Abstract
Deep brain stimulation (DBS) is a surgical procedure that has been shown effective in improving the cardinal motor signs of advanced Parkinson's disease, however, declines in cognitive function have been associated with bilateral subthalamic nucleus (STN) DBS. Despite the fact that most activities of daily living clearly have motor and cognitive components performed simultaneously, postoperative assessments of cognitive and motor function occur, in general, in isolation of one another. The primary aim of this study was to determine the effects of unilateral and bilateral STN DBS on upper extremity motor function and cognitive performance under single- and dual-task conditions in advanced Parkinson's disease patients. Data were collected from eight advanced Parkinson's disease patients between the ages of 48 and 70 years (mean 56.5) who had bilaterally placed STN stimulators. Stimulation parameters for DBS devices were optimized clinically and were stable for at least 6 months prior to study participation. Data were collected while patients were Off anti-parkinsonian medications under three stimulation conditions: Off stimulation, unilateral DBS and bilateral DBS. In each stimulation condition patients performed a cognitive (n-back task) and motor (force tracking) task under single- and dual-task conditions. During dual-task conditions, patients performed the n-back and force-maintenance task simultaneously. Under relatively simple dual-task conditions there were no differences in cognitive or motor performance under unilateral and bilateral stimulation. As dual-task complexity increased, cognitive and motor performance was significantly worse with bilateral compared with unilateral stimulation. In the most complex dual-task condition (i.e. 2-back + force tracking), bilateral stimulation resulted in a level of motor performance that was similar to the Off stimulation condition. Significant declines in cognitive and motor function under modest dual-task conditions with bilateral but not with unilateral STN DBS suggest that unilateral procedures may be an alternative to bilateral DBS for some patients, in particular, those with asymmetric symptomology. From a clinical perspective, these results underscore the need to assess cognitive and motor function simultaneously during DBS programming as these conditions may better reflect the context in which daily activities are performed.
Keywords: Parkinson's disease, deep brain stimulation, force control, cognitive function, dual-task
Introduction
Deep brain stimulation (DBS) is a surgical procedure that has been shown effective in improving the cardinal motor signs of advanced Parkinson's disease (The Deep Brain Stimulation Study Group, 2001). Despite the lack of a clear consensus, current surgical practice is to implant stimulating electrodes in the subthalamic nucleus (STN) during a single surgical session (Benabid et al., 2000). Although effective for Parkinson's disease motor symptom relief, short- and long-term reports indicate that bilateral STN DBS may be associated with significant postoperative morbidity in the form of cognitive deficits, declines in working memory, reduced information processing speed and increased depression and anxiety (Saint-Cyr et al., 2000; Gironell et al., 2003; Hershey et al., 2004; Rodriguez-Oroz et al., 2005; Schupbach et al., 2005); the following provide reviews on the neuropsychological effects of DBS (Voon et al., 2005; Skidmore et al., 2006; Temel et al., 2006b). In a recent meta-analysis examining the cognitive sequelae of STN DBS, it was concluded, that while STN DBS is safe, significant declines in executive function, verbal learning and memory are associated with bilateral STN DBS (Parsons et al., 2006). While some neuropsychological complications associated with bilateral STN DBS such as depression, anxiety and apathy may be transient or responsive to medical or behavioural therapy (Voon et al., 2005), solutions for reversing declines in working memory and other important cognitive functions that persist following bilateral STN DBS have not been identified. A decline in cognitive functioning (e.g. working memory and attention) has been shown to be an important factor that contributes to worsening postural stability, an increased rate of falling (Woollacott and Shumway-Cook, 2002) and decreased manual function in older adults (Voelcker-Rehage and Alberts, 2005; Voelcker-Rehage et al., 2006).
Recently, it was shown that, compared with elderly controls, mild to moderate Parkinson's disease patients without DBS produced more errors while driving a car as part of a route following task (Uc et al., 2007). A potential explanation for increased driving errors for Parkinson's disease patients’ is that they have limited cognitive resources and when presented with an increase in cognitive load or task demands (i.e. motor aspect of driving along with decision-making components of driving) the patients’ performance on one or both tasks degrades. A limited number of studies have examined the effects of Parkinson's disease on dual-task performance. Rochester and colleagues (2004) studied mild and moderate Parkinson's disease patients and found that as overall difficulty of the dual task increased, single-task performance declined (i.e. decreased walking speed and step length). These data suggest the basal ganglia may play an important role in the successful performance of tasks with both motor and cognitive components.
The impacts of a decline in cognitive resources (e.g. working memory) in Parkinson's disease patients and the effects of bilateral STN DBS on these resources and the motor functioning of Parkinson's disease patients have not been well documented. Bilateral STN DBS has been shown to improve reaction time in Parkinson's disease patients using a simple reaction time paradigm (Temel et al., 2006a). However, bilateral STN did not improve reaction time when these same patients completed a choice or complex reaction time task. The absence of an improvement in reaction time under more complex conditions may suggest that bilateral STN DBS may result in ‘cognitive slowing’ or a decline in information processing capability (Temel et al., 2006a). More recently, Hershey and colleagues (2007) assessed spatial working memory during bilateral and unilateral STN DBS. Declines in spatial working memory were present with bilateral DBS. During unilateral stimulation, motor function improved more when DBS was active in the more affected side of the brain (e.g. contralateral to more affected side) compared with the less affected side. However, with DBS in the more affected side of the brain working memory was impaired compared with stimulation in the less affected side of the brain. They concluded that unilateral and bilateral STN DBS can differentially affect cognitive and motor performance (Hershey et al., 2007). These recent data underscore the importance of using assessment tasks with varying levels of difficulty (Temel et al., 2006a) and examination of cognitive and motor effects during both unilateral and bilateral STN DBS (Hershey et al., 2007). To date, little consideration has been given to how a decline in cognitive function, as a result of bilateral STN DBS in particular, may impact cognitive and motor performance in Parkinson's disease patients using a dual-task paradigm; an experimental condition that better replicates the context in which most daily activities are performed.
Using clinical and objective kinematic measures, we have shown that unilateral STN DBS, 11–26 months post-DBS surgery, led to significant improvements in ipsilateral and contralateral motor function in advanced Parkinson's disease patients (Alberts et al., 2004). Our results indicate that unilateral STN DBS improves the control and coordination of grasping forces produced by each limb during the performance of a bimanual dexterity task and leads to an overall improvement in interlimb coordination (Alberts et al., 2004) and maximum force produced by each limb (Alberts et al., 2008). Additional studies provide clinical confirmation of our findings and indicate unilateral STN DBS improves motor functioning of both limbs (Chung et al., 2006; Slowinski et al., 2007; Tabbal et al., 2008). It is clear that unilateral STN DBS improves ipsilateral and contralateral motor function in advanced Parkinson's disease patients (Alberts et al., 2004; Germano et al., 2004; Piper et al., 2005; Chung et al., 2006; Slowinski et al., 2007; Tabbal et al., 2008). The incidence of adverse neuropsychological effects appears to be less in unilateral compared with bilateral STN DBS (Jahanshahi et al., 2000; Morrison et al., 2000; Saint-Cyr et al., 2000; Woods et al., 2002). Identification of the specific effects of unilateral or bilateral STN DBS on cognitive–motor performance is necessary to determine the best approach for individual patients, in particular those patients with slight cognitive dysfunction or advancing age.
