Abstract
A link between developmental language disorders and atypical cerebral lateralization has been postulated since the 1920s, but evidence has been indirect and inconsistent. The current study investigated this proposal using functional transcranial Doppler ultrasonography (fTCD), which assesses blood flow through the middle cerebral arteries serving the left and right cerebral hemispheres. A group of young adults with specific language impairment (SLI; n = 11) were recruited along with three comparison groups: (i) adults with a history of childhood SLI, but who did not meet criteria for language impairment in adulthood (SLI-history; n = 9); (ii) adults with an autism spectrum disorder and a comorbid language impairment (ASD; n = 11) and (iii) adults with no history of developmental disorder (typical; n = 11). There was no difference between the chronological age of the four groups, and the SLI and typical groups were individually matched on gender and handedness. During fTCD measurement, participants were asked to silently generate words starting with a given letter and then later required to verbalize these. All of the participants in the SLI-history group and the majority of participants in the ASD (81.8%) and typical (90.9%) groups had greater activation in the left compared to the right middle cerebral arteries, indicating left hemisphere dominance. In contrast, the majority of participants in the SLI groups had language function lateralized to the right hemisphere (54.5%) or dispersed bilaterally (27.3%). These findings suggest that atypical cerebral dominance is not implicated in all cases of poor language development (i.e. ASD and SLI-history groups), but may act as a biological marker of persisting SLI.
Keywords: specific language impairment, autism, cerebral lateralization, language, functional transcranial Doppler ultrasonography
Introduction
Specific language impairment (SLI) is recognized when there is significant delay in the development of language that cannot be attributed to low intelligence, hearing impairment or limited educational opportunities. Although the disorder is identified on the basis of difficulties in early language development, as children grow older, literacy problems usually become apparent (Bishop and Snowling, 2004). While it is clear that genetic factors play a role in the etiology of SLI (Bishop, 2002), there is little understanding of the neurobiological phenotype.
The notion that developmental disorders of language and literacy might reflect failure to develop normal cerebral lateralization dates back to Orton's earliest writings in the 1920s (Orton, 1966). Orton argued that a failure to develop a dominant language hemisphere led to difficulties with speech, language and reading. A more specific instantiation of this kind of idea came from Annett (1985), who proposed a single-gene account of cerebral lateralization, the Right Shift Theory. According to this theory, the majority of the population inherits either one or two copies of an allele that promotes early left hemisphere development and right hand skill. However, around 18% of people are homozygous for an allele that does not carry any bias. The ultimate degree of cerebral lateralization, and relative hand skill, will also be influenced by chance events, but in general those without the right shift factor will be less likely than the rest of the population to have left hemisphere speech. Like Orton, Annett also maintained that lack of cerebral dominance was a risk factor for language and literacy problems. Although Annett has gathered a substantial amount of data testing the theory, results have been inconsistent, and a comprehensive review of studies of handedness in SLI and dyslexia concluded that there was no association with either hand preference or hand skill (Bishop, 1990). Nevertheless, handedness is only a weak correlate of cerebral lateralization for language, and more direct investigations of brain structure and function have repeatedly revived the idea that there may be atypical cerebral lateralization in children with disorders of language and literacy.
Recent studies have used neuroimaging to investigate cerebral asymmetry in those with SLI, with a particular focus on the perisylvian region, which includes both Broca's and Wernicke's areas. Findings from these studies have been largely inconsistent. Several magnetic resonance imaging (MRI) studies have reported structural atypicalities, noting reduced or reversed volumetric asymmetry of a number of perisylvian structures, including the planum temporal, the pars triangularis and the inferior frontal gyrus (Cohen et al., 1989; Jernigan et al., 1991; Plante et al., 1991; Gauger et al., 1997; Herbert et al., 2003; Jäncke et al., 2007). Functional imaging studies have also documented differences in activation, where children with SLI are more likely than control participants to show symmetrical cerebral activation both while performing language tasks (Tzourio et al., 1994; Chiron et al., 1999; Bernal and Altman, 2003) and during the resting state (Lou et al., 1984, 1990; Ors et al., 2005). However, other studies have either failed to replicate these findings (Preis et al., 1998) or have reported considerable variability between children (Shafer et al., 2000; Trauner et al., 2000), making it difficult to draw any firm conclusions about the existence of cerebral asymmetries in children with SLI.
There are further difficulties with the existing studies of cerebral asymmetry in SLI. Due to the ethical constraints of certain imaging techniques (e.g. SPECT), participants have often been unable to be drawn from the typically developing population, resulting in control groups comprising children with other developmental disorders, such as attention deficit hyperactivity disorder (Lou et al., 1984) or dystrophia (Chiron et al., 1999). Similarly, studies have often included SLI participants with comorbid conditions, making it difficult to establish whether a reduction or reversal of laterality is related to language difficulties or other aspects of atypical development. The specific phenotype under consideration may prove to be important: in a series of structural imaging studies, Leonard et al. (2002, 2006) found that people with poor language comprehension tended to have more symmetrical brain structures than normal, whereas those with purer dyslexic difficulties not accompanied by oral language difficulties tended to have an exaggerated pattern of leftward cerebral asymmetry.
