Abstract
We describe how upper limb amputees can be made to experience a rubber hand as part of their own body. This was accomplished by applying synchronous touches to the stump, which was out of view, and to the index finger of a rubber hand, placed in full view (26 cm medial to the stump). This elicited an illusion of sensing touch on the artificial hand, rather than on the stump and a feeling of ownership of the rubber hand developed. This effect was supported by quantitative subjective reports in the form of questionnaires, behavioural data in the form of misreaching in a pointing task when asked to localize the position of the touch, and physiological evidence obtained by skin conductance responses when threatening the hand prosthesis. Our findings outline a simple method for transferring tactile sensations from the stump to a prosthetic limb by tricking the brain, thereby making an important contribution to the field of neuroprosthetics where a major goal is to develop artificial limbs that feel like a real parts of the body.
Keywords: limb ownership, prosthetics, body representation, plasticity, illusion, referred sensation
Introduction
A major goal in applied neuroscience is to create artificial limb devices that feel and act just like real limbs. This work is associated with great technical challenges and raises fundamental questions related to how the brain distinguishes between parts of one's body and objects in the external world. A major area of research is dedicated to learning how to control the movements of artificial limbs using signals recorded directly from populations of cortical neurons (Nicolelis, 2003; Schwartz, 2004; Hochberg et al., 2006; Lebedev and Nicolelis, 2006; Velliste et al., 2008), signals originating from the nerves (Navarro et al., 2005), or from the muscles in the stump (Sebelius et al., 2005; Carrozza et al., 2006; Miller et al., 2008). However, for a limb to be functionally useful, one must be able to sense the touch and movements; this is never more true that for the hand, where sensations are required to provide feedback from the digits (Johansson, 1996). In principle, by connecting sensors in the artificial limbs to electrodes in the primary somatosensory cortex (London et al., 2008) or peripheral nerves (Navarro et al., 2005; Kuiken et al., 2007), one could effectively create tactile sensibility in the prosthetic limb. However, these emerging approaches are invasive and associated with major technical, surgical and other clinical challenges.
In the present article, we describe a simple procedure to produce tactile sensations in a prosthetic hand in upper limb amputees by simply tricking the brain using the so-called ‘rubber hand illusion’, which has been described in normal individuals (Botvinick and Cohen, 1998; Armel and Ramachandran, 2003; Ehrsson et al., 2004; Tsakiris and Haggard, 2005). In this illusion, synchronous brushstrokes, applied to a rubber hand in full view and to the participant's real hand, which is hidden under a table or behind a screen, produce the experience that the touch is located on the rubber hand and that the rubber hand is one's own hand. This illusion occurs as the brain's perceptual systems attempt to interpret the conflicting visual, tactile and proprioceptive information, culminating in a re-calibration of the location of the touch and the felt position of the hand with the result that touch appears to be felt by the rubber hand (Botvinick and Cohen, 1998). This process is known to involve multisensory areas of the brain including premotor, parietal and cerebellar structures (Ehrsson et al., 2004, 2005). The aim of the present study was to investigate if this multisensory illusion could be evoked in upper limb amputees by stimulating the stump in synchrony with the hand prosthesis.
Prior to experimentation, we were dubious whether this illusion could be elicited in amputees. In normal individuals it is critical to stimulate exactly the same locations on the rubber hand and the real hand for an illusion to be produced (H. H. Ehrsson, unpublished results). So how could the illusion possibly work in upper-limb amputees, who do not even have a hand to stimulate? We reasoned that the substantial plasticity in the cortical arm representation after amputation (Cohen et al., 1991; Elbert et al., 1994; Kew et al., 1994; Yang et al., 1994; Flor et al., 1995; Lotze, 2001), resulting in a less precise somatotopical organization with substantial overlap between hand and distal and proximal upper limb representations in the primary somatosensory cortex and thalamus (Pons et al., 1991; Merzenich et al., 1993; Florence and Kaas, 1995; Wall, 2002) might have the capacity to facilitate the rubber hand illusion. We also thought that the phantom limb sensations that can often be elicited by touching the stump, could help to elicit an illusion, in particular for referred tactile sensations in the phantom hand (Ramachandran et al., 1992; Grüsser et al., 2001). Recent studies have shown that visual feedback of a moving hand apparently originating from the position formally occupied by the amputated limb can elicit a ‘mirror illusion’ of seeing the phantom (Ramachandran and Rogers-Ramachandran, 1996). This visual impression of the missing hand seems to modulate the central representations of the phantom with respect to posture, movement, pain and the vividness of the phantom (Ramachandran and Hirstein, 1998; Giraux and Sirigu, 2003; Hunter et al., 2003; Brodie et al., 2007; Chan et al., 2007). Thus, being able to see the rubber hand being brushed could drive changes in the perceived location of the phantom limb just as it causes changes in position sense of the arm in normal individuals (Botvinick and Cohen, 1998; Ehrsson et al., 2005; Tsakiris and Haggard, 2005; Holmes et al., 2006).
We carried out several pilot experiments on upper limb amputees and these suggested that synchronized brushing of the participant's stump and the finger of the prosthetic hand did indeed produce experiences very similar to the rubber hand illusion. More specifically, we also noted that brushing the stump at the location eliciting referred sensations of the phantom index finger seemed to enhance the illusion. Therefore, we designed a full experiment with 18 upper limb amputees where we quantified the strength of the illusion using questionnaires, and carried out behavioural and physiological experiments to provide objective evidence of the effect ensuring that appropriate control conditions were included.
