Abstract
Ictal hypoxemia has been reported in small series of cases and may contribute to sudden unexpected death in epilepsy (SUDEP). We sought to determine the incidence and severity of ictal hypoxemia in patients with localization-related epilepsy undergoing in-patient video-EEG telemetry. We examined whether seizure-associated oxygen desaturation was a consequence of hypoventilation and whether factors such as seizure localization and lateralization, seizure duration, contralateral spread of seizures, patient position at seizure onset and body mass index influenced ictal-related hypoxemia. A total of 304 seizures with accompanying oxygen saturation data were recorded in 56 consecutive patients with intractable localization-related epilepsy; 51 of 304 seizures progressed to generalized convulsions. Pulse oximetry showed oxygen desaturations below 90% in 101 (33.2%) of all seizures with or without secondary generalization, with 31 (10.2%) seizures accompanied by desaturations below 80% and 11 (3.6%) seizures below 70%. The mean duration of desaturation below 90% was 69.2 ± 65.2 s (47; 6–327). The mean oxygen saturation nadir following secondary generalization was 75.4% ± 11.4% (77%; 42–100%). Desaturations below 90% were significantly correlated with seizure localization [P = 0.005; odds ratio (OR) of temporal versus extratemporal = 5.202; 95% CI = (1.665, 16.257)], seizure lateralization [P = 0.001; OR of right versus left = 2.098; 95% CI = (1.078, 4.085)], contralateral spread of seizures [P = 0.028; OR of contralateral spread versus no spread = 2.591; 95% CI = (1.112, 6.039)] and gender [P = 0.048; OR of female versus male = 0.422; 95% CI = (0.179, 0.994)]. In the subset of 253 partial seizures without secondary generalized convulsions, 34.8% of seizures had desaturations below 90%, 31.8% had desaturations below 80% and 12.5% had desaturations below 70%. The degree of desaturation was significantly correlated with seizure duration (P = 0.001) and with electrographic evidence of seizure spread to the contralateral hemisphere (P = 0.003). Central apnoeas or hypopnoeas occurred with 50% of 100 seizures. Mixed or obstructive apnoeas occurred with 9% of these seizures. End-tidal carbon dioxide (ETCO2) was recorded in seven patients (19 seizures). The mean increase in ETCO2 from preictal baseline was 18.6 ± 17.7 mm Hg (13.2; 2.8–77.8). In these 19 seizures, all oxygen desaturations below 85% were accompanied by an increase in ETCO2. Ictal hypoxemia occurs often in patients with localization-related epilepsy and may be pronounced and prolonged; even with seizures that do not progress to generalized convulsions. Oxygen desaturations are accompanied by increases in ETCO2, supporting the assumption that ictal oxygen desaturation is a consequence of hypoventilation. Ictal hypoxemia and hypercapnia may contribute to SUDEP.
Keywords: partial seizures, hypoxemia, hypercapnia, SUDEP, epilepsy
Mortality in patients with epilepsy is increased with a standardized mortality ratio of 1.6–9.3 in the general epilepsy population. Causes of increased mortality include accidental death, suicide, status epilepticus and sudden unexpected death in epilepsy (SUDEP). SUDEP is the most common cause of death in patients with epilepsy, with the highest incidence of SUDEP in patients being evaluated in epilepsy referral centres and in patients being assessed for epilepsy surgery (Tellez-Zentano et al., 2005). In the latter population, the annual incidence is 2.2–10 per 1000 population per year (Tellez-Zentano et al., 2005). Both cardiac and respiratory dysfunction have been implicated as possible precipitating causes in SUDEP.
Asphyxia with partial seizures without convulsive activity was first described by Hughlings Jackson (1899). Electrical stimulation of the hippocampal gyrus, the ventral and medial aspects of the temporal pole, the anterior portion of the insula and the anterior limbic gyrus results in inhibition of respiratory movements (Kaada and Jasper, 1952). In small series of patients with partial onset seizures, hypoxemia has been observed using pulse oximetry in adults and children (Hewertson et al., 1996; Nashef et al., 1996; Blum et al., 2000). It has not been determined whether the observed oxygen desaturation reflects hypoventilation or is a consequence of seizure-associated peripheral vasoconstriction (Blum et al., 2000).
The study was designed to determine the incidence and severity of hypoxemia in a population of patients with localization-related epilepsy undergoing inpatient video-EEG telemetry. We sought to confirm that seizure-associated oxygen desaturation is a consequence of hypoventilation. We also examined whether factors such as seizure localization and lateralization, seizure duration, contralateral spread of seizures, patient position at seizure onset and body mass index (BMI) might contribute to or exacerbate ictal-related hypoxemia.
