ueda N, Zipes DP, Wu J. Epicardial but not endocardial premature stimulation initiates ventricular tachyarrhythmia in canine in vitro model of long QT syndrome. Heart Rhythm. 2004;1:684–694. doi: 10.1016/j.hrthm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Antzelevitch C, Shimizu W. Cellular mechanisms underlying the long QT syndrome. Curr Opin Cardiol. 2002;17:43–51. doi: 10.1097/00001573-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 25.Kautzner J, Yi G, Camm AJ, Malik M. Short- and long-term reproducibility of QT, QTc, and QT dispersion measurement in healthy subjects. Pacing Clin Electrophysiol. 1994;17:928–937. doi: 10.1111/j.1540-8159.1994.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz PJ, Spazzolini C, Crotti L, Bathen J, Amlie JP, Timothy K, Shkolnikova M, Berul CI, Bitner-Glindzicz M, Toivonen L, Horie M, Schulze-Bahr E, Denjoy I. The Jervell and Lange-Nielsen syndrome: natural history, molecular basis, and clinical outcome. Circulation. 2006;113:783–790. doi: 10.1161/CIRCULATIONAHA.105.592899. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama K, Yamanari H, Otsuka F, Fukushima K, Saito H, Fujimoto Y, Emori T, Matsubara H, Uchida S, Ohe T. Dispersion of regional wall motion abnormality in patients with long QT syndrome. Heart. 1998;80:245–250. doi: 10.1136/hrt.80.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyas H, O’Leary PW, Earing MG, Cetta F, Ackerman MJ. Mechanical dysfunction in extreme QT prolongation. J Am Soc Echocardiogr. 2007 doi: 10.1016/j.echo.2007.08.001. doi:10.1016. [DOI] [PubMed] [Google Scholar]
- 29.Savoye C, Klug D, Denjoy I, Ennezat PV, Le Tourneau T, Guicheney P, Kacet S. Tissue Doppler echocardiography in patients with long QT syndrome. Eur J Echocardiogr. 2003;4:209–213. doi: 10.1016/s1525-2167(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 30.Skulstad H, Edvardsen T, Urheim S, Rabben SI, Stugaard M, Lyseggen E, Ihlen H, Smiseth OA. Postsystolic shortening in ischemic myocardium: active contraction or passive recoil? Circulation. 2002;106:718–724. doi: 10.1161/01.cir.0000024102.55150.b6. [DOI] [PubMed] [Google Scholar]
- 31.Azevedo CF, Amado LC, Kraitchman DL, Gerber BL, Osman NF, Rochitte CE, Edvardsen T, Lima JA. Persistent diastolic dysfunction despite complete systolic functional recovery after reperfused acute myocardial infarction demonstrated by tagged magnetic resonance imaging. Eur Heart J. 2004;25:1419–1427. doi: 10.1016/j.ehj.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Voigt JU, Lindenmeier G, Exner B, Regenfus M, Werner D, Reulbach U, Nixdorff U, Flachskampf FA, Daniel WG. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J Am Soc Echocardiogr. 2003;16:415–423. doi: 10.1016/s0894-7317(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 33.Edvardsen T, Aakhus S, Endresen K, Bjomerheim R, Smiseth OA, Ihlen H. Acute regional myocardial ischemia identified by 2-dimensional multiregion tissue Doppler imaging technique. J Am Soc Echocardiogr. 2000;13:986–994. doi: 10.1067/mje.2000.108466. [DOI] [PubMed] [Google Scholar]
- 34.Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–1164. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]