Methods
Participants
Eighteen volunteers participated in the study (14 males and 4 females; aged between 22 and 74 years). They were healthy with the sole exception being that they had all had one upper limb amputated at a level somewhere between the wrist and the elbow. Eleven had had their right arm amputated and the other seven had had the left arm amputated. The majority had their amputation after a traumatic accident (n = 15), while the remaining three had undergone the surgery to remove tumours. The participating amputees were recruited by phone, practically at random, from a list of upper-limb amputees who had been 20–75 years of age at the time the amputation was conducted, and who were registered at the Red Cross Hospital in Stockholm (www.rks.se/). The only inclusion criteria, apart from not taking any medication, was that they were using a prosthesis at least 4–8 h daily for 5–7 days per week. As this was a relatively unselected group of amputees, the time after amputation, phantom limb pain, specific phantom sensations such as telescoping and ‘maps’ of referred sensations on the stump, and daily prosthetic usage were factors that all varied greatly in the group.
Evaluation conducted prior to the experiments
Before the rubber hand illusion experiments commenced, all participants were interviewed to establish the following: their prosthesis use, and the existence and type/significance of phantom sensations, phantom limb pain (using a visual analogue scale from 0 to 10), and telescoping (i.e. shrinking of phantom arm so that the phantom hand is experienced close to the stump). Referred phantom sensation on the stump (Ramachandran et al., 1992; Grüsser, 2001), also referred to by us as ‘mapping of the phantom hand’, was investigated prior to the experiment. The patients were asked if they felt that their fingers or another part of the hand were being touched when they touched different parts of the stump. Each patient was then asked to touch the stump and define the referred phantom parts of the hand (divided into digits I–V, the palm or the dorsum of the hand). The points on the stump were then marked with a pen, after which the patient verified the mapping by touching the marks. The mapping was also documented on a protocol with a drawing of the stump in neutral position between pro- and supination, and with elbow at 90° flexion/and with the flexion of the elbow at 90°. The mark corresponding to digit II, the index finger, was used during the experiment where simultaneous touching of the stump and the rubber hand occurred. A summary of the patient data is presented in Table 1. All participants had given their written consent and the study was approved by the Central Ethics Committee at the Karolinska Institutet. The experiments were conducted in accordance with the declaration of Helsinki.
Table 1.
Details of participants
| Subject (gender, age) | Handeness | Lower arm stump length (cm)a | Cause of amputation | Time since amputation (years) | Prosthesis usage/type of prosthesisb | Phantom limb | Phantom pain (VAS)c | Telescopy | Mapd |
|---|---|---|---|---|---|---|---|---|---|
| #1 (m, 22) | R | R. mid third (16.5) | Tumour | 0.5 | All day, cosmetic | Yes | Yes (0) | Yes | Yes |
| #2 (f, 74) | R | R. lower third (22.5) | Tumour | 2 | All day, cosmetic | Yes | Yes (2) | Yes | Yes |
| #3 (m, 49) | R | L. wrist | Traumatic | 29 | All day, cosmetic | Yes | No | No | No |
| #4 (m, 39) | R | L. upper third (9) | Traumatic | 10 | All day, cosmetic | Yes | Yes (5) | Yes | Yes |
| #5 (m, 31) | R | R wrist | Traumatic | 7 | All day, cosmetic | Yes | No | Yes | Yes |
| #6 (m, 28) | R | R. lower third | Traumatic | 10 | All day, cosmetic | Yes | Yes (0) | No | Yes |
| #7 (m, 49) | R | R. mid third (16) | Traumatic | 18 | All day, cosmetic | Yes | Yes (0) | Yes | Yes |
| #8 (f, 33) | R | R. mid third (16) | Traumatic | 0.5 | No | Yes | Yes (4) | Yes | Yes |
| #9 (m, 62) | R | R. mid third (13) | Traumatic | 57 | Half day, cosmetic | No | No | No | No |
| #10 (m, 62) | R | R. wrist | Traumatic | 37 | All day, cosmetic | Yes | No | Yes | Yes |
| #11 (m, 62) | R | R. mid third | Traumatic | 51 | Half day, cosmetic | Yes | No | No | No |
| #12 (m, 52) | R | L. mid third | Traumatic | 1.5 | Half day, myoelectric | Yes | No | Yes | Yes |
| #13 (f, 55) | L | L. over third (11) | Traumatic | 10 | All day, hook | Yes | Yes (2–3) | Yes | Yes |
| #14 (m, 60) | R | L. mid third (13) | Traumatic | 52 | All day, myo-electric) | Yes | No | Yes | Yes |
| #15 (f, 39) | R | L. mid third (11) | Traumatic | 10 | All day, cosmetic | Yes | Yes (5.5) | No | No |
| #16 (m, 57) | R | R. lower third (22.5) | Tumour | 1 | All day, cosmetic | Yes | No | No | No |
| #17 (m, 49) | R | R. lower third (24) | Traumatic | 10 | All day, estetic | Yes | No | Yes | No |
| #18 (m,44) | L | L. mid third (18) | Traumatic | 21 | Half day, cosmetic | Yes | Yes (1) | Yes | Yes |
aStump length from elbow in centimetre. bMyo-electric, cosmetic, esthetic (very life like) or hook. cVisual analogue scale of the subjective strength of the phantom limb pain at the time of testing (0–10). dA distinct representation of index finger on the stump.
Experimental procedures: overall structure
Each subject participated in three experiments performed in a single 2-h session. We started with the subjective data collection in the form of a questionnaire, then moved on to a pointing-task experiment, and finally registered physiological changes associated with the illusion (see below). Between each experiment the participants had a 10–15 min long break to relax.