Most studies in this area have focused on children with SLI, leaving open the possibility that the participants’ language difficulties were not persisting, but merely a transient phase of development. A significant proportion of children with language difficulties in early childhood show considerably less or no impairment when tested in the later school years (Stothard et al., 1998). The developing brain is capable of reorganizing the patterns and systems of neural connections (Sur and Leamey, 2002), and it is possible that the variability of findings in this research area may be related to neurological differences between those with transient and those with persisting language impairment.
Finally, it remains uncertain whether atypical cerebral asymmetry is a specific correlate of SLI, or whether it is related more generally to poor language development. Reduced or reversed cerebral dominance has been noted in a number of disorders in which speech and/or language difficulties are hallmark, including Down Syndrome (Heath and Elliott, 1999), Rett Syndrome (Olsson and Rett, 1986), stuttering (Cykowski et al., 2008) and autism spectrum disorder (ASD; Rinehart et al., 2002). ASD, in particular, has attracted a considerable amount of research in this area. For example, Herbert and colleagues (Herbert et al., 2005) conducted a whole-brain MRI morphometric survey of children with autism, and found that patterns of structural asymmetry in these children and a group of children with SLI were more similar to each other than to a typically developing control group. This finding, in conjunction with evidence that children with ASD and SLI demonstrate similar linguistic abnormalities, has led some to consider the possibility that the two disorders have overlapping etiologies (Tager-Flusberg and Joseph, 2003). Others contend that these similarities are superficial and not indicative of a common underlying cause (Whitehouse et al., 2007, 2008). As yet, no study has compared the functional neurology of language in ASD and SLI.
In the current study, we investigated hemispheric dominance for language in adults with SLI using functional transcranial Doppler (fTCD). This method uses ultrasound to measure event-related changes in blood flow in the middle cerebral arteries (MCA). As with other perfusion-sensitive neuroimaging techniques, fTCD works under the premise that an increase in neural activity results in greater glucose and oxygen consumption that must be replenished via enhanced blood flow to the area (Lohmann et al., 2006). fTCD has been used for medical purposes for over two decades (Aaslin et al., 1982), but the technique has only recently been harnessed for the investigation of cerebral lateralization (Deppe et al., 1997). This method gives high correlations with existing ‘gold standard’ measures of cerebral lateralization, such as the Wada technique (Knecht et al., 1998) and fMRI (Deppe et al., 2000).
The adults with SLI were subdivided according to whether they had evidence of persisting difficulties in adulthood, or whether the problems had resolved (SLI-history group). Two additional groups were recruited to help interpret the data from the participants with SLI. The first control group comprised adults with no history of developmental disorder and allowed us to examine whether the SLI group had atypical cerebral lateralization as measured by fTCD. Finally, adults with ASD and a comorbid language disorder allowed us to test recent ideas that ASD and SLI are different manifestations of the same organic cause.
Methods
Participants
Three clinical groups were recruited from previous studies conducted by our research group at Oxford University (Whitehouse et al., in press) or from their participation in the Manchester Language Study (Conti-Ramsden et al., 2007). Each participant had been administered assessments of non-verbal IQ and language/literacy ability at a previous testing session. Although time since this testing session varied between participants (M 2.51 years; SD 1.67 range 0.6–6.92), it is important to note that numerous longitudinal studies have shown that the language/literacy ability of individuals with developmental language disorders remains relatively stable beyond middle childhood (Stothard et al., 1998; Whitehouse et al., in press). The majority of participants were assessed at age 16 years or more (83.9%), and all were seen after their 13th birthday. Non-verbal IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (Wechsler and Chen, 1999) for all but three participants, who were administered the Wechsler Intelligence Scale for Children—Third edition (WISC-III; Wechsler, 1992). Language tests administered differed between participants, but all completed the Test of Word Reading Efficiency (TOWRE; Torgesen et al., 1999). The TOWRE comprises two subtests, one assessing real word reading and the other assessing non-word reading, which combine to provide an overall measure of reading ability. This measure has been found to be a good marker of language impairment in adults (Whitehouse et al., in press). Participants in the current sample with a standard score on the overall measure that was <1.33 SD below the mean (i.e. <80) were deemed to have persisting language impairment. The SLI group was made up of adults with a documented history of developmental language difficulties (n = 11). All of these participants had previously attended a special school for children with language difficulties or a specialist language unit attached to mainstream school and met the above criterion for persisting language impairment. Admission to these schools/services requires a detailed clinical assessment confirming the presence of language difficulties in the absence of an identifiable cause, such as brain injury, a known developmental syndrome, or physical abnormality of the articulators. School records of each participant had been checked during childhood for this diagnostic information. The SLI-history group (SLI-history, n = 9) also comprised adults who were diagnosed with SLI in childhood and had received specialist educational support for language in school, but did not meet the criterion for language impairment when tested in adulthood. The third clinical group were adults with ASD (n = 11), all of whom met the cutoff for persisting language impairment. Four participants in this group were diagnosed with ASD in childhood (i.e. before 12 years of age). The remaining seven participants had originally received a diagnosis of developmental language disorder in childhood, but were re-diagnosed (by clinical services) with ASD in adolescence. We have previously argued that the relatively late diagnosis of these individuals reflects the broadening of autism diagnostic boundaries over the past two decades (Bishop et al., 2008). All ASD participants had their diagnosis confirmed by the Autism Diagnostic Observation Schedule—Generic (Lord et al., 2000), while no participant in the SLI or SLI-history group met criteria for ASD on this measure. A final, non-clinical group was made up of adults with no history of developmental disorder (Typical group, n = 11), recruited from the Oxfordshire area. The groups were highly similar in age (P = 0.99), and the three clinical groups did not differ in non-verbal ability (P = 0.24). The participants in the SLI and typical groups were individually matched for gender and handedness, as assessed by the Edinburgh Handedness Inventory (Oldfield, 1971). Table 1 shows the characteristics of the four groups. Six adults were seen but not included in the final groups because either (i) they did not meet criteria for any group (i.e. had a diagnosis of ASD but had no language impairment, n = 4) or (ii) a temporal skull window that permitted clear Doppler recording could not be found (SLI = 1, ASD = 1).