Methods
We studied 57 consecutive patients with intractable localization-related epilepsy admitted for in-patient video-EEG telemetry to assess candidacy for epilepsy surgery. Anti-epileptic medications were tapered and/or discontinued during the monitoring period to provoke seizures in the majority of patients (n = 52). In four patients, anti-epileptic therapy was not altered due to the occurrence of frequent daily seizures. One patient was not on anti-epileptic therapy at the time of admission. EEG was recorded from scalp locations corresponding to the standard International 10-20 system, including T1 and T2 locations and bilateral mandibular notch surface electrodes, except in six patients who had intracranial electrodes. Synchronized video and single-channel electrocardiogram (using right and left lateral infraclavicular electrodes) were recorded. Oxygen saturation values were recorded using digital pulse oximetry (Nellcor N-395 oximeter) and displayed every second on a dedicated channel of a BMSI 6000 video-EEG recording unit. The accuracy of the pulse oximetry data at very low saturation levels is unspecified by the manufacturer. Therefore, all nominal saturation values of <50% were truncated at 50% for data analysis. Body position and state of the patient at seizure onset (awake or asleep), seizure localization at onset and time to contralateral spread when present, duration of seizure, duration of desaturation (defined as the interval from saturation dropping below 90% to subsequent return above 90%), time to onset of desaturation from seizure onset, oxygen desaturation nadir and ictal heart rate change were recorded. The heart rate was measured in 10-s epochs relative to seizure onset. We monitored nasal airflow and recorded abdominal excursions. The BMI for each patient was calculated.
One of the 57 patients had only simple partial seizures without EEG change and was excluded from further analysis. In one other patient with frontal lobe seizures, 98 brief, stereotyped seizures were recorded without oxygen desaturation. In this patient, only the first 10 consecutive seizures were analyzed. A total of 381 partial onset seizures were captured and analysed in the group of 56 patients.
Summary statistics were reported as mean ± SD (median; range). The generalized estimating equation (GEE) approach (Liang and Zeger, 1986) was used for repeated measurements analysis of binary outcomes to investigate the association between a risk factor (explanatory variable) of interest and the binary response variable. Logistic regression was used to study the association between a risk factor and the binary response variable. A linear mixed-effects model was used for repeated measurement analysis of continuous outcomes to investigate the association between a risk factor and the continuous response variable. All analyses were performed with SAS Version 9.1.
The majority of patients with localization-related epilepsy admitted to the epilepsy monitoring unit have temporal lobe epilepsy. To determine whether seizure localization was associated with desaturation below 90% we obtained an estimate of the sample size that would allow a reasonably robust detection of a difference between temporal and extratemporal seizures. The Fisher's exact test was used to test the null hypothesis that the probability (p1) of a desaturation below 90% in the temporal seizure group equals the probability (p2) of desaturation below 90% in the extratemporal seizure group versus the alternative hypothesis that p1 does not equal p2. We determined that a sample size of 85 seizures in each group (temporal onset seizures and extratemporal onset seizures) would test the null hypothesis p1 = p2 = 0.12 versus the alternative hypothesis p1 ≠ p2 = 0.43 providing a power of 0.995 at a significance level of α = 0.05. Data collection was therefore stopped when 85 extratemporal onset seizures with oxygen saturation data were acquired. At that point, we had recorded a total of 381 partial onset seizures.
In seven patients (19 seizures), in addition to the parameters noted above, end-tidal CO2 (ETCO2) was recorded simultaneously with nasal cannulae using a capnograph (BCI, Inc, Waukesha, WI, USA). The time on the capnograph was synchronized with that on the BMSI 6000. Digitized ETCO2 values, averaged over four breaths, were displayed every 4 s and recorded. ETCO2 was recorded in this small subset of patients primarily to demonstrate the validity of pulse oximetry during seizures. We anticipated that seizure-related hypoventilation resulting in oxygen desaturation would be accompanied by a concomitant rise in ETCO2. Conversely, artefactual seizure-related drops in pulse oximetry values would not be accompanied by increases in ETCO2.
Results
There were 34 (60.7%) female patients in this group of 56 patients. The mean age was 36.9 ± 14 (34.5; 16–63) years. At the time of hospital admission, patients were taking between zero and five anti-epileptic medications. Eleven patients were on monotherapy, one patient was not on anti-epileptic medication and the remaining patients were on polypharmacy.