Experimental procedures: questionnaire
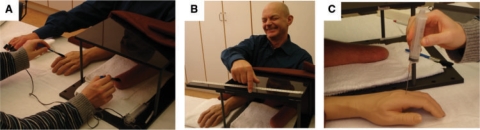
The first experiment aimed to quantify the subjective experience of the hypothesized illusion. The participants were seated with the stump of their arm and their contralateral arm resting prone on the table (Fig. 1A). A rubber, life-size prosthesis with the appearance of a male or female right or left hand was placed on the table parallel to the stump (or the contralateral hand in some experiments, see next paragraph). This prosthesis, also referred to as the ‘rubber hand’ in this article, was not attached to the stump, and the average distance between the index finger of the rubber hand and the participants’ stump was 26 cm. The particular rubber hand used in each experiment was always assigned the appropriate gender and laterality (right or left) for the person being tested. The stump (or contralateral arm) was hidden under a small plastic table. The participant was instructed to relax and look at the rubber hand. An experimenter used two small soft paintbrushes to touch the index finger of the rubber hand and the stump (or the contralateral arm) for 2 min, synchronizing the brushstrokes as closely as possible (Fig. 1A).
Fig. 1.
The experimental set-up. The stump was hidden under a table and synchronous brushstrokes were applied to the stump and the index finger of a rubber hand placed in full view in front of the participant (A). After experiencing the illusion, the participant was asked to demonstrate where he or she had felt the touches by making a horizontal pointing movement along a ruler with eyes closed (B). We also stabbed the rubber hand with a needle whilst simultaneously measuring the associated changes in the participant's skin conductance as an objective measure of any fear and anticipated pain. (See the Methods section for details.)
We employed two experimental conditions in which we always stimulated the index finger of the rubber hand: (i) in the ‘stump condition’ we brushed the stump at a location corresponding to the referred phantom index finger; (ii) in the ‘arm condition’ we brushed the dorsal surface of the contralateral lower arm at a level corresponding to the position of the referred phantom index finger in the stump. In people for whom a map of referred sensations could not be produced on the stump, we brushed the distal stump at a central point. We always started the experiment with the stump and arm conditions, and the order of these conditions was balanced across participants.
At the very end of the experiments, i.e. after the objective tests (explained below) had been conducted, we tested a third condition and collected data with the help of a questionnaire: (iii) the ‘finger-condition’, where we brushed the contralateral index fingers of the rubber hand and the hidden real hand, as in the classical rubber hand illusion (Ehrsson et al., 2004). We included this condition to verify that this particular group of amputees could experience the rubber hand illusion, and to give some idea of the relative strengths of the stump illusion and the conventional rubber hand illusion. The fact that this was tested after the other experiments ensured that the experience of the rubber hand illusion on the intact hand would not bias the results when stimulating the stump. Previous experiments have shown that the rating scores for the rubber hand illusion are quite consistent across experiments and for repeated testing with the same individuals (Ehrsson et al., 2007; Ehrsson et al., unpublished results) indicating the potential order effects are not large.
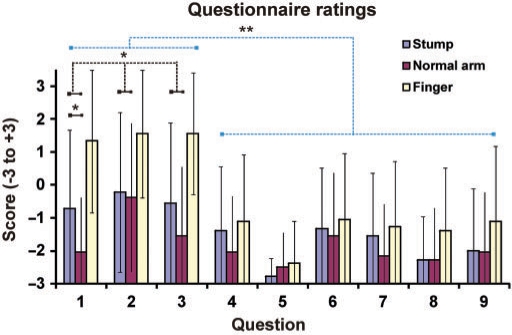
After a 120 s stimulation period conducted as described above, the participants completed a ‘rubber hand illusion questionnaire’. Nine questions, slightly modified from those used by Botvinick and Cohen (1998), were designed which required the participants to rate the strength of their agreement or disagreement with nine perceptual effects (Fig. 2). The first three questions were designed to correspond to the illusion, i.e. to sensing touch on the rubber hand and the experience that the prosthesis was part of the participant's own body (Q1—‘I felt the touch of the brush on the prosthetic hand’; ‘Q2—It seemed as if the brush caused the touch sensations that I was experiencing’; Q3—It felt as if the prosthetic hand was my hand’). The six other statements, which were unrelated to the illusion, served as control statements for suggestibility and task compliance [Q4—It felt as if the stump was moving towards the prosthetic hand; Q5—It felt as if I had three arms; Q6 I could sense the touch of the paintbrush somewhere between the stump and the prosthetic hand; Q7 The stump started to feel ‘rubbery’; Q8 It was almost as if I could see the hand prosthesis moving towards the stump; Q9 The prosthesis started to change shape, colour and appearance so that it started to (visually) resemble the stump]. The questions were translated into Swedish and adapted for the three conditions by changing the words stump/arm/finger as appropriate. The order in which the questions were presented to the test subjects was varied and balanced across individuals.
Fig. 2.
The results of the questionnaire. The responses to Questions 1–3 reflect the experiences of the illusion: Q1—‘I felt the touch of the brush on the prosthetic hand’; ‘Q2—It seemed as if the brush caused the sensation touch that I experienced’; Q3—It felt as if the prosthetic hand was my hand’. The responses to Questions 4–9 served as controls for suggestibility and task compliance (see Methods section). The scores for the illusion questions (Q1–Q3) were significantly greater (P < 0.01) than those for the control conditions after the period of synchronous stimulation on the stump and the prosthetic hand (blue). Further, on average, the scores on the three illusion-related questions were greater in the stump condition than in the control condition when contralateral intact arm was stimulated (P < 0.05). Finally, it can be noted that the illusion ratings when stroking the participants’ stumps were lower than when testing the classical rubber hand illusion by stroking their intact contralateral hand (yellow). For details, see the Results section.