Table 1.
Participant characteristics
| SLI (n = 11) | SLI-history (n = 9) | ASD (n = 11) | Typical (n = 11) | |
|---|---|---|---|---|
| Gender (male : female) | 7 : 4 | 7 : 2 | 10 : 1 | 7 : 4 |
| CA at behavioral testing | 18.15 (3.75) | 18.33 (14.33) | 19.98 (5.03) | – |
| Range | 15.7–30.03 | 13.72–26.77 | 13.07–27.78 | – |
| CA at Doppler testing | 20.67 (3.84) | 21.18 (2.79) | 21.10 (3.74) | 20.88 (3.29) |
| Range | 17.54–31.57 | 19.58–28.31 | 16.47–30 | 18.01–27.16 |
| Non-verbal IQ | 89.82 (11.37) | 103.67 (20.22) | 88.91 (15.9) | – |
| Range | 69–108 | 65–119 | 62–109 | – |
| TOWRE standard score | 65.5 (10.56) | 90 (7.71) | 70.55 (7.93) | – |
| Range | 53–79 | 81–104 | 56–81 | – |
| Handedness (Left : Ambidextrous : Right) | 2 : 1 : 8 | 2 : 0 : 7 | 0 : 0 : 11 | 2 : 1 : 8 |
CA = Chronological age; TOWRE = Test of Word Reading Efficiency.
The majority of adults in the SLI (n = 9), SLI-history (n = 9) and ASD (n = 10) groups were part of longitudinal studies conducted at Oxford (Whitehouse et al., in press) or Manchester (Conti-Ramsden et al., 2007), and therefore childhood data were also available on these participants. Participants were tested during middle childhood (SLI: M 8.64, SD 2.14; SLI-history: M 8.23, SD 2.48; ASD: M 9.6, SD 2.7; P = 0.15). The behavioural assessment battery administered during childhood varied between participants, but all received at least one test of non-verbal ability and of receptive language ability. Non-verbal IQ in childhood was assessed by either the WISC-III (Wechsler, 1992) or Ravens Colored Progressive Matrices (Raven et al., 1986), while receptive language was gauged by the WISC-Revised (Wechsler, 1974), Test for Reception of Grammar (Bishop, 1982) British Picture Vocabulary Scale (Dunn et al., 1982) or BPVS-2 (Dunn et al., 1997). Participants’ scores on these measures were converted to a scaled score with an M of 100 and a SD of 15. Although it is not ideal to collate data from different tests, we undertook this procedure to obtain a general idea of level of childhood impairment. The three groups did not differ in childhood non-verbal IQ (SLI: M 107.94, SD 12; SLI-history: M 101.11, SD 17.09; ASD: M 93.55, SD 16.38; P = 0.15). There was, however, a significant difference on receptive language (P = 0.05). Bonferroni post-hoc tests found a trend for the ASD group (ASD: M 71.8, SD 0.97; P = 0.22) to have worse receptive language than the SLI group (M 82.67, SD 11.93; P = 0.1). The comparison between the ASD and SLI-history group (M 82.11, SD 9.44) also approached significance (P = 0.13). However, there was no difference between the SLI and the SLI-history groups (P = 1). As indicated in Table 1, some participants in the clinical groups had non-verbal IQ scores in the intellectually handicapped range (<70) when seen in the teenage/adult years. We retained these individuals in the study because they had all demonstrated normal range non-verbal IQ in childhood; a decline in non-verbal IQ with age is commonly reported in SLI (Botting, 2005), though it remains unclear whether this is a statistical artifact, a reflection of changing content of IQ tests with age, or a genuine decline.