A systematic comparison of unilateral to bilateral STN DBS on motor and cognitive performance under dual-task conditions has not been completed. We are aware of only one published study that examined bilateral DBS on dual-task performance of two cognitive tasks (Witt et al., 2004). Witt and colleagues (2004) reported that bilateral STN DBS did not improve the performance of a dual cognitive task (i.e. random number generation task paired with a card sorting task). Motor function was not characterized under dual-task conditions in their study, however, the authors suggest that the effects of STN DBS on cognitive–motor dual-task performance should be, ‘investigated in further studies using motor and non-motor tasks simultaneously with more concurrent attention demanding tasks’ (p. 700). The primary aim of this study was to determine the effects of unilateral and bilateral STN DBS on upper extremity motor function and cognitive performance under varying levels of cognitive–motor dual-task complexity. Based on previous reports of impaired cognitive functioning following bilateral STN DBS, it was hypothesized that compared with unilateral STN DBS bilateral STN DBS would result in greater declines in cognitive and motor function when moving from a single- to dual-task.
Methods
Participants
A total of eight participants with advanced Parkinson's disease between the ages of 48 and 70 years (mean 56.5) years participated in this study. Table 1 contains patient demographics, time since DBS, stimulation parameters for the left and right stimulators and clinical ratings for all patients. Bolded stimulation parameters in Table 1 refer to parameters used during the unilateral stimulation condition. All patients had undergone simultaneous bilateral STN DBS at least 12 months prior to data collection. Surgical procedures for DBS implantation have been reported in detail previously (Starr et al., 1998a, b). The dorsal–ventral, anterior and lateral borders of the STN were identified electrophysiologically during DBS surgery. The DBS lead was placed such that contact 2 was at the dorsal border of the STN. Placement of electrodes was verified using Cicerone visualization software described previously (Miocinovic et al., 2007). Stimulation parameters for DBS devices were clinically determined using the methods described by Moro and colleagues (2006) and were stable for at least 6 months prior to study participation. Because participants needed to make verbal responses, patients with dysarthria or speech impairments were excluded from the study. Each patient was compensated $100 for his/her time. Participants signed an informed consent approved by the Georgia Institute of Technology and Cleveland Clinic Institutional Review Boards prior to study enrolment. Four patients were implanted at Emory University in Atlanta and four patients were implanted at the Cleveland Clinic.
Table 1.
Patient demographics and UPDRS Part III motor scores
| Patient | Gender | Age (years) | DBS implant duration (months) | Left stimulation parameters | Right stimulation parameters | UPDRS total | Unilateral stimulation |
|
|---|---|---|---|---|---|---|---|---|
| Off/Uni/Bi-DBS (%) | Contralateral improvement (%) | Ipsilateral improvement (%) | ||||||
| 1 | M | 70 | 16 | 2+/185 Hz/60 μs/3.2 V | 2+/185 Hz/60 μs/3.0 V | 45/31/26 | 58 | 21 |
| (31/42) | ||||||||
| 2 | M | 59 | 12 | 2+/135 Hz/90 μs/2.0 V | 2+/135 Hz/90 μs/2.2 V | 52/34/24 | 55 | 19 |
| (35/54) | ||||||||
| 3 | F | 48 | 14 | 3 + 2−/135 Hz/60 μs/3.2 V | 3 + 2−/135 Hz/60 μs/2.8 V | 56/39/32 | 47 | 15 |
| (30/43) | ||||||||
| 4 | M | 49 | 12 | 2 − 3+/135 Hz/60 μs/3.2 V | 2+/135 Hz/60 μs/2.8 V | 63/42/36 | 50 | 20 |
| (33/43) | ||||||||
| 5 | F | 56 | 13 | 2+/180 Hz/60 μs/3.2 V | 2+/180 Hz/60 μs/2.8 V | 62/41/37 | 51 | 16 |
| (34/40) | ||||||||
| 6 | F | 59 | 22 | 2+/135 Hz/60 μs/3.5 V | 2+/135 Hz/60 μs/3.0 V | 59/35/26 | 52 | 22 |
| (41/56) | ||||||||
| 7 | M | 54 | 14 | 2+/185 Hz/90 μs/2.5 V | 2+/135 Hz/90 μs/2.5 V | 56/41/31 | 48 | 17 |
| (27/45) | ||||||||
| 8 | M | 57 | 12 | 2+/185 Hz/90 μs/3.1 V | 2+/135 Hz/90 μs/3.0 V | 60/39/32 | 43 | 21 |
| (35/47) | ||||||||
| Mean (SD) | 56.5 (6.8) | 14.4 (3.4) | (33/46) | 50.5 (4.7) | 18.9 (2.6) | |||
Bold parameters were used during unilateral stimulation.
Apparatus
A 6 df force-torque transducer (Mini-40 Model, ATI Industrial Automation, Garner, NC, USA) was used to measure normal grip force (Fz) during a force-tracking motor task. Grip force was measured with a resolution of 0.06 N at a sampling rate of 256 Hz. A customized LabView program was used to collect and display the force data to the participant.
The n-Back task
A number of variations of the n-back task have been utilized in previous studies (Owen et al., 2005). The n-back task utilized in the current study was based on the methods originally used in its development; this version requires the participant to repeat the n-th item back (e.g. 0-, 1-, 2-back) in a sequentially presented list of items (Dobbs and Rule, 1989). Task difficulty is manipulated by requiring the participants to remember items further back in the list. In the current study, the number of intervening letters varied from zero to two. The letters were presented at a rate of one item per ∼1.5 s. Using this method of n-back testing requires encoding, maintenance, updating and output, however, is does not require comparison or decision making like other versions of the task. Two English-speaking experimenters conducted the n-back task. Experimenter 1 read aloud the randomized letter sets of the n-back task while experimenter 2 monitored the participant's responses for accuracy. Participants were asked to respond by articulating the letter presented directly before (0-back), 1 cycle before (1-back) or 2 cycles before (2-back). If the participant made an error or failed to respond within ∼1.5 s, experimenter 2 said ‘start over’; and experimenter 1 began a new set of letters. Approximately 19–23 trials (letters) were presented during a 30 s block. After performing the n-back task for 30 s participants rested for 15–45 s and then repeated the n-back task under the same level of difficulty (0, 1- or 2-back). Participants performed five 30 s blocks at each n-back condition (0, 1- and 2-back). These five blocks were collected sequentially and were randomized across participants. To control for practice effects, all participants completed three practice blocks (30 s each) at each n-back difficulty level prior to data collection. All participants reported task comprehension and demonstrated stable performance between the second and third familiarization blocks. All practice and test blocks consisted of a unique list of randomized letters to prevent any memorization of letters.
Force-maintenance task
Participants used a precision grip (thumb and index finger only) to exert an isometric force against the force transducer. The transducer was oriented in a comfortable position relative to the patient and then affixed to the table to prevent any movement. Three maximum precision grip efforts, 5 s each, were collected with the most affected hand as determined through clinical evaluation and the patient's self-report. For all patients, the self-reported more affected limb was also rated as the more affected side during the clinical examination. Participants were given at least 2 min rest between maximum efforts. The greatest force achieved from the three efforts was considered the maximum and was used in calculating a 20% target force level. The 20% target force level was selected as Galganski and colleagues (1993) found no differences in younger adults’ and older adults’ SD at this force level and based on our previous studies with younger and older adults, this force level could be maintained relatively easily with minimal fatigue (Voelcker-Rehage and Alberts, 2006, 2007). The target force level and actual grip force produced by the most affected hand of the patient were displayed on a 21 in. LCD monitor located ∼18–24 in. (44–59 cm) directly in front of the participants. Participants were instructed to match their grip force to the target force line as accurately as possible. An auditory stimulus ‘ready, go’ signalled the participants to start matching their force to the target force. Participants performed one to five practice repetitions prior to test blocks to be certain all task requirements were understood. Ten force-maintenance blocks for each limb, 30 s each, were performed with at least 30 s of rest between each block.
Dual task: n-back and force maintenance simultaneously
Participants performed 15 dual-task blocks in which they were asked to simultaneously perform the n-back task and force-maintenance task. The force-maintenance task was performed in random combination with each of the three n-back conditions (0-, 1-, 2-back; five repetitions each). Participants were instructed to perform both tasks as accurately as possible and to devote half of their attention to the cognitive task and half of their attention to the motor task. Participants were given at least 30 s of rest between each block.