The participants were then required to rate the extent to which these questions did or did not apply to their experience using a 7-point visual analogue scale. On this scale, −3 meant ‘absolutely certain that it did not apply’, 0 meant ‘uncertain whether or not it applied’ and +3 meant ‘absolutely certain that it applied’. An ANOVA was used to analyse the data. We also compared the mean score of the three illusion questions with the mean score of the control questions using a paired one-tailed t-test with the a priori hypothesis that the scores should be greater in the illusion statements. We also used a paired one-tailed t-test to test our prediction that a greater mean score would be obtained for the illusion statements in the stump condition than for the arm condition.
We also looked for factors that could predict if an amputee would feel the illusion. We thus divided the data obtained from the rubber hand illusion questionnaire into participants with or without phantom limb pain, with and without telescoping and with and without a map of referred sensations on the stump. All but one of the participants reported phantom sensations before the experiments, and most people used a cosmetic prosthesis, so the data could not be divided according to either of these dimensions. We also ran correlation analyses for the time since amputation and VAS pain scores.
Experimental procedures: post-stimulation pointing responses
The motivation for the next experiment was to obtain behavioral evidence that the illusion caused a shift in the perceived location of the touches in space, i.e. towards the position of the prosthesis. For periods of 60 s, we exposed the 18 participants to the stump-illusion condition and a control condition, presented three times each in a counterbalanced order across participants. The control stimulation consisted of asynchronous touches applied to the rubber hand and the stump as it has been well established that asynchronous stimulation strongly reduces the rubber hand illusion in normal participants (Botvinick and Cohen, 1998; Ehrsson et al., 2004; Tsakiris and Haggard, 2005). Immediately before and after the stimulation trials, the participants were required to close their eyes and point to where they had felt the touches using their index finger as follows. The participants kept their arm-stump in position on the table, and then stretched their other hand about 45° out from the body's parasagittal plane. The participants then moved their index finger in a straight line to the position where they had felt the brushstrokes, making a single continuous movement (Fig. 1B). A scale mounted on the table was used to measure the end-point of each pointing movement. The pointing error was calculated as the distance between the indicating index finger and the stump after the stimulation period minus the distance between the indicating index finger and the stump before the stimulation period. Pointing errors were analysed with a paired t-test. We used a one-tailed test because the only hypothesis under investigation was that the illusion should be stronger in the synchronous condition.
Experimental procedures: skin conductance response
The third experiment was included to obtain physiological evidence for the illusion that would be independent of written or pointing movement responses. Thus, two Ag-AgCl skin conductance electrodes were attached to the pulps of second and third fingers and the skin conductance recorded using a portable system (AT64 Portable SCR; Advanced Technology, Illinois, USA). We used Biopacs isotonic gel (Gel 101) to ensure that good contact was made and the participants wore the electrodes for 5 min before registration was initiated. The data was sampled (at 8 Hz), and stored and analysed digitally. Before the experiments commenced the participants had been informed that they would never be stabbed with the needle and that they would not experience any painful stimulation (see below).
Two conditions were defined (the same ones as in the pointing task above): (i) the stump condition with synchronous touches applied to the stump and the rubber index finger; and (ii) the asynchronous condition where the touches applied to the stump and rubber finger were presented alternately. The motivation for including a control condition was to exclude general arousal associated with seeing a needle approaching the rubber hand.
The two conditions were repeated three times in a pseudo-randomized order [(1, 2, 2, 1, 1, 2) or (2, 1, 1, 2, 2, 1)] to minimize the effect of presentation order. Further, the order of presentations was balanced across individuals. Each condition lasted for a random period of 40, 60 or 80 s, with the period length matched between the conditions. At the end of the simulation the rubber hand was suddenly ‘stabbed’.
The threatening stimulus consisted of a needle (1.2 × 50 mm; Sterican® Braun Melsungen AB) attached to a syringe (100 ml), which was used to stab the rubber hand just above the knuckle of the index finger (Fig. 1C). Great care was taken to move the syringe in the same way from trial to trial. The procedure of moving the needle and stabbing the hand (the ‘threat stimuli’) lasted for about 2 s.
For each trial we identified a peak value in the skin conductance response (SCR) within 1–4 s of the onset of the threat stimuli. As a baseline we used the value 1 s before the stimuli was presented. We included all trials and analysed the data from the two conditions in exactly the same way, thus we compared the magnitude of the SCR (Dawson et al., 2007).
For the statistical analysis, we compared the mean SCR associated with the two conditions across individuals using a paired t-test. A one-tailed test was used because we had an a priori hypothesis of greater autonomic arousal in the illusion condition given that asynchronous stimulation is known to significantly reduce the rubber hand illusion (Botvinick and Cohen, 1998; Ehrsson et al., 2004; Tsakiris and Haggard, 2005). In all statistical tests, we set alpha to 5%.
Results
Questionnaire
The results of the statistical analysis of the questionnaire data are presented in Fig. 2. In the illusion condition, where the stump is brushed (blue), the subjects (N = 18) provided stronger ratings for the three illusion questions than for the six control questions [P = 0.002 paired one-tailed t-test comparing the means of questions 1–3 versus questions 4–9; an ANOVA gave F(8,153) = 3.27, P = 0.0018]. Furthermore, the ratings for the illusion questions were greater in the stump condition than in the arm condition, where the contralateral lower arm was stimulated (red bars; P < 0.04 paired one-tailed t-test comparing the mean of questions 1–3 for the stump and normal arm conditions). Post hoc paired t-tests showed that the scores were significantly different for question 1 (‘I felt the touch of the brush on the prosthetic hand’) (P = 0.012 one-tailed), but not for questions 2 (‘It seemed that the brush that touched the prosthetic hand generated the feeling of touch that I experienced’) (P = 0.36) and 3 (‘I felt that the prosthetic hand was my own hand’) (P = 0.08). When inspecting the results of individual participants, 6 out of the 18 showed confirmative scores (≥+1) for all three illusion questions; these participants were defined as having a strong illusion (their mean score was +2.17 on the illusion statements).