To examine the validity of using the TOWRE criteria for language impairment at adulthood, we compared participant scores on other language tests for which data were available. Once again, there was variability in assessments at adulthood, but all participants in the SLI, SLI-history and ASD groups were administered some form of receptive and expressive language assessment. The participants recruited from the Manchester Language Study (SLI: n = 6; SLI-history: n = 4; ASD: n = 2) were administered either the third or fourth version of the Clinical Evaluation of Language Fundamentals (Semel et al., 1995, 2003), a comprehensive language assessment that generates separate indices for receptive and expressive language ability. All other participants were administered the Test for Reception of Grammar—Electronic (Bishop, 2005), an assessment of syntactic comprehension (receptive language), and the memory for sentences subtest of the NEPSY (Korkman et al., 1998), which requires participants to repeat sentences of increasing complexity (expressive language). All of these tests are widely used in the diagnosis of developmental language disorders. Participants’ scores on these four measures were converted to a scaled score with a M of 100 and a SD of 15. Scores from the two expressive language tasks were combined, as were the data from the two receptive language tasks. ANOVA revealed a main effect for group for both the receptive, F(2,30) = 11.11, P < 0.001, and expressive language scores, F(2,30) = 17.81, P < 0.001. Bonferroni post-hoc tests found that test scores were significantly higher (P < 0.01) in the SLI-history group (receptive language: M 98.67, SD 12.05; expressive language: M 88.78; SD 7.29) in comparison with the participants in the SLI (receptive language: M 69.73, SD 11.67; expressive language: M 65.64; SD 10.26) and ASD groups (receptive language: M 78, SD 17.04; expressive language: M 67.27; SD 10.18). There were no significant differences between the scores of the SLI and ASD groups (for both comparisons, P > 0.5). Individual scores on these measures are presented in an online appendix.
Apparatus and stimuli
Changes in blood flow velocity through the right and left MCAs were measured using a Doppler ultrasonography device (DWL Multidop T2: manufacturer, DWL Elektronische Systeme, Singen, Germany), requiring two 2 MHz transducer probes mounted on a flexible headset placed at the temporal skull windows. The experimental task was presented on a Dell laptop computer. Letters were used as stimuli and were presented in the middle of the screen in size 96 font. The experiment was controlled by Presentation software (Neurobehavioral systems), which sent marker pulses to the Multidop system to mark the start of each epoch.
Procedure
Participants were tested individually at the laboratory at Oxford University or in a quiet room at their home, sitting approximately 80 cm from a computer screen. The word generation paradigm used in this study is described by Knecht et al. (1998). Briefly, participants received a cue to attend to the screen, which was replaced after 5 s by a letter from the alphabet. Upon the presentation of the letter, participants were required to silently generate as many words starting with that letter as possible. Following a 15 s delay, participants received a cue to say out loud the words they thought of during the silent generation period. Figure 1 shows the timeline for this procedure, including the period of interest for Doppler recording. A total of 23 trials were presented, with Q, X and Z the only letters omitted. This procedure was approved by the Central University Research Ethics Committee of Oxford University and informed consent was obtained from each participant. All participants tolerated the Doppler headset and no other testing difficulties were encountered.
Fig. 1.
Timeline for the word generation task, also showing the period of interest for Doppler recording (LI period 8–18 s).
Data analysis
Data were analysed offline using the software, Average version 1.85 (Deppe et al., 1997). The blood flow envelope from each probe was downsampled at a rate of 25 Hz. Following an artefact rejection procedure, where epochs with unusually high or low levels of activity were removed from the data, time-locked epochs were averaged.
A laterality Index (LI) was computed and served as a measure of the direction and degree of laterality. Difference plots were created by subtracting the % cerebral blood flow velocity measured by the right probe averaged across all accepted trials, from that measured by the left probe. The LI was calculated as the mean blood flow velocity difference in a 2 s window centred on the peak value during the silent word generation phase of the task (Fig. 1). A positive LI indicated greater left than right hemisphere activation, with a negative index signifying the reverse. More extreme scores (i.e. strongly positive or negative) indicate a greater degree of laterality.
Results
The analyses first concentrate on the behavioral data, comparing the number of words generated by participants in each group, averaged across the 23 trials. A one-way ANOVA revealed a main effect of group, F(3,41) = 13.32, P < 0.001. Bonferroni post-hoc tests found that the Typical group (M 4.4, SD 0.9) generated significantly more words than the SLI (M 2.93, SD 0.62) and the ASD groups (M 2.22, SD 0.61), but not the SLI-history group (M 3.55, SD 1.18, comparison: P = 0.18). The ASD group generated significantly fewer words then the SLI-history group, but did not differ from the SLI group (P = 0.67). All differences were significant at the P < 0.01 level.
Analyses then turned to the fTCD data. The number of accepted epochs did not differ between the SLI (M 22, SD 2.1, range 16–23), SLI-history (M 22.44, SD 0.88, range 21–23), ASD (M 21.36, SD 2.66, range 15–23) and typical groups (M 22.36, SD 1.03, range 20–23), F(3,41) = 0.84, P = 0.54. ANOVA was then used to examine group differences in the mean activation recorded by the two Doppler probes (Table 2). There was no main effect for group in the measurements taken by either the right (P = 0.49) or left probe (P = 0.66). Table 2 shows that considerable within-group variability is likely to have contributed to the lack of group differences. Nevertheless, the lack of statistical significance suggests that there were no systematic differences between groups in the overall level of blood flow activity in the right or left hemisphere.
Table 2.