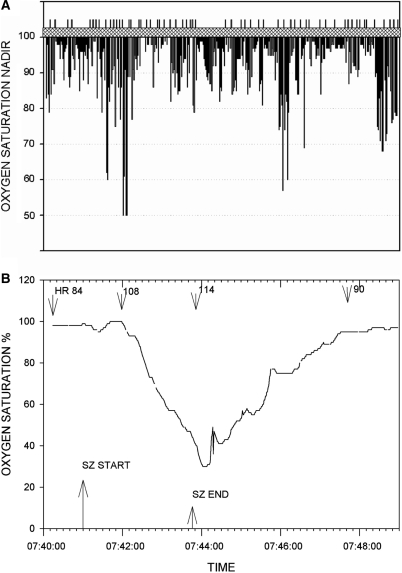
Oxygen saturation data was available in 304 seizures of partial onset, 51 of which progressed to generalized convulsions. We first looked at the entire group of partial onset seizures with or without secondarily generalized convulsions. A total of 101 of these 304 seizures were associated with desaturations below 90%; 30 seizures occurred in sleep, 69 during wakefulness and with two seizures the patient's state could not be determined. Of these 101 seizures, 31 (30.7%) seizures were associated with desaturations below 80% and 11 (10.9%) seizures with desaturations below 70% (Fig. 1A and B). The mean oxygen saturation nadir following secondary generalization was 75.4% ± 11.4% (77%; 42–100%). Review of oxygen saturation data acquired during continuous video-EEG telemetry confirmed that no patient had oxygen desaturation below 90% unless temporally associated with a seizure.
Fig. 1.
(A) Oxygen saturation nadir (percent saturation) for each seizure. Recorded saturation values below 50% were truncated at 50%. The vertical lines above the hatched bar separate data from the 56 individual patients. (B) Pronounced oxygen desaturation with a complex partial left temporal onset seizure without secondary generalization. Patient was a 19-year-old male with a BMI of 19.9. Seizure onset occurred with the patient awake and sitting in bed. He became unresponsive with lip smacking, a slight head turn to the left followed by forceful head turning to the right. He remained sitting for the duration of the seizure. The heart rate (b.p.m.) at various times is shown. Two other complex partial seizures in this patient (one left and one right temporal onset) were accompanied by oxygen desaturations below 50%. Oxygen saturation percent is shown on the ordinate.
The mean duration for all seizures was 80.6 ± 76.1 s (64.0; 3–610). There was a statistically significant correlation between the level of desaturation and seizure duration (P = 0.001). Seizure localization was statistically significantly associated with desaturation below 90% [P = 0.005, odds ratio (OR) of temporal versus extratemporal onset = 5.202; 95% CI = (1.665, 16.257)]. Seizure lateralization was statistically significantly associated with desaturation below 90% [P = 0.001, OR of right versus left = 2.098; 95% CI = (1.078, 40.85)]. Contralateral spread of seizures was statistically significantly associated with desaturation below 90% [P = 0.028, OR of contralateral spread versus no contralateral spread of seizures = 2.591; 95% CI = (1.112, 6.039)]. Patient position at seizure onset (recumbent versus semi-recumbent or sitting), state of the patient at seizure onset (awake versus asleep), age of patient and BMI were not associated with desaturation below 90%. There was a statistically significant association of gender with desaturation below 90% [P = 0.048, OR of female versus male = 0.422; 95% CI = (0.179, 0.994)]. Heart rate change (ictal peak heart rate − baseline heart rate) was not associated with desaturation below 90%. In the smaller group of seizures with more pronounced desaturations (<85%), a statistically significant association of desaturation with seizure lateralization or localization was not present, possibly because of lower power in this group. In a given patient with a seizure related desaturation of <85%, the probability that a subsequent seizure would be accompanied by a desaturation of <85% was 0.433.
For all seizures, the mean delay from seizure onset to the onset of desaturation below 90% was 66.7 ± 37.6 s (59; 6–226). The mean saturation nadir occurred 98.3 ± 63.3 s (86; 8–521) after seizure onset. The delay to desaturation from seizure onset is, in part, a reflection of circulation time and processing delay in the oximeter. For all seizures, the mean duration of desaturation (from <90% to ≥90%) was 69.2 ± 65.2 s (47; 2–327). Oxygen saturation recovery to within 2% of preictal baseline value was further delayed. The mean duration from desaturation below 90% to recovery to preictal baseline level was 104.4 ± 78.4 s (90; 8–407). These data are summarized in Table 1.
Table 1.