The strength of the stump illusion seemed to be relatively weak compared to previously published data on the traditional rubber hand illusion (Botvinick and Cohen, 1998; Ehrsson et al., 2004), and the condition in the present study where tactile stimulation was applied to the participants’ contralateral index finger (Fig. 2, yellow). As can be seen in Fig. 2, this group of amputees could clearly experience the normal rubber hand illusion (scores for statements 1–3 of above +1, which was much higher than for the control statements). Furthermore, the ratings for the illusion questions were greater in the finger condition than in the stump condition (P = 0.005 paired two-tailed t-test comparing the mean for statements 1–3 for the finger and stump arm conditions; post hoc paired t-tests showed that the scores were significantly different for questions 1 (P = 0.038 two-tailed) and 3 (P = 0.002 two-tailed), but not for the second question (P = 0.097 two-tailed). However one should interpret these statistical comparisons with caution because we always tested the finger condition after the stump condition, thus not controlling for any order effects.
Post-stimulation pointing responses
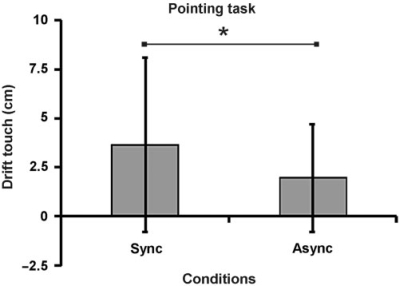
The errors observed in the pointing task were consistent with the questionnaire data presented above. When the participants were asked to close their eyes and point towards where they felt the touch of the paintbrush after a period of stump stimulation, the error was greater than after the asynchronous control condition (P = 0.015 paired one-tailed t-test; see Fig. 3; P = 0.0019, Wilcoxon Signed Rank Test, one-tailed). The error in pointing in the illusion condition was 3.6 cm, which corresponds to 14% of the actual distance between the stump and the index finger of the prosthesis, which is in the same order as previously reported proprioceptive drift measures for the rubber hand illusion (Ehrsson et al., 2005; Tsakiris and Haggard, 2005).
Fig. 3.
Behavioural evidence that people perceived a change in the location of the sensation of touch from the stump (and phantom in the cases of referred sensations) towards the rubber hand. When asked to indicate where they had sensed the touches of the paintbrush, by pointing with the intact hand with their eyes closed, the participant indicated greater drift in the perceived location of the touch towards the rubber hand after the illusion condition with synchronous stimulation (Sync) than after the asynchronous control condition (Async; P < 0.05).
Skin conductance responses
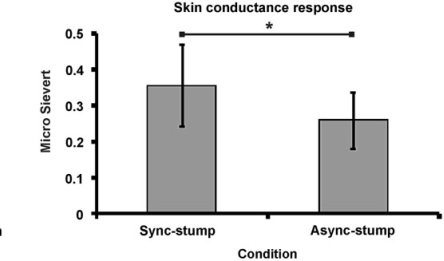
Finally, we turn to the physiological evidence. When we stabbed the prosthesis after a period of synchronous stimulation of the stump and hand prosthesis a greater skin conductance response was measured than after the asynchronous condition (P = 0.042 paired one-tailed t-test; see Fig. 4; P = 0.007, Wilcoxon Signed Rank Test, one-tailed). This is objective evidence that participants experienced anxiety when they saw the prosthetic hand being stabbed in the synchronous (illusion) condition.
Fig. 4.
Objective physiological evidence that the participants experienced an increase in the ownership of the prosthetic hand when we brushed the stump and the prosthetic hand synchronously. Greater psychologically induced sweating, as measured with the skin conductance response (in micro Sievert), was observed when the prosthetic hand was stabbed with a needle in the illusion condition (sync-stump) than in the asynchronous control condition (async-stump; P < 0.05).
Results across all three tests
Because three experiments were performed in each individual, one can pool the data and treat the combined data-set as a repeated measure within subject design; one can then use a multivariate analysis of variance to analyse the data. We ran this complementary analysis in SPSS [General linear model, within subject(s) design with repeated measures; the within subject factor illusion (illusion condition or control condition) and the three measures (questionnaire, pointing task and SCR)]. From the data obtained with the questionnaire we took the mean value of the three illusion statements from the stump and arm condition, respectively. From the pointing error and GSR experiments we took the mean values from the synchronous and asynchronous conditions. The multivariate analysis of variance was significant, i.e. there was a significant effect of illusion across the three measures F(3,15) = 3.759, P = 0.034 (two-tailed), Wilk's Lambda = 0.429.
Factors predicting the illusion
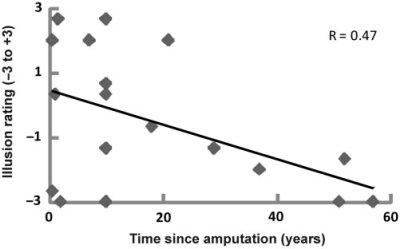
Interestingly, the shorter the time that had elapsed since amputation, the greater the likelihood of experiencing a strong illusion (Fig. 5). More precisely, we observed a significant correlation between the reported strength of the illusion, as indicated by the mean score on illusion-questions 1–3, and the number of years to have lapsed since the amputation (Pearson's correlation = −0.474, N = 18, P = 0.047, two-tailed; see Fig. 5). Similarly, there was a significant correlation between the time since amputation and the difference in illusion scores between the stump and the control (lower arm) condition (Pearson's correlation = −0.506, n = 18, P = 0.032, two-tailed). No significant correlation was found between the time since amputation and the pointing errors or skin conductance responses. We noted that participants with referred sensations tended to report stronger illusions than people without (mean illusion score −0.4 versus −0.9), but this was not significant. Also of the six participants with high scores on the illusion statements, five had a map of referred sensations. There was no difference in the illusion score ratings when dividing the data into people with or without telescoping, and with or without phantom limb pain; there was no correlation with pain VAS ratings.