Procedure
All data were collected during two visits to the Neural Control Laboratory at Georgia Tech or the Cleveland Clinic. Individual patient experimental sessions were separated by not more than 7 days. All patients reported to the laboratory in the clinically defined off phase in terms of anti-parkinsonian medication (e.g. at least 12 h since last medication) and On DBS (both stimulators On). After completing the informed consent process, patients were evaluated clinically using the UPDRS Part III Motor Exam administered by an experienced movement disorders neurologist. The neurologist was blinded as to DBS status. The order of testing for Day 1 during bilateral STN DBS was: (i) clinical evaluation; (ii) force-maintenance task; (iii) n-back testing (three levels of difficulty) and (iv) dual-task conditions with three levels of difficulty. The order of the dual-task conditions (i.e. force maintenance with 0-, 1- or 2-back) was randomized for each patient. Upon completing the initial bilateral DBS testing session, patients were randomized to either one or both of the DBS systems to be turned Off. During unilateral DBS the stimulator contralateral to the most affected side, determined clinically and through patient self-report, remained On while the stimulator ipsilateral to the most affected side was turned Off. Therefore, the unilateral stimulation condition replicated the scenario in which the patient would undergo if unilateral DBS were to be performed i.e. the most affected side was treated (Slowinski et al., 2007). After turning one or both stimulators Off, the patient rested in the laboratory for 3 h to minimize any residual stimulation effects (Temperli et al., 2003; Alberts et al., 2004). Following the 3 h rest period clinical, cognitive and biomechanical testing were repeated. Upon completion of this testing session the patient's stimulator(s) was/were turned On and patients took their anti-parkinsonian medication. Total time spent in the laboratory was ∼5–6 h (testing time ∼2 h and 3 h rest during the washout period). Following each single- and dual-task condition, patients rated their level of mental and physical fatigue on a scale of 1–10; 1 = no fatigue and 10 = exhausted.
Within 7 days of the initial experimental session patients returned to the laboratory to complete the second data collection session. Identical to the initial visit, patients arrived Off anti-parkinsonian medication and On bilateral stimulation. To account for potential practice effects on the second day of testing, the patients repeated the bilateral On DBS-testing protocol that was performed on Day 1 (e.g. clinical evaluation, n-back testing, force-maintenance testing and dual-task conditions). Statistical analysis of bilateral On DBS indicated that cognitive and motor performance did not differ from Day 1 and Day 2 experimental sessions. Therefore, cognitive and motor performance was stable across experimental sessions. Because performance was stable across the experimental sessions data were collapsed across days for the bilateral On DBS condition. On Day 1, the order of testing for four patients was: bilateral DBS followed by unilateral DBS, Day 2 order of testing was bilateral DBS followed by Off DBS. For the remaining four patients, after bilateral STN DBS, the order was reversed.
Data analysis
Force tracking
All force data were filtered with a phase-symmetric low-pass filter using Woltring's algorithm (detailed in previous studies, Voelcker-Rehage et al., 2006; Voelcker-Rehage and Alberts, 2007) using existing Matlab analysis programs developed in our laboratory. Force data were assessed for accuracy from 3 s after the start of the block until completion of the block; this period allowed the patient sufficient time to achieve the target force. The primary motor outcome variables for the force-tracking task were time within the target range (TWR) and relative root mean square error (RRMSE). The TWR is calculated by determining the time the patient's force trace is within ±2.5% of the target line. The TWR provides an overall accuracy measure of force tracking. To account for differences in the amplitude of the target force (e.g. inter- and intra-patient variability due to stimulation status), the RRMSE, as defined in equation (1), was used as a method of normalizing performance relative to force amplitude. The RRMSE is considered to reflect the overall variability of force-tracking performance; a lower RRMSE suggests control of distal musculature and hand functionality (Kriz et al., 1995; Kurillo et al., 2004). In equation (1), FT(t) is the target force provided to the patient, F0(t) is the force produced by the patient and T is the time of the block.
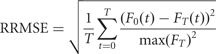 |
(1) |
The n-Back performance
The n-back performance was measured by determining the percentage of correct letters recalled during a 30 s block and the total number of errors (NE) committed during a block (Voelcker-Rehage et al., 2006).
Dual-task analysis
To examine participants’ performance under the dual-task conditions, the dual-task loss (DTL) was computed using a standard measure to compare performance on single- and dual-task conditions (Lindenberger et al., 2000). The DTLs were computed as the percentage of loss in motor and cognitive performance during dual-task conditions relative to performance in the single-task conditions in the following manner:
DTLforce = [(mean dual taskforce – mean baselineforce)/mean baselineforce] × 100.
DTLn-back = [(mean dual taskn-back – mean baselinen-back)/mean baselinen-back] × 100.
Statistical analysis
Motor (RRMSE, TWR) and cognitive [percentage of correctly repeated letters (PRL), NE] performance data were analysed with repeated measures ANOVAs. Greenhouse Geyser adjustment was reported when the sphericity assumption was violated. Post hoc contrasts (Bonferroni adjustment) were used to determine differences between the DBS status and level of task difficulty to determine the conditions that were most affected by the different states of DBS. Analyses were conducted separately for the motor and cognitive task.
Two 3 (stimulation: Off DBS, unilateral DBS, bilateral DBS) × 3 (task difficulty: 0-, 1-, 2-back) × 2 (context: single task, dual task) repeated measure ANOVAs were used to determine differences between different states of DBS in n-back difficulty and between single- and dual-task context using PRL and NE. Additionally, two 3 (stimulation) × 4 (task difficulty: force only, force at 0-, 1- and 2-back difficulty) repeated measure ANOVAs were carried out using the RRMSE and TWR scores.
To examine whether DTLs for the force task and the n-back difficulties were significantly different from zero, a series of one-sample t-tests (test value = 0) were conducted separately for each DBS condition. Repeated measures ANOVAS with corresponding post hoc tests were used to compare the DTLs for task difficulties (0-, 1-, 2-back) and DBS status.
Results
Clinical ratings
Table 1 contains total and symptom related UPDRS motor scores for each patient during Off, unilateral and bilateral DBS. While there was a tendency for patients to rate their level of cognitive and motor fatigue levels slightly higher (i.e. more fatigue) as the testing session progressed, no statistically significant differences were present between stimulation conditions. In terms of clinical motor function, the UPDRS-III scores decreased significantly as a result of unilateral and bilateral DBS. Unilateral DBS resulted in a 33% improvement in clinical rating while bilateral DBS improved ratings by 46% compared with Off DBS. Statistical analysis (t-tests for paired samples) revealed that the additional 13% improvement in UPDRS motor score with bilateral DBS was statistically better than unilateral DBS [t(7) = 9.11, P < 0.01]. Both bilateral and unilateral were significantly better than Off DBS [tbi-off(7) = 18.40, P < 0.01; tuni-off(7) = 15.52, P < 0.01].