Desaturations with partial onset seizures
| Partial seizures with or without secondary generalization | Partial seizures without secondary generalization | |
|---|---|---|
| Total number of seizures | 304 | 253 |
| Seizures with desaturation <90% | 101 (33.2%) | 88 (34.8%) |
| Seizures with desaturation <80% | 31 (10.2%) | 28 (11.1%) |
| Seizures with desaturation <70% | 11 (3.6%) | 11 (4.4%) |
| Seizures with no desaturation < 90% Seizure duration (s) | 78.1 ± 90.4 (48; 3–610) | 62.9 ± 84.7 (39; 3–610) |
| Seizures with desaturation < 90% Seizure duration (s) | 93.0 ± 60.4 (75; 12–410) | 83.1 ± 49.0 (70; 12–163) |
| Delay to desaturation below 90% from seizure onset (s) | 66.7 ± 37.6 (59; 6–226) | 58.5 ± 31.1 (56; 6–175) |
| Time to desaturation nadir from seizure onset (s) | 98.3 ± 63.3 (86; 8–521) | 79.7 ± 38.5 (71; 8–205) |
| Duration of desaturation (s) (Below 90% to above 90%) | 69.2 ± 65.2 (47; 2–327) | 54.7 ± 63.2 (34; 2–296) |
| Duration of desaturation (s) (Below 90% to preictal baseline) | 104.4 ± 78.4 (90.8; 8–407) | 90.8 ± 80.0 (69; 8–366) |
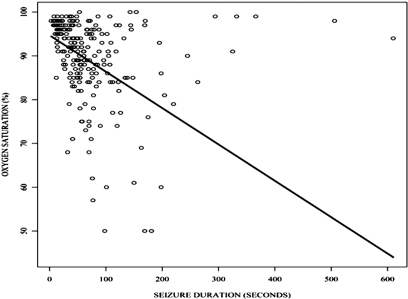
We next looked at the subset of partial onset seizures that did not progress to generalized convulsions. Of the 304 seizures for which oxygen saturation data was available, 253 (83.2%) did not result in secondarily generalized convulsions. Desaturation nadirs below 90% occurred with 88 (34.8%) of the 253 seizures. In 28 (31.8%) of these seizures, oxygen saturations fell below 80% and in 11 (12.5%) seizures saturations fell below 70%. The mean desaturation nadir in these 88 seizures was 80.7%. Fifty-five of the 88 seizures with desaturations below 90% were complex partial seizures of temporal onset. Eleven were electrographic seizures of temporal onset without any discernable behavioural manifestations. In another 11 seizures of temporal onset there was inadequate testing to determine whether impairment of consciousness was present. The remaining seizures included three complex partial frontal onset seizures, one gelastic seizure and seven seizures with inadequate electrographic localization. Desaturations below 90% occurred with 52 of 101 (51.5%) recorded seizures of right temporal onset and in 23 of 58 seizures of left temporal onset (39.7%). In the remaining two patients, there was near simultaneous electrographic involvement of the left and right temporal scalp regions at seizure onset. In this group of seizures that did not generalize, seizure duration statistically significantly correlated with the level of desaturation (P ≤ 0.001) (Fig. 2). The level of desaturation also correlated with electrographic spread of the seizure to the contralateral hemisphere (P = 0.003). Seizure duration and electrographic spread to the contralateral hemisphere were statistically significantly correlated (P = 0.003). BMI and the state of the patient at seizure onset were associated with only the more severe desaturations. The BMI was statistically significantly associated with desaturations below 85% [P = 0.021; OR = 1.116; 95% CI = (1.017, 1.225)]. The state of the patient was statistically significantly associated with desaturations below 85% [P = 0.001; OR of awake versus asleep = 3.674; 95% CI = (1.719, 7.851)].
Fig. 2.
Plot of seizure duration versus level of desaturation. Oxygen saturation = 94.704 – 0.083 × seizure duration.
To determine a possible effect of anti-epileptic drug load on the degree of oxygen desaturation, the two-sided Wilcoxon signed-rank test was used to determine whether there was a statistically significant difference between oxygen saturation nadirs for the first and last partial seizure in each patient. Fifty-one patients had more than one seizure with saturation data. The mean saturation nadir associated with the first partial seizure was 89.1 ± 9.9% (92%; 50–100%) and for the last seizure the mean nadir was 90.8 ± 9.0% (94%, 57–100%). In seizures that progressed to generalized convulsions, the desaturation nadir prior to the onset of secondary generalization was used. There was no statistically significant difference in desaturation nadirs between the first and last seizures (P-value = 0.366).