Mean (SD) and range of activation (% cerebral blood flow velocity) measured by the Doppler probes over the right and left MCAs for the four participant groups
| Left MCA | Right MCA | |
|---|---|---|
| SLI | 3.76 (4.93) | 2.04 (4.17) |
| Range | –3.43 to 9.2 | –3.89 to 7.91 |
| SLI-history | 1.17 (3.78) | 0.81 (5.05) |
| Range | –4.11 to 10.01 | –6.24 to 8.14 |
| ASD | 3.65 (3.88) | 1.9 (4.9) |
| Range | –2.6 to 7.53 | –5.74 to 10.69 |
| Typical | 1.77 (5.91) | –0.32 (5.61) |
| Range | –4.72 to 13.75 | –6.25 to 10.02 |
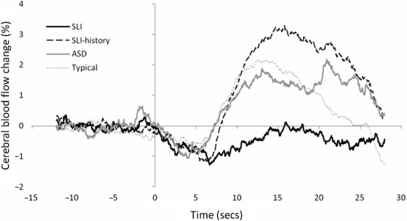
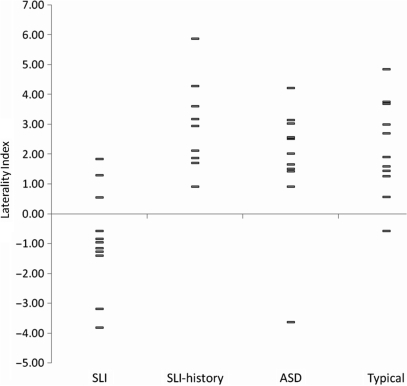
Figure 2 shows the difference plots of the four participant groups. Inspection of the figure suggests that the SLI group showed greater right than left hemisphere activation during the word generation task, while the three control groups showed the reverse. The mean LI of participants in the four groups, presented in Fig. 3, were compared using ANOVA. There was a significant main effect of group, F(3,41) = 9.61, P < 0.001. Bonferroni post-hoc tests found that the mean LI of the group with persisting SLI (M –0.85, SD 1.68) was significantly lower than that of the SLI-history (M 2.95, SD 1.52, Cohen's d = 2.37), ASD (M 1.76, SD 2.02, d = 1.4) and typical groups (M 2.20, SD 1.57, d = 1.88) (for all comparisons, P < 0.01). No other comparison reached significance. The same pattern of group differences is returned if the analysis is restricted to right-handed participants only, F(3,32) = 9.15, P < 0.001 (Bonferroni post-hoc tests were significant at P < 0.02 level).
Fig. 2.
Average difference in activation between the left and right MCAs averaged across all trials.
Fig. 3.
The LI of participants in each group. Each bar represents one participant. A positive LI indicates left hemisphere asymmetry for language function, while a negative score indicates right hemisphere lateralization.
The LI is a mean of up to 23 epochs and to determine whether this significantly differed from zero, a 95% confidence interval was computed around the LI of each participant. If confidence intervals overlapped with zero, the participants were deemed to have bilateral activation. Based on these criteria, 6 of 11 (54.5%) participants in the SLI group had language abilities lateralized to the right hemisphere, three had bilateral activation (27.3%) and two participants had left hemisphere lateralization (18.2%). Of the left-handed participants in this group (n = 3), one participant each had left, right and bilateral language function. In comparison, nine, nine and 10 participants in the SLI-history (100%), ASD (81.8%) and typical (90.9%) groups, respectively, had left lateralized language. One participant in each of the ASD and typical groups were found to have right hemisphere lateralization (9.1% of each group), while another ASD participant was deemed bilateral (9.1%). Chi-square analysis found these group differences to be statistically significant, χ2 = 21.83, df = 6, P < 0.001.
Eight of 42 participants in this study were either left-handed or ambidextrous. To examine the relation between handedness and cerebral lateralization (as measured by fTCD), we compared the LI between those with (n = 34) and without right-hand dominance (n = 8) independent of diagnosis. An independent samples t-test found no significant difference in cerebral lateralization for language between these two participant groups (right-handed participants: M 1.65, SD 2.31; left-handed/ambidextrous (M 0.58, SD 1.38), t(40) = 1.25, P = 0.22.
A final analysis examined whether there was any association between cerebral lateralization (i.e. the LI) and the mean number of words produced on the word generation task. There was no significant correlation between these measures in the SLI (r = –0.15, P = 0.65), SLI-history (r = –0.03, P = 0.93), ASD (r = –0.26, P = 0.43) or Typical samples (r = 0.29, P = 0.39), nor when the data were collapsed across these groups (r = 0.16, P = 0.32).
Discussion
We found a striking departure from normal patterns of cerebral lateralization in participants with SLI, which was specific to this group and not seen in participants with either a history of SLI or with language impairment associated with autism. This cannot be explained in terms of an excess of left-handers in the SLI group, since they were matched to the typical group on handedness. One question that is raised is whether the lack of lateralization might be a consequence rather than a cause of language impairment, reflecting the difficulty that the SLI group had in performing the word generation task. This seems unlikely for three reasons. First, studies that vary word retrieval difficulty in a within-subjects design have not found an effect of task difficulty on lateralization (Dräger and Knecht, 2002; Dräger et al., 2004). Second, the autism group did as poorly as the SLI group in the word generation task, yet showed strong left hemisphere language lateralization. Third, the current study found no significant association between word generation performance and lateralization.