Fig. 5.
Significant relationship between the time since amputation and the strength of the illusion as indicated by the illusion-items in the questionnaire (Pearson's correlation = −0.474, N = 18, P = 0.047, two-tailed P < 0.05). See the Results section for details.
Discussion
Our results suggest that the rubber hand illusion can be induced in upper limb amputees by simultaneously touching the stump and the finger of the prosthesis. Six of the 18 participants reported strong sensations of touch from the prosthesis and reported developing a sense of ownership of the artificial hand. When the quantitative questionnaire data from all 18 participants was analysed we observed a significant effect in the whole group. Furthermore, when asked to point towards where they felt the touches after a period of stimulation, the participants tended to point towards a location closer to the prosthesis in the illusion condition. Similarly, in this condition we registered greater psychological sweating, as measured with the skin conductance response, when the prosthesis was stabbed with a needle.
A methodological strength of the present study was that we simply did not rely on what the amputees told us. The questionnaire and the inclusion of a control condition were designed to control for task-compliance, suggestibility effects and confabulation. We also employed two objective tests of the illusion. By asking the participants to close their eyes and point to where they had sensed the touch we obtained a behavioural index of the illusion. Furthermore, the registration of changes in the conductance of the skin when we ‘injured’ the prosthetic hand provided us with an objective measure of autonomic system arousal. Previous studies have shown that when an owned rubber hand is threatened, it elicits anxiety that can be reliably measured as increase in skin conductance responses (Armel and Ramachandran, 2003) and activity in brain areas related to pain anticipation (Ehrsson et al., 2007) making this a good test for body ownership illusions.
One should emphasize that the illusion, in most amputees, was much weaker and less vivid than the traditional rubber hand illusion (Botvinick and Cohen, 1998; Ehrsson et al., 2004), which was also tested in this group of subjects using their contralateral hand after all the other experiments had been completed (Fig. 1). In fact, if one looks at the mean scores of the data obtained with the questionnaire, on average our group of participants denied experiencing the illusion in the stump condition (average score below 0). Importantly, however, these scores were significantly higher than in the control statements, and higher than illusion statements in the arm condition serving as the control (see Fig. 1). The fact that only 6 out of the 18 amputees reported a strong illusion during stump stimulation can explain the moderate effect size.
In our view, it is not surprising that the stump version of the rubber hand illusion should be more difficult to elicit than the conventional illusion tested using the intact arm of the respondents. We used an unselected group of amputees which was heterogeneous with respect to many factors such as phantom limb sensations, time since amputation, age, and level of amputation. Furthermore, one should recall that in normal volunteers the rubber hand illusion only works if one applies brushstrokes simultaneously to the corresponding parts of the hand. In addition, the illusion is reduced if the rubber hand is not aligned in parallel with the real hand (Ehrsson et al., 2004; Tsakiris and Haggard, 2005; Costantini and Haggard, 2007), or if the direction of the brushstrokes on the two hands is not the same (Costantini and Haggard, 2007). It was not possible to precisely match these factors when producing tactile sensations in the phantom index finger by brushing the map of referred sensations on the stump. Even if the illusion is weaker in amputees, it does seem to work well in some individuals which is an important observation in its own right.
In this respect, it was interesting to note that the shorter the time period since amputation, the greater the illusion (Fig. 5). The experience of a phantom limb is known to slowly fade over decades, so maybe the more vivid phantom limb experiences during the first few years after amputation enhances the illusion. It is also possible that as years go by the amputee's perceptual systems learn to accept the new body image with a missing hand and, therefore, the sight of the rubber hand illusion is less efficient in capturing the tactile sensations.
We will now turn to the possible mechanisms that could be responsible for mediating the rubber hand illusion in upper limb amputees. It could be so that the brushstrokes applied to the stump elicit referred tactile sensations in the phantom index finger and that the sight of the brush touching the rubber hand ‘captured’ these tactile sensations so that these sensations were now felt in the location where the brush touched the rubber hand. Indeed, before the experiments were conducted, we carefully characterized the presence of a “map” of referred phantom sensations on the stump of each participant, and if the person had such a map with the index finger (12 out of 18), we marked and stimulated that very spot during the experiments. In this scenario, the mechanism responsible for the illusion would be similar to that underlying the rubber hand illusion in normal individuals in that the brain would receive correlated somatosensory stimulation on the rubber finger seen and the index finger felt. This information would then be integrated and interpreted in multisensory areas leading to a spatial remapping of the sense of touch to the rubber hand (Botvinick and Cohen, 1998; Botvinick, 2004; Ehrsson et al., 2004; Ehrsson et al., 2005; Makin et al., 2008).
There are several routes by which the tactile information from the stump could reach the deafferented primary sensorimotor cortex, a likely candidate site for the phantom limb sensations, including reorganized thalamocortical and corticocortical pathways. We know from an earlier study that vibrotactile stimulation on the body surface ipsilateral to an amputation eliciting referred sensations in the phantom was associated with activation of the deafferented primary somatosensory cortex (Kew et al., 1997). The organization of the somatotopic maps in the primary somatosensory cortex and thalamus are known to undergo substantial structural and functional changes after amputation (Ramachandran and Hirstein, 1998; Flor et al., 2006). Although a representation of the missing limb seems to remain for many years after amputation (e.g. Cohen et al., 1991; Roux et al., 2003), the cortical zones representing body parts adjacent to the missing one start to expand into this area (Merzenich et al., 1984; Pons et al., 1991; Yang et al., 1994; Flor et al., 1995). Thus, by brushing the stump, tactile information reaches cortical tissue that used to process information from the missing hand. In turn, this cortex still probably has long-range direct and indirect anatomical connections to hand representations in other areas involved in the rubber hand illusion, such as the intraparietal cortex and premotor cortex (Ehrsson et al., 2004, 2005; Makin et al., 2008).