Nineteen of 56 patients had no desaturations below 90% with any of 85 seizures. In this group of 19 patients, 41 of the 85 seizures were of extra-temporal onset. The mean BMI in patients having no desaturations with any seizure was 26.8 ± 5.2 (25.4; 20.4–37.1). In patients with desaturations accompanying any seizure, the mean BMI was 26.2 ± 6.4 (25.4; 15.7–42.2). The BMI was not statistically significantly associated with whether or not the patients had desaturations with any seizure [P = 0.691, OR = 0.981, 95% CI = 0.892–1.079)].
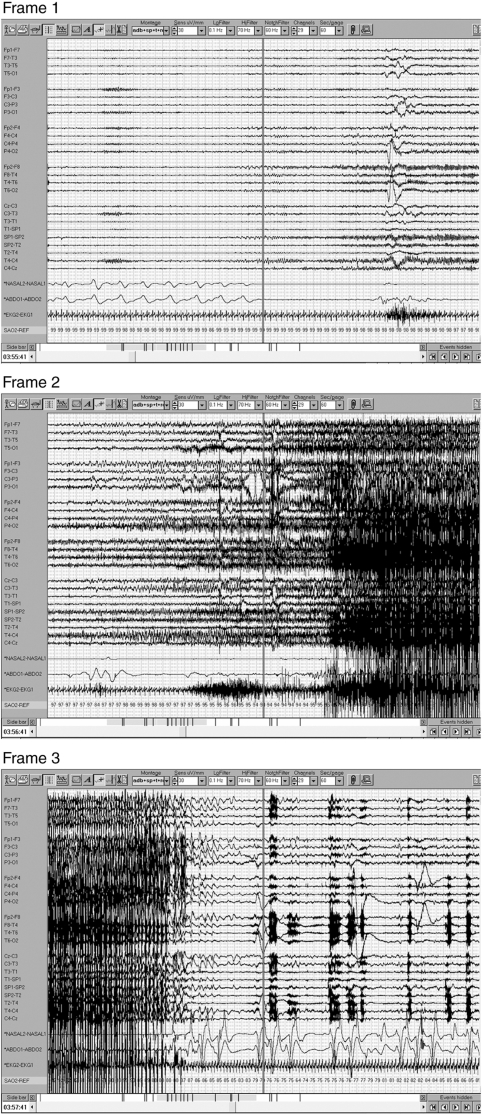
Nasal airflow and abdominal excursion data were available in 100 seizures. In the remaining seizures, these data were obscured by artefact and not interpretable or were unavailable. In 44 (44%) of these 100 seizures, there was evidence of central apnoea during the seizure (Fig. 3). Six (6%) seizures were accompanied by hypopnoeas (airflow amplitude reduced by at least 30% relative to pre-ictal baseline). There were two (2%) obstructive apnoeas (absent airflow with continuing abdominal excursions) and seven (7%) mixed apnoeas (central and obstructive components during the apnoea). There were no apnoeas or hypopnoeas detected with 41 (41%) of 100 seizures.
Fig. 3.
Complex partial seizure with secondary generalization recorded in a 31-year-old woman; 60 s per frame. Frame 1 shows right temporal onset seizure with central apnoea. Secondary generalization occurs in Frame 2 and oxygen desaturation is seen. Seizure ends in Frame 3 with resumption of breathing and continued but improving oxygen desaturation.
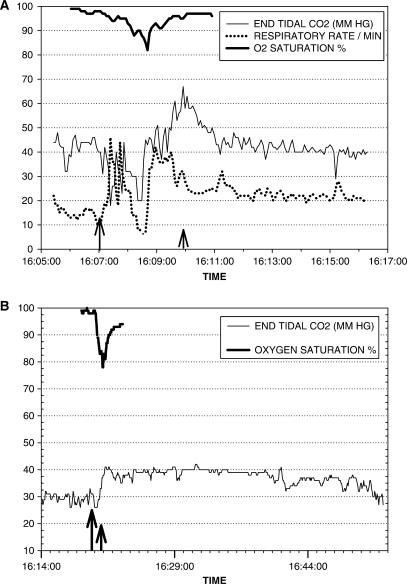
In seven patients (19 seizures), we recorded simultaneous ictal ETCO2 and oxygen saturation data. The mean preictal ETCO2 for the group, averaged over 1 min from 1 to 2 min prior to seizure onset, was 32.8 ± 8.2 mm Hg (30.3; 16.2–47.2). One patient had been hyperventilating vigorously prior to seizure onset with mean preictal ETCO2 of 16.2 and 22.6 mm Hg, respectively for two seizures. The mean increase in ETCO2 from the preictal baseline to ictal peak ETCO2 was 18.6 ± 17.7 mm Hg (13.2; 2.8–77.8). In seven of the 19 seizures, ETCO2 values were above 50 mm Hg (Fig. 4A). The mean oxygen saturation nadir during these seizures was 77% ± 8.8% (79%; 50–86%). The most pronounced increases in ETCO2 occurred in patients with secondarily generalized seizures. However, increases in ETCO2 occurred with every partial onset seizure that had a simultaneous drop in oxygen saturation to 85% or less. In some patients, the increase in ETCO2 beyond preictal values persisted >30 min following termination of the seizure (Fig. 4B).