The striking difference between the SLI group and the SLI-history group was surprising, given that both groups had childhood language difficulties severe enough to merit special education. One suggestion is that this might relate to severity of language impairment: in general, those whose language disorders resolve tend to have milder problems than those who persist (e.g. Stothard et al., 1998). It is possible, too, that there are qualitative differences in the type of language impairment between these two groups: in general, the poorest literacy outcomes are associated with classic SLI where there are major problems with structural aspects of language, and better outcomes are found in those with pragmatic language difficulties, who often have clinical features resembling those of autism, but in milder form (Whitehouse et al., in press). Further research will be needed to distinguish these possibilities, but what we can say is that the wide variation in findings on cerebral lateralization in previous structural and functional imaging studies may well be a consequence of inclusion of heterogeneous cases.
The data from the SLI group were also compared with data collected from a group of adults with ASD who had language deficits of comparable severity. As Fig. 3 shows, the vast majority of ASD participants had language function lateralized to the left-hemisphere, a clear difference to the participants in the SLI group. These data add neurological support to the accumulating evidence from family studies (Whitehouse et al., 2007) that the linguistic similarities between ASD and SLI may reflect the similar expression of different etiologies rather than a common neurobiological basis. Intriguingly, the differences between SLI and ASD in cerebral lateralization for language function are in contrast to the similarities that have been noted in structural studies of these populations (Herbert et al., 2005). Importantly, however, little is known about how structural abnormalities translate to differences in functionality; indeed structural asymmetries in children with SLI rarely correlate with the severity of language deficits (Gauger et al., 1997; Preis et al., 1998). Studies of SLI and ASD that use structural and functional neuroimaging techniques in combination will provide important data in this area.
The distributions of LIs for SLI versus other groups in Fig. 3 is reminiscent of Annett's right shift theory, where a subset of the population is predicted to have no bias to either side, while the remainder have a shift to left hemisphere lateralization (and right handedness). However, the notion that SLI is caused by lack of a right shift factor is inconsistent with strong evidence that atypical cerebral lateralization is entirely compatible with normal, or even superior, cognitive skills. In an impressively comprehensive study, Knecht et al. (2000) compared 264 individuals with left hemisphere language, 31 with bilateral language and 31 with right hemisphere language, as assessed by fTCD, on tests of academic achievement, artistic talent, verbal fluency, intelligence, speed of linguistic processing and mastery of foreign languages. No significant differences were found. We can make sense of these results if we assume that right-hemisphere or bilateral speech does not itself lead to language impairment, but rather is an indicator, albeit an imperfect one, of some other factor that is causally linked to disorder. To illustrate this point, we can use arguments developed to explain distributions of handedness. Suppose, following Laland et al. (1995) we assume that in the general population there is no genetic variation underlying human handedness, but rather a simple population bias that results in a strong, but not 100%, probability of right-handedness. Because hand preference in the general population is determined solely by chance, we expect to see no significant cognitive correlates of atypical handedness. But now suppose that there are pathological factors that can interfere with the normal bias to right-handedness. For instance, a focal brain lesion that randomly impairs function of the left or right side will alter the population handedness ratio towards 50 : 50 (Satz, 1973). If the brain lesion is rare, but has a large detrimental effect on cognitive performance, then we would expect a pattern of results analogous to that obtained here: atypical handedness would not be predictive of cognitive deficit, but cognitive deficit would be associated with atypical handedness. This example is used here because the logic has been well worked-through in the literature on pathological left-handedness (e.g. Bishop, 1984), but we would not wish to imply that focal brain damage is the mechanism underlying the atypical distribution of cerebral lateralization seen in the SLI group. Rather, given the strong genetic etiology of SLI, we would argue that genes that increase risk for SLI also disrupt the processes by which cerebral lateralization is usually established. Thus the argument is not that atypical cerebral lateralization is pathological in itself, but rather that there are pathological processes that lead to atypical cerebral lateralization, and at the same time impair language development.
An important limitation of the current study is the small sample sizes, which are a consequence of difficulties recruiting adults with a history of SLI (see Whitehouse et al., in press). Although this means there are large standard errors around estimates of means and proportions, it is important to note that all comparisons revealed highly significant differences with large effect sizes. It is also noteworthy that, consistent with previous research, group differences in handedness were negligible (Bishop, 2005) and could not explain LI differences. This confirms the view that handedness is too indirect a measure to be useful for the investigation of language lateralization.
In conclusion, the current study provides evidence that when SLI persists beyond adolescence, it is associated with reduced or reversed cerebral dominance. Comparisons with a group of language-impaired adults with ASD found that atypical lateralization is not simply related to poor language development but may be specific to the SLI population.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
This work was supported by the Wellcome Trust. Our sincerest thanks to the team from the Manchester Language Study—Zoë Simkin, Michelle St Clair and Gina Conti-Ramsden—for their help with participant recruitment and providing access to their database. We would like to thank all of the participants for taking part in this study, Helen Watt and Georgina Holt for their help in testing the participants and Emma Whitehouse for comments on an earlier draft of this manuscript.