Cognitive functioning and DBS during single- and dual-task conditions
Percentage of correct letters
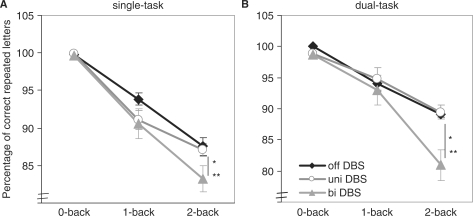
The results from the repeated measures ANOVA (cf. Fig. 1) revealed that overall n-back performance decreased with increasing task difficulty [F(1.12,7.85) = 192.97, P < 0.01, η2 = 0.96]. The main effect of DBS status was significant [F(2,14) = 18.52, P < 0.01, η2 = 0.73]. Post hoc contrasts revealed a significantly lower n-back performance for bilateral DBS as compared with Off and unilateral DBS (always P < 0.01). Importantly, also the task difficulty × stimulation interaction was significant [F(4,14) = 13.96, P = 0.01, η2 = 0.67], resulting from a greater performance decrease with increasing n-back difficulty for bilateral DBS than for the Off and unilateral DBS. In addition, 2-back performance at bilateral DBS was significantly lower than performance at unilateral DBS in single- and dual-task context. Thus, in the most complex condition (2-back at dual task) bilateral DBS led to significantly lower performances compared with unilateral DBS or the Off state (cf. Fig. 1).
Fig. 1.
(A) Results of the n-back task in the single-task condition at Off DBS, unilateral DBS and bilateral DBS (means and standard errors). (B) Results of the n-back task in the dual-task condition at Off DBS, unilateral DBS and bilateral DBS (means and standard errors). A cross marks a significant differences between Off and unilateral DBS, an asterisk marks a significant difference between Off and bilateral DBS, and a double asterisk marks a significant difference between unilateral and bilateral DBS.
Number of errors
Errors in cognitive function were primarily due to responding with the incorrect letter and the participant reporting to experimenter 1 that they did not remember the letter to be recalled. Less than 0.5% of the errors were the result of the patient not responding within the ∼1.5 s time period. Bilateral DBS led to a higher amount of errors as compared with Off DBS (for the 1- and 2-back task) and as compared with unilateral DBS (2-back only) in the single-task context. For the NE the effect of task difficulty [F(2,14) = 178.18, P < 0.01, η2 = 0.96] and stimulation [F(1.06,7.43) = 11.95, P < 0.01, η2 = 0.63] was significant, whereas the context effect [F(1,14) = 1.70, P = 0.23] was not. Participants produced more errors as the difficulty of the n-back task increased, but not as the context changed from single- to dual-task. The NE, however, significantly differed between the DBS states. Bilateral DBS resulted in the greatest amount of errors (P = 0.01).
Motor function and DBS during single- and dual-task conditions
Relative root mean square error
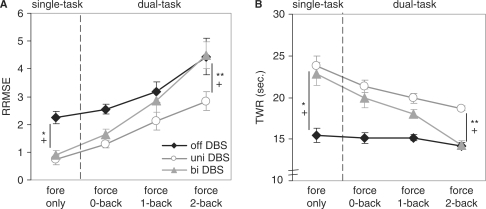
Variability in force tracking increased significantly as task difficulty increased [F(3,42) = 30.94, P < 0.01, η2 = 0.82]. Additionally, the force variability differed between the three DBS conditions [F(2,42) = 44.62, P < 0.01, η2 = 0.86], with unilateral DBS showing the highest force-tracking performance and with Off DBS showing the lowest performance (always P < 0.03). The deterioration in force-maintenance performance across the task difficulties, however, was different between the DBS states [F(6,42) = 3.54, P = 0.01, η2 = 0.34] (cf. Fig. 2A). As shown in Fig. 2A bilateral DBS revealed a tremendous performance decrease across the task conditions. Whereas in the force only condition bilateral DBS showed performance levels comparable with unilateral DBS [Off DBS was significantly worse than unilateral and bilateral DBS (P < 0.01)], in the 2-back condition bilateral DBS performance level was comparable with the Off DBS state and significantly worse than unilateral DBS (P < 0.05) (cf. Fig. 2A).
Fig. 2.
(A) Results of the RRMSE force in the single- and dual-task conditions at Off DBS, unilateral DBS and bilateral DBS (means and standard errors). (B) Results of the TWR force in the single- and dual-task conditions at Off DBS, unilateral DBS and bilateral DBS (means and standard errors). A plus marks a significant differences between Off and unilateral DBS, an asterisk marks a significant difference between Off and bilateral DBS and a double asterisk marks a significant difference between unilateral and bilateral DBS.
Time within target range
Results from the TWR confirmed results of RRMS. During the Off DBS condition, performance was essentially the same across all task condition (e.g. fairly low accuracy for all conditions), performance during bilateral stimulation decreased dramatically across all task difficulties and was similar to performance levels of Off DBS during the most difficult condition, 2-back.
DTLs different from zero
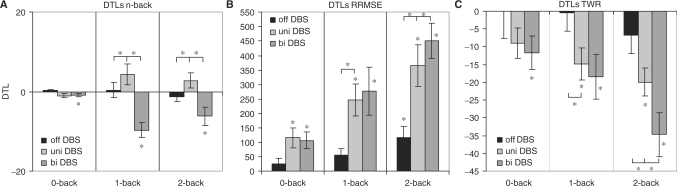
Bilateral stimulation resulted in a significant decline in cognitive function when moving from single- to dual-task conditions, while no change in cognitive performance was present for Off or unilateral DBS during this same transition from single- to dual-task conditions. Results from the one-sample t-tests indicated that Parkinson's disease patients showed no significant DTLs for n-back task performance during Off DBS [t0-back(7) = 1.53, P = 0.17; t1-back(7) = 0.19, P = 0.86; t2-back(7) = −1.04, P = 0.33] or with unilateral DBS [t0-back(7) = −2.27, P = 0.06; t1-back(7) = 1.66, P = 0.14; t2-back(7) = 1.46, P = 0.19], however, during bilateral DBS [t0-back(7) = −2.55, P = 0.04; t1-back(7) = −4.99, P < 0.01; t2-back(7) = −2.73, P = 0.03] significant cognitive DTLs were present (cf. Fig. 3A); the DTLs were not different between the 1- and 2-back conditions [t(7) = −1.56, P = 0.16].
Fig. 3.
DTLs and standard errors for (A) the n-back task, (B) the force-maintenance task (RRMSE) and (C) the force maintenance task (TWR) task at Off DBS, unilateral DBS and bilateral DBS. An asterisk marks DTLs significantly greater than zero and significant differences between the states of stimulation (*P < 0.05). Positive DTLs for force TWR and n-back indicate gains in dual-task performance, negative DTLs for force TWR and n-back indicate DTLs. Positive values for force RRMSE indicate DTLs, negative values indicate dual-task gains.
As expected, force-tracking performance did decline as task complexity increased from single- to dual-task conditions while Off DBS and with unilateral and bilateral stimulation conditions. However, the greatest declines in motor performance were associated with bilateral DBS. The DTLs in force-tracking performance (RRMSE) at Off DBS were significant for the 2-back condition [t0-back(7) = 1.29, P = 0.24; t1-back(7) = 2.17, P = 0.17; t2-back(7) = 2.89, P = 0.02]. Results under unilateral and bilateral DBS revealed significant DTLs in force-maintenance performance for all three n-back difficulties [unilateral DBS: t0-back(7) = 3.30, P = 0.01; t1-back(7) = −4.42, P < 0.01; t2-back(7) = 5.01, P < 0.01; bilateral DBS: t0-back(7) = 3.54, P = 0.01; t1-back(7) = 3.33, P = 0.01; t2-back(7) = 7.42, P < 0.01] (cf. Fig. 3B). Also for TWR, the greatest performance decrements occurred during the 2-back dual-task condition and the smallest with the 0-back difficulty (cf. Fig. 3C).
Task difficulty and stimulation differences in DTLs
DTLsn-back, did not significantly change with increasing task difficulty [F(2,14) = 0.50, P = 0.58], but with the type of stimulation [F(2,14) = 17.47, P < 0.01, η2 = 0.71]. DTLs were significantly higher with bilateral DBS as compared with Off and unilateral DBS (P < 0.03). The task difficulty × stimulation interaction was also significant [F(4,28) = 7.14, P < 0.01, η2 = 0.51] (cf. Fig. 3A).