Fig. 4.
(A) Partial onset seizure with secondary generalization. There is an increase in ETCO2 above 65 mm Hg accompanying a drop in oxygen saturation. Respiratory rate increases during the period of hypercarbia. The longer arrow indicates seizure onset; the shorter arrow indicates seizure end. (B) Left temporal onset complex partial seizure. There is an abrupt increase in ETCO2 with concomitant drop in oxygen saturation. ETCO2 elevation persists above preictal levels for >30 min after the end of the seizure.
Ictal tachycardia occurred with most seizures. The mean preictal heart rate (averaged over 10 s, 1 min prior to seizure onset) was 81 ± 17 beats per minute (b.p.m.) (72; 42–144). The mean ictal heart rate increase was 38 ± 28 b.p.m. (36; –24 to +126) with a range of –24 to +126 b.p.m. The duration of ictal tachycardia (defined as the time interval during which the heart rate was >100 b.p.m.) was a mean of 129 ± 324 s (70; 10–3670) with a range of 10–3670 s (25 and 75 percentile values of 40 and 120.5 s, respectively). Premature atrial beats, premature ventricular beats and sinus pauses occurred with some seizures in 10 patients. One patient developed a bradyarrhythmia and asystole following a secondarily generalized seizure of left temporal onset. Oxygen saturation dropped below 50% following that seizure.
Discussion
In this group of patients, seizure-associated oxygen desaturation was common and occasionally severe. Pronounced desaturations occurred in some patients with partial seizures that did not progress to generalized convulsions. In a subset of seizures with simultaneous recording of oxygen saturation and ETCO2, an increase in ETCO2 occurred with every seizure that was associated with an oxygen desaturation of 85% or less. The concurrent increase in seizure-associated ETCO2 makes it likely that ictal oxygen desaturation is due to alveolar hypoventilation. In some patients, hypoventilation persisted despite increases in ventilatory rate (Fig. 4A).
Desaturations occurred most commonly with seizures of temporal lobe onset and were uncommon with extra-temporal onset seizures. Seizures occurred more commonly with right rather than left temporal onset seizures. Both the duration of the seizure and electrographic evidence of contralateral spread appeared to influence the degree of desaturation.
Our data suggest that severe desaturations are seen more commonly with seizure spread to the contralateral hemisphere. The descending pathways from limbic areas to the brainstem respiratory centres are primarily ipsilateral (Hopkins and Holstege, 1978). Therefore, seizure-related bilateral impairment of these descending pathways may be a requisite for pronounced respiratory inhibition. There is evidence for significant projections from limbic regions to brainstem areas involved in control of respiratory activity. There are ipsilateral direct projections from the central nucleus of the amygdala to the pontine and medullary tegmentum in the cat (Hopkins and Holstege, 1978). Structures receiving these descending influences include the nucleus of the solitary tract that has neurons projecting to the phrenic motor pool, the parabrachial pontine region that is involved in respiratory phase switching (Bassal and Bianchi, 1982) and the lateral periaqueductal grey that in turn projects to the nucleus retro ambiguous, which modulates upper airway activity and abdominal respiratory muscles (Holstege, 1989). Physiological studies indicate that the hippocampus also influences breathing. Hippocampal neurons include cells that have phase-locked activity with the respiratory cycle (Frysinger and Harper, 1989). Stimulation of the hippocampus entrains the respiratory rhythm (Ruit and Neafsey, 1988). Hippocampal activity is increased before the termination of apnoea, indicating a role in resumption of breathing (Poe et al., 1994).
Obstructive and mixed apnoeas were observed less commonly than central apnoeic events in our group of patients. In a rat model of penicillin-induced seizures, there was reduced activity in the vagal and hypoglossal nerves following a decrease or cessation of phrenic nerve activity. These changes were consistent with seizure-related obstructive apnoea followed by central apnoea. The central apnoeas were profound and could not be reversed by augmentations in ventilatory drive from increasing carbon dioxide (St-John et al., 2006).