An interesting alternative scenario worth discussing is that the visual information of seeing the rubber hand touched would, ‘in itself’, be sufficient to give rise to the sense of touch experienced in the prosthesis. This certainly never happens in normal individuals, but Ramachandran and colleagues have reported some cases where amputees, who saw a mirror image of a hand that was superimposed on their stump being touched, reported sensing touch on their phantom limb, despite the fact that their stump was not being touched at all (Ramachandran and Rogers-Ramachandran, 1996, 2008). This even seems to work if another person's arm is placed next to the hidden stump and touched in full view (Ramachandran and Rogers-Ramachandran, 2008). This would presumably be some form of ‘synaesthesia’ in amputees where the visual information causes tactile experiences de novo, which would be a different mechanism from the rubber hand illusion that relies on the binding of visual and tactile events [(Ramachandran et al. have put forward the hypothesis that mirror neurons, in combination with the absence of tactile signals in the somatosensory cortices of amputees could explain this effect (Ramachandran and Rogers-Ramachandran, 2008)]. Our experiments were not designed to try to reproduce Ramachandran's findings, however, our results from the pointing task and the recordings of the skin conductance response suggest that this ‘synaesthesia effect’ is an unlikely explanation for our results because the effects we observed were stronger in the condition with synchronous stimulation than in the asynchronous condition. This difference can be explained more satisfactorily in terms of the binding of correlated visual and tactile information, as in the rubber hand illusion. Furthermore, none of the participants spontaneously described feeling the touch on their prosthetic hand when they saw the hand being brushed in the absence of a physical stimulation of the stump in the asynchronous condition.
Anecdotal reports exist of amputees describing how they can sometimes experience how their phantom ‘fills’ the prosthesis when they strap it on, and how this can even help them when using it (e.g. Sacks, 1987). Likewise it is not uncommon to hear amputees describe how their prosthesis sometimes feels like part of their body when they use it in various everyday situations. Could this have contributed to the present results? We think this is highly unlikely because (i) in the present experiments the hand prosthesis was not attached to the stump; (ii) the ‘rubber hand’ used was not the amputee's own prosthesis, and, most importantly, (iii) we employed control conditions so that any so-called ‘spontaneous filling in of the phantom’ or eventual bias arising from the participants’ previous experiences of using their strapped-on prostheses should be eliminated comparing the experimental conditions.
Our observations open up interesting new avenues for the development of prosthetic limb devices. In principle, it should be possible to design prosthesis equipped with tactile sensors in the fingertips (Brock and Chiu, 1985; Dario and Butazzo, 1987; Edin et al., 2008; Carpaneto et al., 2003) that can be connected to an array of tactile simulators on the stump that would reproduce the present illusion in everyday usage. In such a device, every time the finger of the prosthesis touched an object a tactile stimulation would be delivered instantaneously to the stump, thereby tricking the multisensory brain into experiencing the sensation of touch from the artificial finger. This method could provide a relatively easy way to restore rudimentary tactile sensibility in the prosthesis, which would complement existing approaches to the provision of sensory feedback from prosthetic limb devices (e.g. Riso, 1999; Lundborg and Rosén, 2001). It is also possible that having a sense of ownership of the prosthesis would be helpful to the user in its own right. If the prosthesis is experienced as being ‘one's hand’, it would probably be easier and more intuitive to use. Furthermore, a feeling of ownership of the prosthesis could have a ‘cosmetic’ value to the user because he or she would not be continuously reminded that the prosthesis is artificial, thereby reducing body dissatisfaction.
Funding
European Research Council; the Human Frontier Science Program; the Swedish Research Council (No. 5188); the Swedish Foundation for Strategic Research; the SMART-hand EU-project (contract no NMP4-CT-2006-0033423); Skåne County Council Research and Development Foundation.