DTLsforce (RRMSE) displayed a significant main effect of task difficulty [F(2,14) = 26.10, P < 0.01, η2 = 0.79], DTLs in force-maintenance performance were smallest for the 0-back condition, and highest for the 2-back condition, a significant main effect of stimulation [F(1.17,8.22) = 12.14, P < 0.01, η2 = 0.63], and a significant task difficulty × stimulation interaction [F(4,28) = 8.71, P < 0.01, η2 = 0.55]. Differences between DBS states were significant in the 1-back condition (significantly lower DTLs while Off DBS than unilateral DBS; P = 0.02) and the 2-back condition (always P < 0.05), with highest DTLs at bilateral DBS. Results of the TWR confirmed results of RRMSE (cf. Fig. 3B and C).
Discussion
The aim of this study was to determine the effects of unilateral and bilateral STN DBS on cognitive and motor function under dual-task conditions. The use of a dual-task paradigm with varying levels of cognitive difficulty better replicates the performance of activities of daily living (ADLs), which typically consist of cognitive and motor components performed concurrently. Under relatively simple dual-task conditions there were no differences in cognitive or motor performance between unilateral and bilateral stimulation. However, as dual-task complexity increased, cognitive and motor performance was significantly worse with bilateral compared with unilateral stimulation. In fact, in the most complex dual-task condition (i.e. 2-back + force maintenance), bilateral stimulation resulted in a level of motor performance that was similar to no stimulation at all. This pattern of results was present in all eight patients studied. Overall, these data suggest that under modestly complex cognitive–motor task conditions, unilateral DBS was associated with better cognitive and motor performance than bilateral DBS.
DBS and working memory during single-task conditions
Bilateral STN DBS led to a significant decline in working memory under the most difficult n-back condition compared with unilateral and Off DBS conditions. These data are consistent with a previous study indicating that bilateral STN DBS results in decreased memory performance when memory load demands were highest (Hershey et al., 2004). At first glance, the current data are in conflict with two studies that have shown STN stimulation improves working memory (Pillon et al., 2000; Rivaud-Pechoux et al., 2000). However, an important difference between the studies showing a decline in working memory and those showing improvements is the level of task difficulty. Impairments in working memory during the most demanding n-back condition (2-back) provides support for the hypothesis that bilateral STN DBS interferes with patients’ ability to handle higher demands placed on cognitive control processes (Hershey et al., 2004). The fact that unilateral STN DBS did not lead to the same level of decline in working memory may indicate that the non-stimulated basal ganglia may be able to compensate for the disruption of activity caused by stimulation. Alternatively, disruption from activation of an adjacent white matter tract may not be enough to disrupt function during unilateral stimulation. Based on recent data suggesting that DBS contralateral to the more affected side of the body may result in greater impairments in spatial working memory compared with stimulation contralateral to the less affected side (Hershey et al., 2007), additional studies are necessary to determine if the same pattern of results occurs under dual-task conditions.
DBS and motor function during single-task conditions
Unilateral and bilateral DBS resulted in a significant improvement in clinical ratings and in force-maintenance performance compared with Off DBS. The mean improvement in UPDRS Part III motor scores was 33% and 46% for unilateral and bilateral DBS, respectively. This level of improvement in clinical ratings associated with unilateral STN DBS is consistent with our earlier findings (Alberts et al., 2004) and more recent data (Chung et al., 2006; Slowinski et al., 2007). The contralateral improvement (51%) in motor symptoms was greater than the ipsilateral (19%) improvement under unilateral stimulation. The degree of improvement in ipsilateral clinical ratings is within the range recently reported (Chung et al., 2006; Slowinski et al., 2007). Force-maintenance performance was significantly improved during unilateral and bilateral stimulation compared with Off DBS. Few data are available in which unilateral and bilateral DBS have been compared in terms of upper extremity function (Bastian et al., 2003). During fast reaching movements Bastian and colleagues (2003) found there was no additive effect of bilateral STN DBS compared with unilateral. The current data are consistent with these findings as force-tracking performance for the most affected limb was similar under unilateral and bilateral stimulation. The lack of an additive effect of bilateral stimulation on discrete unimanual upper extremity task performance suggests that contralateral basal ganglia thalamocortical pathways are largely responsible for mediating contralateral upper extremity motor performance or whatever ipsilateral pathway is required to obtain maximal benefit is also fully activated during contralateral stimulation.
DBS and dual-task performance
In general, cognitive and motor performance declined as task complexity increased across stimulation conditions. These results were expected as previous studies indicate that even in healthy older adults declines in cognitive and motor function occur when moving from single- to dual-task conditions (Chen et al., 1996; Lindenberger et al., 2000; Morris et al., 2000; Marchese et al., 2003; Rochester et al., 2004; Voelcker-Rehage et al., 2006). The overall performance decrements during bilateral DBS with increasing dual-task difficulty are comparable to the pattern of performance decrements observed in older adults. Voelcker-Rehage et al. (2006) showed in a study with older adults that motor and cognitive performance decrease with increasing task difficulty under dual-task conditions. However, the relative loss in cognitive and motor performance when moving from single- to dual-task conditions was greater during bilateral DBS than the DTL older adults experienced. It should be noted that only one of the patients in the current study would be considered an ‘older adult’; seven of eight patients were under 60 years of age and yet their loss in cognitive functioning with bilateral STN DBS was greater under dual-task conditions. Also, healthy young adults exhibit no decrease in cognitive or motor performance during dual-task conditions, neither in the cognitive or motor domain.
Motor performance did not change dramatically under dual-task conditions while Off DBS, until the most difficult condition. Relatively stable motor performance while Off DBS is likely a reflection of a floor effect as patients’ performance was relatively poor under single-task conditions and there was little room for further decline. Motor performance during dual-task conditions was significantly worse compared with single-task conditions under both unilateral and bilateral DBS. The overall accuracy of force tracking (TWR) declined while the variability of force output (RRMSE) increased as task complexity increased. During the 0-back dual-task condition only a slight decline in motor performance was displayed by patients. However, as the cognitive demands increased, the difference between unilateral and bilateral stimulation on motor performance became apparent. During the 1-back dual-task condition, motor performance under bilateral stimulation was only slightly better than no stimulation. During the most difficult condition, 2-back dual task, overall motor performance, TWR and RRMSE, was significantly worse than unilateral DBS. In fact, during the 2-back dual-task condition with bilateral stimulation motor performance was nearly identical to performance levels while Off stimulation. A decline in motor performance with bilateral DBS under dual-task conditions, in particular during the 2-back condition, may have been an imbalance in the level of attention allocated for the performance of the cognitive task (e.g. greater emphasis placed on n-back compared with force task despite experimental instructions and response to follow-up questions regarding attention allocation across the two tasks). The n-back is known to place demands on executive processes as information must be processed, requires online monitoring and manipulation of remembered information; therefore, it is assumed to place great demands on key processes within working memory (McElree, 2001; Blokland et al., 2008). Previous imaging studies have shown that the frontal cortex, in particular, the dorsolateral prefrontal cortex is activated during a working memory task such as the n-back task (Jansma et al., 2000; Owen et al., 2005). Disruption of information processing in the non-motor regions of STN and adjacent areas that project to the frontal cortex may be responsible for the varying levels of decline reported in cognitive functioning during STN DBS.