Seizure-related arterial PCO2 increases are likely higher than values indicated during ETCO2 recordings. The ETCO2 is lower than arterial PCO2 by 2–4 mm Hg and the capnograph recordings are susceptible to seizure-related displacement and blockage of the nasal prongs, resulting in lowered ETCO2 values. Ictal ETCO2 values > 50 mm Hg were seen with seven of 19 seizures. These data suggest a mechanism for seizure-related cardiac dysfunction. In healthy young adults, acute hypercapnia to 7 kPa (52.5 mm Hg) resulted in prolongation of the QTc interval and QT dispersion (Kiely et al., 1996). This increased QT dispersion, reflecting differences in regional myocardial repolarization, represents a putative substrate for arrhythmia (Kiely et al., 1996). In a sheep model of bicuculline-induced epileptic sudden death, all animals had central apnoeas (Johnston et al., 1997). Animals dying early had pronounced elevations of PaCO2 and drops in PaO2 compared with surviving animals. Hypercarbia contributed to death in these animals. Malignant arrhythmias were not seen and sinus tachycardia persisted until death (Johnston et al., 1997).
Ictal tachyarrhythmia is the most commonly reported arrhythmia associated with seizures (Blumhardt et al., 1986; Maromi et al., 2004). This was also observed in our study, with increases in heart rate above 100 b.p.m. persisting for a median of 70 s. Pronounced tachycardia to 180 b.p.m. occurred with some seizures. Ictal bradycardia and asystole are rare (Rocamora et al., 2003) and in our series, occurred with one seizure that was associated with oxygen desaturation to below 50%.
The patient BMI was associated only with the more severe desaturations accompanying partial seizures, suggesting that a high BMI may exacerbate the level of ictal desaturation in some patients. However, pronounced ictal desaturations did accompany partial seizures in patients with a BMI in the normal range. Ictal oxygen desaturations are more likely to occur in males than in females for reasons that remain unclear.
There was considerable variability in the anti-epileptic drug regimens used by our patients on admission to the epilepsy-monitoring unit. Only 11 patients were on monotherapy. Polytherapy with a variety of anti-epileptic drugs in the majority of patients precluded any determination of whether a particular anti-epileptic drug or class of drugs might influence the degree of ictal-associated hypoxemia. Anti-epileptic drug levels were not monitored; however, since anti-epileptic drugs were reduced or stopped on admission the anti-epileptic drugs’ load would be decreased at the tail-end of the monitoring period. We found no difference in the desaturation nadirs between the first and last seizure of each patient. This suggests that the anti-epileptic drug load did not influence the severity of seizure-related oxygen desaturation.
Our data indicate that there is a high probability of recurrent ictal desaturations in a patient who has had a seizure-related desaturation below 85%. Patients with medically refractory epilepsy who have severe seizure-related hypoxemia and hypercapnia identified in epilepsy-monitoring units may be at increased risk for SUDEP. The pursuit of epilepsy surgery to control seizures may be of benefit in this patient population. This would require further study, however, as the role of epilepsy surgery in reducing the risk of SUDEP is still questioned (Ryvlin and Montavont, 2008). Other therapeutic measures to ameliorate hypoxemia in patients with refractory partial seizures could include the use of selective serotonin reuptake inhibitors. Serotonin is implicated in modulating the brainstem respiratory network (Richter et al., 2003). In DBA/2 mice with audiogenic seizures, there is seizure-related respiratory arrest. Fluoxetine reduced respiratory arrest at doses that did not stop seizures (Tupal and Faingold, 2006). Cardiac pacing has been required in patients with ictal bradycardia and asystole (Rugg-Gunn et al., 2004). Nocturnal supervision, including regular checks and the use of listening devices, may be protective against SUDEP (Langan et al., 2005). The potential benefits of other interventions, such as home pulse oximetry monitoring with appropriate alarms or continuous positive airway pressure devices, may be worth evaluating in patients with documented severe seizure-associated oxygen desaturations who are awaiting potentially curative therapies.
Funding
The National Center for Research Resources; a component of the National Institutes of Health (UL1 RR024146); National Institutes of Health Roadmap for Medical Research.