References
- Armel KC, Ramachandran VS. Projecting sensations to external objects: evidence from skin conductance response. Proc Biol Sci. 2003;270:1499–506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M. Neuroscience. Probing the neural basis of body ownership. Science. 2004;305:782–83. doi: 10.1126/science.1101836. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Brock D, Chiu S. Environment perception of an articulated robot hand using contact sensors. Robot Manuf Automat. 1985;15:89–96. [Google Scholar]
- Brodie EE, Whyte A, Niven CA. Analgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a ‘virtual’ limb upon phantom limb pain, sensation and movement. Eur J Pain. 2007;11:428–36. doi: 10.1016/j.ejpain.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Carpaneto J, Micera S, Zaccone F, Vecchi F, Dario PA. Sensorized thumb for force closed-loop control of hand neuroprostheses. IEEE Trans Neural Syst Rehabil Eng. 2003;11:346–53. doi: 10.1109/TNSRE.2003.819938. [DOI] [PubMed] [Google Scholar]
- Carrozza MC, Cappiello G, Micera S, Edin BB, Beccai L, Cipriani C. Design of a cybernetic hand for perception and action. Biol Cybern. 2006;95:629–44. doi: 10.1007/s00422-006-0124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina PF, et al. Mirror therapy for phantom limb pain. N Engl J Med. 2007;22:2206–7. doi: 10.1056/NEJMc071927. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991;114:615–27. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- Costantini M, Haggard P. The rubber hand illusion: sensitivity and reference frame for body ownership. Conscious Cogn. 2007;16:229–40. doi: 10.1016/j.concog.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Dario P, Buttazzo G. An anthropomorphic robot finger for investigating artificial tactile perception. Int J Robot Res. 1987;6:25–48. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. The handbook of psychophysiology. Cambridge, UK: Cambridge University Press; 2007. pp. 152–91. [Google Scholar]
- Edin BB, Ascari L, Beccai L, Roccella S, Cabibihan JJ, Carrozza MC. Bio-inspired sensorization of a biomechatronic robot hand for the grasp-and-lift task. Brain Res Bull. 2008;15:785–95. doi: 10.1016/j.brainresbull.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Holmes NP, Passingham RE. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J Neurosci. 2005;25:10564–73. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–7. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Weich K, Weiskopf N, Dolan RJ, Passingham RE. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc Natl Acad Sci USA. 2007;104:9828–33. doi: 10.1073/pnas.0610011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. Neuroreport. 1994;20:2593–7. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–4. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, Staehelin JT. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–81. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- Florence SL, Kaas JH. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci. 1995;15:8083–95. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraux P, Sirigu A. Illusory movements of the paralyzed limb restore motor cortex activity. Neuroimage. 2003;20:S107–11. doi: 10.1016/j.neuroimage.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Winter C, Mühlnickel W, Denke C, Karl A, Villringer K, et al. The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience. 2001;102:263–72. doi: 10.1016/s0306-4522(00)00491-7. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Holmes NP, Snijders HJ, Spence C. Reaching with alien limbs: visual exposure to prosthetic hands in a mirror biases proprioception without accompanying illusions of ownership. Percept Psychophys. 2006;68:685–701. doi: 10.3758/bf03208768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JP, Katz J, Davis KD. The effect of tactile and visual sensory inputs on phantom limb awareness. Brain. 2003;126:579–89. doi: 10.1093/brain/awg054. [DOI] [PubMed] [Google Scholar]
- Johansson RS. Sensory control of dexterous manipulation. In: Wing AM, Haggard P, Flanagan JR, editors. Hand and brain: the neurophysiology and psychology of hand movements. San Diego: Academic; 1996. pp. 381–412. [Google Scholar]
- Kew JJ, Ridding MC, Rothwell JC, Passingham RE, Leigh PN, Sooriakumaran S, et al. Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. J Neurophysiol. 1994;72:2517–24. doi: 10.1152/jn.1994.72.5.2517. [DOI] [PubMed] [Google Scholar]
- Kew JJ, Halligan PW, Marshall JC, Passingham RE, Rothwell JC, Ridding MC, et al. Abnormal access of axial vibrotactile input to deafferented somatosensory cortex in human upper limb amputees. J Neurophysiol. 1997;77:2753–64. doi: 10.1152/jn.1997.77.5.2753. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371–80. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Nicolelis MA. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006;29:536–46. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- London BM, Jordan LR, Jackson CR, Miller LE. Electrical stimulation of the proprioceptive cortex (area 3a) used to instruct a behaving monkey. IEEE Trans Neural Syst Rehabil Eng. 2008;16:32–6. doi: 10.1109/TNSRE.2007.907544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain. 2001;124:2268–77. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Rosén B. Sensory substitution in prosthetics. Hand Clin. 2001;17:481–8. [PubMed] [Google Scholar]
- Makin T, Holmes N, Ehrsson HH. On the other hand: dummy hands and peripersonal space. Behav Brain Res. 2008;191:1–10. doi: 10.1016/j.bbr.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Miller LA, Stubbefield KA, Lipschutz RD, Lock BA, Kuiken TA. Improved myoelectric prosthesis control using targeted reinnervation surgery: a case series. IEEE Trans Neural Syst Rehabil Eng. 2008;16:46–50. doi: 10.1109/TNSRE.2007.911817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–58. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA. Brain-machine interfaces to restore motor function and probe neural circuits. Nat Rev Neurosci. 2003;4:417–22. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–60. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121:1603–30. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992;258:1159–60. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–86. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Sensations referred to a patient's phantom arm from another subjects intact arm: Perceptual correlates of mirror neurons. Med Hypotheses. 2008;70:1233–34. doi: 10.1016/j.mehy.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Riso RR. Strategies for providing upper extremity amputees with tactile and hand position feedback—moving closer to the bionic arm. Technol Health Care. 1999;7:401–9. [PubMed] [Google Scholar]
- Roux FE, Lotterie JA, Cassol E, Lazorthes Y, Sol JC, Berry I. Cortical areas involved in virtual movement of phantom limbs: comparison with normal subjects. Neurosurgery. 2003;53:1342–52. doi: 10.1227/01.neu.0000093424.71086.8f. [DOI] [PubMed] [Google Scholar]
- Sacks O. The man who mistook his wife for a hat and other clinical tales. London: Harper Perennial; 1987. [Google Scholar]
- Schwartz AB. Cortical neural prosthetics. Annu Rev Neurosci. 2004;27:487–507. doi: 10.1146/annurev.neuro.27.070203.144233. [DOI] [PubMed] [Google Scholar]
- Sebelius FC, Rosén BN, Lundborg GN. Refined myoelectric control in below-elbow amputees using artificial neural networks and a data glove. J Hand Surg. 2005;30:780–9. doi: 10.1016/j.jhsa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Haggard P. The rubber hand illusion revisited: visuotactile integration and self-attribution. J Exp Psychol Hum Percept Perform. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Brain Res Rev. 2002;39:181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- Yang TT, Gallen C, Schwartz B, Bloom FE, Ramachandran VS, Cobb S. Sensory maps in the human brain. Nature. 1994;368:592–93. doi: 10.1038/368592b0. [DOI] [PubMed] [Google Scholar]