Recent data collected using a rat model of Parkinson's disease, indicate that bilateral stimulation of the STN leads to an inhibition of 5-hydroxytrptamine neurons, which resulted in depression related behavioural changes (Temel et al., 2007). Given its small size, stimulation within the STN, even with leads located within the sensorimotor territory, can result in spread of current to limbic and associative areas as well as to surrounding structures and fibre systems that may also affect cognition. Until recently, the pattern of current spread and its effects on neuronal activity on target nuclei associated with STN DBS was not well characterized. Theoretical (McIntyre et al., 2004a, b) and experimental data (Hashimoto et al., 2003), however, suggest that the therapeutic mechanisms of DBS may work by activating axons surrounding the electrode. This axonal activation is non-discriminately applied to fibres leaving, passing through or adjacent to the stimulated nucleus. Activation of these fibre systems is proposed to produce a regularization of neural activity patterns in the pallidum during STN DBS (Montgomery and Baker, 2000; Vitek, 2002b; Hashimoto et al., 2003; Grill et al., 2004; Rubin and Terman, 2004). These stimulation effects are subsequently transmitted throughout the basal ganglia and thalamocortical networks, modulating neural activity throughout the brain (Fukuda et al., 2001; Hershey et al., 2003; Phillips et al., 2006). Recently, finite element modelling of tissue and DBS current was used to determine the volume of tissue activated (VTA) during therapeutic and non-therapeutic stimulation parameters with STN DBS (Butson et al., 2007). With therapeutically effective DBS parameters, a significant portion of the VTA with stimulation was outside the STN and spread to the zona incerta and Fields of Forel. This article did not further parse the STN into motor and non-motor regions and calculate that amount of VTA within each region so it is unknown what segments of STN were being stimulated. These data do, however, support the hypothesis that non-motor pathways are likely activated during STN DBS and may contribute to the present observations.
The transmission of pathological information within the basal ganglia thalamocortical circuits is hypothesized to underlie the symptoms of Parkinson's disease (Albin et al., 1989; DeLong, 1990; Llinas et al., 1999; Vitek and Giroux, 2000; Timmermann et al., 2003). Analysis of globus pallidus internus (GPi) neuronal activity during STN DBS in parkinsonian primates has led to the hypothesis that DBS masks this intrinsic activity by replacing the pathological activity associated with Parkinson's disease with a more regular pattern of activity in the GPi. This regularized activity is then transmitted throughout the motor circuit producing an ‘informational lesion’ and prevents the passage of pathological information from the pallidum (Hashimoto et al., 2003; Grill et al., 2004). While DBS may prevent the transmission of pathological information within the motor circuit, current spread to non-motor regions of STN is likely to create the same type of informational lesion, thus disrupting potentially non-pathological information processing. Such a ‘lesion’ may not produce a detectable deficit in cognitive function following unilateral procedures or even with bilateral DBS when the patients are able to focus their attention on the performance of a cognitive or motor task; as it is the case during most clinical examinations. However, as the cognitive demands of the task increase and information-processing demands increase, function of redundant non-motor circuits may be compromised and during bilateral stimulation the redundancy of non-motor circuits may be decreased. Thus, patients are unable to draw on cognitive resources as they are now compromised as a result of bilateral disruption of non-motor circuits.
Importance of cognitive–motor assessment
At first glance, the current data indicating a decline in cognitive–motor performance associated with bilateral STN DBS under dual-task conditions appears paradoxical in light of reported improvements from other studies in clinical measures of quality of life and performance of ADLs following STN DBS. It is acknowledged that the vast majority of studies indicate bilateral STN DBS results in significant improvements in the patient's perception of their quality of life (Drapier et al., 2005; Erola et al., 2005; Lyons and Pahwa, 2005; Fraix et al., 2006; Gronchi-Perrin et al., 2006; Kleiner-Fisman et al., 2006; Siderowf et al., 2006; Martinez-Martin and Deuschl, 2007). However, an analysis of the change in individual subscales of the PDQ-39 indicated that only physical aspects of quality of life improved significantly following bilateral STN DBS (Drapier et al., 2005), while the subscales characterizing emotional well-being, social support and cognition improved slightly (but not significantly) and communication actually worsened after bilateral STN DBS. Although the PDQ-39 has been validated (Peto et al., 1995), it has been shown to be susceptible to placebo effects, especially in the context of a surgical intervention (McRae et al., 2004). More recently, preliminary data suggest patients’ perceptions of their day-to-day function to be improved subtly, however, caregivers perceived the patients’ as exhibiting subtle declines in day-to-day functioning (Duff-Canning et al., 2008). Collectively, these studies indicate that the PDQ-39 is limited and may not adequately capture patients’ quality of life following DBS.
Despite the overall excellent motor and quality of life improvements using clinical measures, there is a contrast between the improvement in motor disability and the difficulties experienced by patients as they try to reintegrate into a more normal life (Agid et al., 2006). Schupbach and colleagues (2006) recently examined a group of relatively young Parkinson's disease patients, average age = 52, following bilateral STN DBS. Clinical measures of motor function and quality of life improved significantly, however, patients experienced difficulties in psychosocial function, personal relationships and functioning in a socio-professional environment. A striking finding from their study was the relatively large percentage of patients who did not return back to work following DBS. Of the 29 patients studied, 16 were working prior to DBS. However, after DBS surgery, in spite of improvements on clinical measures of motor function and quality of life, 7 of those 16 patients (44%) did not return to work following DBS. It was noted that after surgery, ‘patients experienced slight and subtle intellectual symptoms that became apparent in the course of repeated and thorough unstructured interviews’, (p. 1814) (Schupbach et al., 2006). For those not returning to the workplace, they reported, ‘I don't have the same ability to concentrate as before’, and they had difficulty ‘ordering complex actions and thoughts, anticipating and planning ahead’ and ‘limited attention, and working memory’ and were ‘easily distracted’ (Schupbach et al., 2006). These features of cognitive function are not assessed in the self-report measures of the PDQ-39. Rather, the PDQ-39, for the most part, asks patients to rate their performance on a discrete motor task over the past month, the context in which this task is performed is, in general, not taken into consideration during the administration of this exam. We propose that these conditions do not adequately reflect the context in which most ADLs are performed (i.e. concurrent performance of a cognitive and motor task). Better replicating the context in which ADLs are performed during the programming and adjustment of stimulation parameters is necessary to further improve cognitive and motor outcomes of those patients currently undergoing DBS surgery. This may be particularly relevant in the near future if it is demonstrated that DBS is capable of altering the course of Parkinson's disease (Schupbach et al., 2007; Wallace et al., 2007). An earlier application of DBS would likely result in a younger group of patients being implanted; patients who are still active in the workforce. For these patients in particular, the detection and prevention of subtle cognitive and cognitive–motor declines during DBS programming may allow them to maintain their level of performance and remain in the workforce longer. Thereby, maintaining their quality of life and decreasing non-treatment related costs (i.e. lost worker productivity) associated with Parkinson's disease and DBS for Parkinson's disease.
One limitation of this study is that we only tested patients who had undergone STN DBS. Though the STN is the most commonly targeted site for treatment of advanced Parkinson's disease, we do not know if these same effects are present with bilateral GPi DBS. Fewer reports of cognitive dysfunction have been reported with GPi DBS, however, this may be a function of the relatively smaller number of GPi cases rather than the effect of stimulation site (Vitek, 2002a). The patients’ level of attention to the cognitive and motor task under dual-task conditions may also have influenced the results. While they were instructed to focus attention on both tasks equally, it is possible they attended more to the cognitive or motor task. When asked about the focusing of attention after the experiment, all patients responded that they tried to weigh each task equally. Learning and fatigue certainly impact cognitive and motor performance. However, we were careful to provide adequate rest breaks between blocks, thus minimizing the effects of fatigue. Furthermore, fatigue is not likely to have affected patients’ performance during bilateral DBS conditions as they were always tested in this condition before the unilateral or Off DBS conditions. The lingering effects of medication on cognitive and motor performance between the stimulation conditions are unknown, since patients were first tested while On bilateral DBS. However, these potential beneficial effects would be expected to lead to a greater retention of motor benefits during bilateral DBS, thus potentially decreasing the effects of the dual-task on cognitive and motor performance. This was not the case as cognitive–motor performance was, in general, better during unilateral compared with bilateral STN DBS, even though the patients were off medication longer during the unilateral DBS condition. In terms of learning effects, in general the n-back task is not subject to learning effects once the task requirements have been comprehended by the patient. Nevertheless, practice blocks were given at each level of task difficulty and all the patients reported understanding task requirements. In terms of motor learning impacting performance on the force-maintenance task, the task is relatively simple as the patient produces a constant force and is provided real-time feedback regarding their performance thus patients can learn the task relatively quickly. If motor learning or task experience were to impact force-maintenance performance, data collected later in the day under unilateral DBS would be expected to be better than with bilateral DBS. This was not the case as performance during force maintenance only blocks was similar during unilateral and bilateral DBS.