Acknowledgements
The contents of National Institutes of Health Roadmap for Medical Research are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Glossary
Abbreviations:
- BMI
body mass index
- ETCO2
end-tidal CO2
- SUDEP
sudden unexpected death in epilepsy
References
- Bassal M, Bianchi AL. Inspiratory onset or termination induced by electrical stimulation of the brain. Respir Physiol. 1982;50:23–40. doi: 10.1016/0034-5687(82)90004-4. [DOI] [PubMed] [Google Scholar]
- Blum AS, Ives JR, Goldberger AL, Al-Aweel IC, Krishnamurthy KB, Drislane FW, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia. 2000;41:536–41. doi: 10.1111/j.1528-1157.2000.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Blumhardt LD, Smith PEM, Owen L. Electrographic accompaniments of temporal lobe epileptic seizures. Lancet. 1986;1:1051–6. doi: 10.1016/s0140-6736(86)91328-0. [DOI] [PubMed] [Google Scholar]
- Frysinger RC, Harper RM. Cardiac and respiratory correlations with unit discharge in epileptic human temporal lobe. Epilepsia. 1990;31:162–71. doi: 10.1111/j.1528-1167.1990.tb06301.x. [DOI] [PubMed] [Google Scholar]
- Hewertson J, Boyd SG, Samuels MP, Neville BG, Southall DP. Hypoxemia and cardiorespiratory changes during epileptic seizures in children. Dev Med Child Neurol. 1996;38:511–22. doi: 10.1111/j.1469-8749.1996.tb12112.x. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical evidence for a strong ventral parabrachial projection to nucleus raphe magnus and adjacent tegmental field. Brain Res. 1988;447:154–8. doi: 10.1016/0006-8993(88)90977-8. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529–47. doi: 10.1007/BF00239551. [DOI] [PubMed] [Google Scholar]
- Jackson JH. On asphyxia in slight epileptic paroxysms: on the symptomatology of slight epileptic fits supposed to depend on discharge-lesions of the uncinate gyrus. Lancet. 1899;1:79–80. [Google Scholar]
- Johnston SC, Siedenberg R, Min JK, Jerome EH, Laxer KD. Central apnea and acute cardiac ischemia in a sheep model of epileptic sudden death. Ann Neurol. 1997;42:588–94. doi: 10.1002/ana.410420409. [DOI] [PubMed] [Google Scholar]
- Kaada BR, Jasper H. Respiratory responses to stimulation of temporal pole, insula and Hippocampal and limbic gyri in man. Arch Neurol Psychiatry. 1952;68:609–19. doi: 10.1001/archneurpsyc.1952.02320230035004. [DOI] [PubMed] [Google Scholar]
- Kiely DG, Cargill RI, Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest. 1996;109:1215–21. doi: 10.1378/chest.109.5.1215. [DOI] [PubMed] [Google Scholar]
- Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology. 2005;64:1131–3. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Nashef L, Walker F, Allen P, Sander JW, Shorvon SD, Fish DR. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry. 1996;60:297–300. doi: 10.1136/jnnp.60.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Ho RT, Abou-Khalil BW, Drislane FW, Liporace J, Romeo A, et al. EEG and ECG in sudden unexpected death in epilepsy. Epilepsia. 2004;45:338–45. doi: 10.1111/j.0013-9580.2004.05503.x. [DOI] [PubMed] [Google Scholar]
- Poe GR, Rector DM, Harper RM. Hippocampal reflected optical patterns during sleep and waking states in the freely behaving cat. J Neurosci. 1994;14:2933–42. doi: 10.1523/JNEUROSCI.14-05-02933.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–8. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Rocamora R, Kurthen M, Lickfett L, Von Oertzen J, Elger CE. Cardiac asystole in epilepsy: clinical and neurophysiological features. Epilepsia. 2003;44:179–85. doi: 10.1046/j.1528-1157.2003.15101.x. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR, Duncan JS. Cardiac arrhythmias in focal epilepsy: a prospective long-term study. Lancet. 2004;264:2212–6. doi: 10.1016/S0140-6736(04)17594-6. [DOI] [PubMed] [Google Scholar]
- Ruit KG, Neafsey EJ. Cardiovascular and respiratory responses to electrical and chemical stimulation of the hippocampus in anesthetized and awake rats. Brain Res. 1988;457:310–21. doi: 10.1016/0006-8993(88)90701-9. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Montavont A. La chirurgie de l’épilepsie réduit-elle la surmortalité des épilepsies partielles pharmacorésistantes? Neurochirurgie. 2008;54:282–6. doi: 10.1016/j.neuchi.2008.02.047. [DOI] [PubMed] [Google Scholar]
- St-John WM, Rudkin AH, Homes GL, Leiter JC. Changes in respiratory-modulated neural activities, consistent with obstructive and central apnea, during fictive seizures in an in situ anaesthetized rat preparation. Epilepsy Res. 2006;70:218–28. doi: 10.1016/j.eplepsyres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Téllez-Zenteno JF, Ronquillo LH, Wiebe S. Sudden unexpected death in epilepsy: evidence-based analysis of incidence and risk factors. Epilepsy Res. 2005;65:101–15. doi: 10.1016/j.eplepsyres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–6. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]