Glossary
Abbreviations:
- ASD
autism spectrum disorders
- fTCD
functional transcranial Doppler
- MCA
middle cerebral artery
- SLI
specific language impairment
References
- Aaslid R, Markwalder TM, Nomes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–77. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Annett M. Left, right, hand and brain: the Right Shift Theory. Hillsdale, NJ: Erlbaum; 1985. [Google Scholar]
- Bernal B, Altman NR. Speech delay in children: a functional MR imaging study. Radiology. 2003;229:651–8. doi: 10.1148/radiol.2293021746. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Test for reception of grammar. Manchester: Department of Psychology: University of Manchester; 1982. [Google Scholar]
- Bishop DVM. Using nonpreferred hand skill to investigate pathological left handedness in an unselected population. Dev Med Child Neurol. 1984;26:214–26. doi: 10.1111/j.1469-8749.1984.tb04434.x. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Handedness and developmental disorder. Oxford: Blackwell Scientific and Philadelphia: J.B. Lippincott; 1990. [Google Scholar]
- Bishop DVM. The role of genes in the etiology of specific language impairment. J Commun Disord. 2002;35:311–28. doi: 10.1016/s0021-9924(02)00087-4. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Handedness and specific language impairment: a study of 6-year-old twins. Dev Psychobiol. 2005;46:362–39. doi: 10.1002/dev.20062. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Test for reception of grammar—electronic. London: Psychological Corporation; 2005. [Google Scholar]
- Bishop DVM, Snowling MJ. Developmental dyslexia and Specific Language Impairment: same or different? Psychol Bull. 2004;130:858–86. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Whitehouse AJO, Watt HJ, Line EA. Autism and diagnostic substitution: Evidence from a study of adults with a history of developmental language disorder. Dev Med Child Neurol. 2008;50:341–5. doi: 10.1111/j.1469-8749.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Botting N. Non-verbal cognitive development and language impairment. J Child Psychol Psychiatry. 2005;46:317–26. doi: 10.1111/j.1469-7610.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- Chiron C, Pinton F, Masure MC, Duvelleroy-Hommet C, Leon F, Billard C. Hemispheric specialization using SPECT and stimulation tasks in children with dysphasia and dystrophia. Dev Med Child Neurol: 1999;41:512–20. doi: 10.1017/s0012162299001139. [DOI] [PubMed] [Google Scholar]
- Cohen M, Campbell R, Yaghmai F. Neuropathological abnormalities in developmental dysphasia. Ann Neurol. 1989;25:567–70. doi: 10.1002/ana.410250607. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, Falcaro M, Simkin Z, Pickles A. Familial loading in specific language impairment: patterns of differences across proband characteristics, gender and relative type. Genes Brain Behav. 2007;6:216–28. doi: 10.1111/j.1601-183X.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Kochunov PV, Ingham RJ, Ingham JC, Mangin J-F, Rivière D, et al. Perisylvian sulcal morphology and cerebral asymmetry patterns in adults who stutter. Cerebral Cortex. 18:571–83. doi: 10.1093/cercor/bhm093. [DOI] [PubMed] [Google Scholar]
- Deppe M, Knecht S, Henningsen H, Ringelstein EB. AVERAGE: a Windows(R) program for automated analysis of event related cerebral blood flow. J Neurosci Methods. 1997;75:147–54. doi: 10.1016/s0165-0270(97)00067-8. [DOI] [PubMed] [Google Scholar]
- Deppe M, Knecht S, Papke K, Lohmann H, Fleischer H, Heindel W, et al. Assessment of hemispheric language lateralization: a comparison between fMRI and fTCD. J Cereb Blood Flow Metab. 2000;20:263–8. doi: 10.1097/00004647-200002000-00006. [DOI] [PubMed] [Google Scholar]
- Dräger B, Jansen A, Bruchmann S, Förster AF, Pleger B, Zwitserlood P, et al. How does the brain accommodate to increased task difficulty in word finding?: a functional MRI study. NeuroImage. 2004;23:1152–60. doi: 10.1016/j.neuroimage.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Dräger B, Knecht S. When finding words becomes difficult: is there activation of the subdominant hemisphere? NeuroImage. 2002;16:794–800. doi: 10.1006/nimg.2002.1095. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM, Whetton C, Pintilie D. British Picture Vocabulary Scale. Windsor: NFER-Nelson Publishing Co; 1982. [Google Scholar]
- Dunn LM, Dunn LM, Whetton C, Burley J. The British Picture Vocabulary Scale. 2nd. Windsor: NFER-Nelson; 1997. [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. J Speech Lang Hear Res. 1997;40:1272–84. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Heath M, Elliott D. Cerebral specialization for speech production in persons with Down syndrome. Brain Lang. 1999;69:193–211. doi: 10.1006/brln.1998.2131. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–26. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Bakardjiev A, Hodgson J, Adrien KT, et al. Larger brain and white matter volumes in children with developmental language disorder. Dev Sci. 2003;6:F11–F22. [Google Scholar]
- Jäncke L, Siegenthaler T, Preis S, Steinmetz H. Decreased white-matter density in a left-sided fronto-temporal network in children with developmental language disorder: evidence for anatomical anomalies in a motor-language network. Brain Lang. 2007;102:91–8. doi: 10.1016/j.bandl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jernigan T, Hesselink JR, Sowell E, Tallal P. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Arch Neurol. 1991;48:539–45. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E-B, et al. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Ebner A, Henningsen H, Huber T, Jokeit H, Ringelstein E.-B. Noninvasive determination of language lateralization by functional transcranial Doppler sonography: a comparison with the Wada test. Stroke. 1998;29:82–6. doi: 10.1161/01.str.29.1.82. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp SI. NEPSY: a developmental neuropsychological assessment. San Antonio: Psychological Corporation; 1998. [Google Scholar]