The fundamental goal of DBS is to alter pathological neural activity within the basal ganglia to provide maximum motor response with minimal side effects. The alteration of pathological motor information leads to improvements in motor function. However, the spread of DBS current to non-motor regions of STN or alteration of neuronal activity patterns from the sensorimotor portion of the STN could be responsible for emerging cognitive side effects, especially under bilateral DBS or during the performance of motor tasks under complex conditions. Current methods of assessing cognitive and motor function in a clinical environment may not be sufficiently demanding to reveal changes in cognitive performance that occur under dual-task conditions and can result in diminished motor function. We are currently investigating the effectiveness of using an abridged version of dual-task paradigm in the selection of DBS parameters in a clinical setting.
Funding
National Institute of Health (R03 AG022178 and NS037959).
Supplementary Material
Glossary
Abbreviations:
- DBS
deep brain stimulation
- DTL
dual-task loss
- GPi
globus pallidus internus
- RRMSE
relative root mean square error
- STN
subthalamic nucleus
- TWR
time within the target range
- VTA
volume of tissue activated
References
- Agid Y, Schupbach M, Gargiulo M, Mallet L, Houeto JL, Behar C, et al. Neurosurgery in Parkinson's disease: the doctor is happy, the patient less so? J Neural Transm Suppl. 2006;70:409–14. doi: 10.1007/978-3-211-45295-0_61. [DOI] [PubMed] [Google Scholar]
- Alberts JL, Elder CM, Okun MS, Vitek JL. Comparison of pallidal and subthalamic stimulation on force control in patient's with Parkinson's disease. Motor Control. 2004;8:484–99. doi: 10.1123/mcj.8.4.484. [DOI] [PubMed] [Google Scholar]
- Alberts JL, Okun MS, Vitek JL. Parkinsonism Relat Disord. Vol. 14. 2008. The persistent effects of unilateral pallidal and subthalamic deep brain stimulation on force control in advanced Parkinson's patients; pp. 481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Kelly VE, Revilla FJ, Perlmutter JS, Mink JW. Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson's disease. Mov Disord. 2003;18:1000–7. doi: 10.1002/mds.10493. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P. Deep brain stimulation of the subthalamic nucleus for Parkinson's disease: methodologic aspects and clinical criteria. Neurology. 2000;55:S40–4. [PubMed] [Google Scholar]
- Blokland GA, McMahon KL, Hoffman J, Zhu G, Meredith M, Martin NG, et al. Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: a twin fMRI study. Biol Psychol. 2008;79:70–9. doi: 10.1016/j.biopsycho.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–70. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-C, Schultz AB, Ashton-Miller JA, Giordani B, Alexander NB, Guire KE. Stepping over obstacles: dividing attention impairs performance of old more than young adults. J Gerontol. 1996;51A:M116–22. doi: 10.1093/gerona/51a.3.m116. [DOI] [PubMed] [Google Scholar]
- Chung SJ, Jeon SR, Kim SR, Lee MC. Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced parkinson's disease. Eur Neurol. 2006;56:127–32. doi: 10.1159/000095704. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–5. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychol Aging. 1989;4:500–3. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Drapier S, Raoul S, Drapier D, Leray E, Lallement F, Rivier I, et al. Only physical aspects of quality of life are significantly improved by bilateral subthalamic stimulation in Parkinson's disease. J Neurol. 2005;252:583–8. doi: 10.1007/s00415-005-0704-4. [DOI] [PubMed] [Google Scholar]
- Duff-Canning SJ, Poon YY, Chang T, Mailis N, Lozano AM, Hodaie M, et al. He said, she said: differences between self and caregiver ratings of postoperative behavioral changes in Parkinson's disease patients undergoing bilateral subthalamic nucleus deep brain stimulation. Vol. 23. Chicago, Illinois: Wiley-Blackwell; 2008. Twelfth international congress of Parkinson's disease and movement disorders; p. S127. [Google Scholar]
- Erola T, Karinen P, Heikkinen E, Tuominen J, Haapaniemi T, Koivukangas J, et al. Bilateral subthalamic nucleus stimulation improves health-related quality of life in Parkinsonian patients. Parkinsonism Relat Disord. 2005;11:89–94. doi: 10.1016/j.parkreldis.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Fraix V, Houeto JL, Lagrange C, Le Pen C, Krystkowiak P, Guehl D, et al. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2006;77:443–9. doi: 10.1136/jnnp.2005.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Mentis M, Ghilardi MF, Dhawan V, Antonini A, Hammerstad J, et al. Functional correlates of pallidal stimulation for Parkinson's disease. Ann Neurol. 2001;49:155–64. doi: 10.1002/1531-8249(20010201)49:2<155::aid-ana35>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol. 1993;69:2108–15. doi: 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]
- Germano I, Gracies JM, Weisz D, Tse W, Koller W, Olanow C. Unilateral stimulation of the subthalamic nucleur in Parkinson's disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- Gironell A, Kulisevsky J, Rami L, Fortuny N, Garcia-Sanchez C, Pascual-Sedano B. Effects of pallidotomy and bilateral subthalamic stimulation on cognitive function in Parkinson disease. A controlled comparative study. J Neurol. 2003;250:917–23. doi: 10.1007/s00415-003-1109-x. [DOI] [PubMed] [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–40. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- Gronchi-Perrin A, Viollier S, Ghika J, Combremont P, Villemure JG, Bogousslavsky J, et al. Does subthalamic nucleus deep brain stimulation really improve quality of life in Parkinson's disease? Mov Disord. 2006;21:1465–8. doi: 10.1002/mds.20943. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–23. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–4. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–21. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hershey T, Wu J, Weaver PM, Perantie DC, Karimi M, Tabbal SD, et al. Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Exp Neurol. 2007;210:402–8. doi: 10.1016/j.expneurol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Ardouin CM, Brown RG, Rothwell JC, Obeso J, Albanese A, et al. The impact of deep brain stimulation on executive function in Parkinson's disease. Brain. 2000;123:1142–54. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Coppola R, Kahn RS. Specific versus nonspecific brain activity in a parametric N-back task. Neuroimage. 2000;12:688–97. doi: 10.1006/nimg.2000.0645. [DOI] [PubMed] [Google Scholar]
- Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- Kriz G, Hermsdorfer J, Marquardt C, Mai N. Feedback-based training of grip force control in patients with brain damage. Arch Phys Med Rehabil. 1995;76:653–9. doi: 10.1016/s0003-9993(95)80635-0. [DOI] [PubMed] [Google Scholar]
- Kurillo G, Zupan A, Bajd T. Force tracking system for the assessment of grip force control in patients with neuromuscular diseases. Clin Biomech. 2004;19:1014–21. doi: 10.1016/j.clinbiomech.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–36. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra P. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–7. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KE, Pahwa R. Long-term benefits in quality of life provided by bilateral subthalamic stimulation in patients with Parkinson disease. J Neurosurg. 2005;103:252–5. doi: 10.3171/jns.2005.103.2.0252. [DOI] [PubMed] [Google Scholar]
- Marchese R, Bove M, Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson's disease: a posturographic study. Mov Disord. 2003;18:652–8. doi: 10.1002/mds.10418. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin P, Deuschl G. Effect of medical and surgical interventions on health-related quality of life in Parkinson's disease. Mov Disord. 2007;22:757–65. doi: 10.1002/mds.21407. [DOI] [PubMed] [Google Scholar]