- Laland KN, Kumm J, Van Horn JD, Feldman MW. A gene-culture model of human handedness. Behav Genet. 1995;25:433–45. doi: 10.1007/BF02253372. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Given B, Virginia B, Eden G. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129:3329–42. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Lombardino LJ, Walsh K, Eckert MA, Mockler JL, Rowe LA, et al. Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. J Commun Disord. 2002;35:501–31. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- Lohmann H, Ringelstein EB, Knecht S. Functional transcranial doppler. In: Baumgartner RW, editor. Handbook on Neurovascular Ultrasound. Basel: Karger; 2006. pp. 251–60. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lou HC, Henriksen L, Bruhn P. Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Arch Neurol. 1984;41:825–9. doi: 10.1001/archneur.1984.04050190031010. [DOI] [PubMed] [Google Scholar]
- Lou HC, Henriksen L, Bruhn P. Focal cerebral dysfunction in developmental learning disabilities. Lancet. 1990;335:8–11. doi: 10.1016/0140-6736(90)90136-s. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Br J Psychol. 1971;66:53–9. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olsson B, Rett A. Shift to righthandedness in Rett syndrome around age 7. Am J Med Genet. 1986;24:133–41. doi: 10.1002/ajmg.1320250515. [DOI] [PubMed] [Google Scholar]
- Ors M, Ryding E, Lindgren M, Gustafsson P, Blennow G, Rosen I. SPECT findings in children with specific language impairment. Cortex. 2005;41:316–326. doi: 10.1016/s0010-9452(08)70269-7. [DOI] [PubMed] [Google Scholar]
- Orton JL, editor. The Orton Society; 1966. “Word-Blindness” in School children and other papers on strephosymbolia (Specific Language Disability—Dyslexia), 1925–1946, by Samuel Torrey Orton, MD. Towson, MD. [Google Scholar]
- Plante E, Swisher L, Vance R, Rapcsak S. MRI findings in boys with specific language impairment. Brain Lang. 1991;41:52–66. doi: 10.1016/0093-934x(91)90110-m. [DOI] [PubMed] [Google Scholar]
- Preis S, Jäncke L, Schittler P, Huang Y, Steinmetz H. Normal intrasylvian anatomical asymmetry in children with developmental language disorder. Neuropsychologia. 1998;36:849–55. doi: 10.1016/s0028-3932(98)00033-5. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Raven's Coloured Progressive Matrices. London: H.K. Lewis; 1986. [Google Scholar]
- Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. An examination of lateralisation in children with high-functioning autism and Asperger's disorder. J Autism Dev Disord. 2002;32:321–32. doi: 10.1023/a:1016387020095. [DOI] [PubMed] [Google Scholar]
- Satz P. Left-handedness and early brain insult: an explanation. Neuropsychologia. 1973;11:115–17. doi: 10.1016/0028-3932(73)90071-7. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WH. Clinical evaluation of language fundamentals—Third edition (CELF-3). San Antonio, TX: Psychological Corporation; 1995. [Google Scholar]
- Semel E, Wiig EH, Secord WH. Clinical evaluation of language fundamentals—Fourth edition (CELF-4). San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Shafer VL, Schwartz RG, Morr ML, Kessler KL, Kurtzberg D. Deviant neurophysiological asymmetry in children with language impairment. Neuroreport. 2000;11:3715–8. doi: 10.1097/00001756-200011270-00025. [DOI] [PubMed] [Google Scholar]
- Stothard SE, Snowling MJ, Bishop DVM, Chipchase BB, Kaplan CA. Language impaired preschoolers: A follow-up into adolescence. J Speech Lang Hear Res. 1998;41:407–18. doi: 10.1044/jslhr.4102.407. [DOI] [PubMed] [Google Scholar]
- Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–62. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philos Trans R Soc London [Biol] 2003;358:303–14. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Wagner R, Rashotte C. Test of word reading efficiency (TOWRE). New York: Psychological Corporation; 1999. [Google Scholar]
- Trauner D, Wulfeck B, Tallal P, Hesselink J. Neurological and MRI profiles of children with developmental language impairment. Dev Med Child Neurol. 2000;42:470–5. doi: 10.1017/s0012162200000876. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Heim A, Zilbovicius M, Gerard C, Mazoyer BM. Abnormal regional CBF response in left hemisphere of dysphasic children during a language task. Pediatr Neurol. 1994;10:20–6. doi: 10.1016/0887-8994(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Third UK edition. London: Psychological Corporation; 1992. [Google Scholar]
- Wechsler D, Chen H-Y. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Whitehouse AJO, Barry JG, Bishop DVM. The broader language phenotype of autism: A comparison with Specific Language Impairment. J Child Psychol Psychiatry. 2007;48:822–30. doi: 10.1111/j.1469-7610.2007.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJO, Barry JG, Bishop DVM. Further defining the language impairment of autism spectrum disorders: is there a specific language impairment subtype? J Comm Dis. 2008;41:319–36. doi: 10.1016/j.jcomdis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJO, Line EA, Watt HJ, Bishop DVM. Qualitative aspects of developmental language impairment relates to language and literacy outcome in adulthood. Int J Lang Comm Dis. doi: 10.1080/13682820802708080. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.