- McElree B. Working memory and focal attention. J Exp Psychol Learn Mem Cogn. 2001;27:817–35. [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004a;91:1457–69. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004b;115:589–95. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- McRae C, Cherin E, Yamazaki TG, Diem G, Vo AH, Russell D, et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry. 2004;61:412–20. doi: 10.1001/archpsyc.61.4.412. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97:561–7. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Jr, Baker KB. Mechanisms of deep brain stimulation and future technical developments. Neurol Res. 2000;22:259–66. doi: 10.1080/01616412.2000.11740668. [DOI] [PubMed] [Google Scholar]
- Moro E, Poon YY, Lozano AM, Saint-Cyr JA, Lang AE. Subthalamic nucleus stimulation: improvements in outcome with reprogramming. Arch Neurol. 2006;63:1266–72. doi: 10.1001/archneur.63.9.1266. [DOI] [PubMed] [Google Scholar]
- Morris M, Iansek R, Smithson F, Huxham F. Postural instability in Parkinson's disease: a comparison with and without a concurrent task. Gait Posture. 2000;12:205–16. doi: 10.1016/s0966-6362(00)00076-x. [DOI] [PubMed] [Google Scholar]
- Morrison CE, Borod JC, Brin MF, Raskin S, Germano I, Weisz D, et al. A program for neuropsychological investigation of deep brain stimulation (PNIDBS) in movement disorder patients: development, feasibility, and preliminary data. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:204–19. [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurol. 2006;5:578–88. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res. 1995;4:241–8. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Baker KB, Lowe MJ, Tkach JA, Cooper SE, Kopell BH, et al. Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus – initial experience. Radiology. 2006;239:209–16. doi: 10.1148/radiol.2391041990. [DOI] [PubMed] [Google Scholar]
- Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, et al. Neuropsychological changes between ‘off’ and ‘on’ STN or GPi stimulation in Parkinson's disease. Neurology. 2000;55:411–8. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- Piper M, Abrams GM, Marks WJ., Jr Deep brain stimulation for the treatment of Parkinson's disease: overview and impact on gait and mobility. NeuroRehabilitation. 2005;20:223–32. [PubMed] [Google Scholar]
- Rivaud-Pechoux S, Vermersch AI, Gaymard B, Ploner CJ, Bejjani BP, Damier P, et al. Improvement of memory guided saccades in parkinsonian patients by high frequency subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry. 2000;68:381–4. doi: 10.1136/jnnp.68.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester L, Hetherington V, Jones D, Nieuwboer A, Willems AM, Kwakkel G, et al. Attending to the task: interference effects of functional tasks on walking in Parkinson's disease and the roles of cognition, depression, fatigue, and balance. Arch Phys Med Rehabil. 2004;85:1578–85. doi: 10.1016/j.apmr.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–9. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci. 2004;16:211–35. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson's disease. Brain. 2000;123:2091–108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- Schupbach M, Gargiulo M, Welter ML, Mallet L, Behar C, Houeto JL, et al. Neurosurgery in Parkinson disease: a distressed mind in a repaired body? Neurology. 2006;66:1811–6. doi: 10.1212/01.wnl.0000234880.51322.16. [DOI] [PubMed] [Google Scholar]
- Schupbach WM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM, et al. Stimulation of the subthalamic nucleus in Parkinson's disease: a 5 year follow up. J Neurol Neurosurg Psychiatry. 2005;76:1640–4. doi: 10.1136/jnnp.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach WM, Maltete D, Houeto JL, du Montcel ST, Mallet L, Welter ML, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–71. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- Siderowf A, Jaggi JL, Xie SX, Loveland-Jones C, Leng L, Hurtig H, et al. Long-term effects of bilateral subthalamic nucleus stimulation on health-related quality of life in advanced Parkinson's disease. Mov Disord. 2006;21:746–53. doi: 10.1002/mds.20786. [DOI] [PubMed] [Google Scholar]
- Skidmore FM, Rodriguez RL, Fernandez HH, Goodman WK, Foote KD, Okun MS. Lessons learned in deep brain stimulation for movement and neuropsychiatric disorders. CNS Spectr. 2006;11:521–36. doi: 10.1017/s1092852900013559. [DOI] [PubMed] [Google Scholar]
- Slowinski JL, Putzke JD, Uitti RJ, Lucas JA, Turk MF, Kall BA, et al. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2007;106:626–32. doi: 10.3171/jns.2007.106.4.626. [DOI] [PubMed] [Google Scholar]
- Starr PA, Vitek JL, Bakay RA. Ablative surgery and deep brain stimulation for Parkinson's disease. Neurosurgery. 1998a;43:989–1013. doi: 10.1097/00006123-199811000-00001. discussion 1013–5. [DOI] [PubMed] [Google Scholar]
- Starr PA, Vitek JL, Bakay RA. Deep brain stimulation for movement disorders. Neurosurg Clin N Am. 1998b;9:381–402. [PubMed] [Google Scholar]
- Tabbal SD, Ushe M, Mink JW, Revilla FJ, Wernle AR, Hong M, et al. Exp Neurol. Vol. 211. 2008. Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in parkinson disease; pp. 234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel Y, Blokland A, Ackermans L, Boon P, van Kranen-Mastenbroek VH, Beuls EA, et al. Differential effects of subthalamic nucleus stimulation in advanced Parkinson disease on reaction time performance. Exp Brain Res. 2006a;169:389–99. doi: 10.1007/s00221-005-0151-6. [DOI] [PubMed] [Google Scholar]
- Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Parkinsonism Relat Disord. Vol. 12. 2006b. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review; pp. 265–72. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- The Deep Brain Stimulation Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345:956–63. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Impaired navigation in drivers with Parkinson's disease. Brain. 2007;130:2433–40. doi: 10.1093/brain/awm178. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47:S131–40. [PubMed] [Google Scholar]
- Vitek JL. Deep brain stimulation for Parkinson's disease: a critical re-evaluation of STN versus GPi DBS. Stereotact Funct Neurosurg. 2002a;78:119–31. doi: 10.1159/000068959. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002b;17(Suppl 3):S69–72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Alberts JL. Age-related changes in grasping force modulation. Exp Brain Res. 2005;166:61–70. doi: 10.1007/s00221-005-2342-6. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Alberts JL. Effect of motor practice on dual-task performance in older adults. J Gerontol B Psychol Sci Soc Sci. 2007;62:P141–8. doi: 10.1093/geronb/62.3.p141. [DOI] [PubMed] [Google Scholar]
- Voelcker-Rehage C, Stronge AJ, Alberts JL. Age-related differences in working memory and force control under dual-task conditions. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:366–84. doi: 10.1080/138255890969339. [DOI] [PubMed] [Google Scholar]
- Voon V, Saint-Cyr J, Lozano AM, Moro E, Poon YY, Lang AE. Psychiatric symptoms in patients with Parkinson disease presenting for deep brain stimulation surgery. J Neurosurg. 2005;103:246–51. doi: 10.3171/jns.2005.103.2.0246. [DOI] [PubMed] [Google Scholar]
- Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129–45. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, et al. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol. 2004;61:697–700. doi: 10.1001/archneur.61.5.697. [DOI] [PubMed] [Google Scholar]
- Woods SP, Fields JA, Troster AI. Neuropsychological sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a critical review. Neuropsychol Rev. 2002;12:111–26. doi: 10.1023/a:1016806711705. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.