Abstract
OFF-l-dopa dyskinesias have been a surprising side-effect of intrastriatal foetal ventral mesencephalic transplantation in patients with Parkinson's disease. It has been proposed that excessive and unregulated dopaminergic stimulation of host post-synaptic striatal neurons by the grafts could be responsible for these dyskinesias. To address this issue we transplanted foetal dopaminergic neurons from mice lacking the dopamine transporter (DATKO) or from wild-type mice, into a rat model of Parkinson's disease and l-dopa-induced dyskinesias. Both wild-type and DATKO grafts reinnervated the host striatum to a similar extent, but DATKO grafts produced a greater and more diffuse increase in extra-cellular striatal dopamine levels. Interestingly, grafts containing wild-type dopaminergic neurons improved parkinsonian signs to a similar extent as DATKO grafts, but provided a more complete reduction of l-dopa induced dyskinesias. Neither DATKO nor wild-type grafts induced OFF-l-dopa dyskinesias. Behavioural and receptor autoradiography analyses demonstrated that DATKO grafts induced a greater normalization of striatal dopaminergic receptor supersensitivity than wild-type grafts. Both graft types induced a similar downregulation and normalization of PEnk and fosb/Δfosb in striatal neurons. In summary, DATKO grafts causing high and diffuse extra-cellular dompamine levels do not per se alter graft-induced recovery or produce OFF-l-dopa dyskinesias. Wild-type dopaminergic neurons appear to be the most effective neuronal type to restore function and reduce l-dopa-induced dyskinesias.
Keywords: Parkinson's disease, transplantation, dyskinesia, dopamine, synapse
Introduction
l-dopa-induced dyskinesias (LIDs) are the most disabling side-effects of pharmacological treatment in patients with Parkinson's disease (Fahn, 2003). According to the most accepted hypothesis, LIDs are caused by the pulsatile, non-physiological dopaminergic stimulation of denervated striatal medium spiny neurons during chronic l-dopa therapy (for review see Olanow et al., 2006). Studies in the 6-OHDA rat model of Parkinson's disease have demonstrated that LID are associated with alterations of synaptic plasticity in corticostriatal synapses (Picconi et al., 2003). l-dopa treatment restores striatal long-term potentiation (LTP) lost after dopaminergic denervation, but in rats with LID these synapses do not depotentiate following LTP (Picconi et al., 2003). Various pieces of evidence have linked striatal LTP and synaptic depotentiation with the activity of D1 and NMDA receptors (Chase and Oh, 2000; Oh and Chase, 2002). Additionally, these aberrant responses have been associated with upregulation of preproenkephalin (PEnk) (Fisher et al., 1991), prodynorphin (PDyn), and with changes in gene expression of fosB/ΔfosB-related transcription factors in the post-synaptic striatal neurons (Cenci et al., 1998; Andersson et al., 1999; Lundblad et al., 2004; Pavon et al., 2006).
Intrastriatal foetal ventral mesencephalic (VM) transplantation can be effective in improving both parkinsonian signs and LID in patients with Parkinson's disease (Lindvall et al., 1994; Olanow et al., 2003; Mendez et al., 2005). In rodent models, foetal grafted dopaminergic neurons restore regulated dopamine (DA) release (Strecker et al., 1987; Forni et al., 1989; Di Loreto et al., 1996; Stromberg et al., 2000; Bjorklund and Isacson, 2002), and normalize the expression of fos/ΔfosB-related genes, PEnk and PDyn (Lee et al., 2000; Maries et al., 2006) in striatal areas reinervated by the graft. These biochemical effects re-establish a balanced striatal neurotransmission in areas reinervated by the grafts, which are associated with significant behavioural improvements (Lee et al., 2000; Steece-Collier et al., 2003; Maries et al., 2006). Surprisingly, double-blind clinical trials of human VM transplantation showed lack of efficacy in some groups of grafted Parkinson's disease patients, and the presence of a form of OFF-l-dopa dyskinesia (Freed et al., 2001; Olanow et al., 2003). It was proposed that excessive and unregulated production of DA from grafted foetal neurons affecting non-reinervated striatal areas could be responsible for the generation of the OFF-l-dopa dyskinesias (Freed et al., 2001; Maries et al., 2006), but the molecular mechanisms underlying these side-effects are not known. To address this issue we have implanted reuptake-deficient dopaminergic neurons in the striatum of 6-OHDA-lesioned rats.
The dopamine transporter (DAT) plays a major role in the regulation of DA handling at striatal synapses, and mice lacking the DA transporter have a 5-fold increase in basal striatal extracellular DA levels due to the absence of reuptake (Jones et al., 1998a). These high levels are possible because neither degradative enzymes nor other monoamine transporters can compensate for the lack of reuptake thereby decreasing the rate of DA clearance from the extra-cellular space by about 300 times (Jones et al., 1998a). In this scenario, each DA molecule released has a much longer diffusion range (up to several milimetres) (Jones et al., 1998a). Due to this lack of reuptake, and despite an increased synthesis rate, pre-synaptic DA levels inside the terminals are <10% of normal levels, leading to a dramatic reduction of the DA pool available for depolarization-evoked release (Jones et al., 1998a).
In order to investigate the consequences of an excessive and poorly regulated dopaminergic striatal reinervation from foetal grafts, we used the transplantation of foetal VM cells from DATKO mice into the striatum of 6-OHDA-lesioned, l-dopa-primed dyskinetic rats. Behavioural studies, in vivo microdialysis, receptor autoradiography and molecular and histological analyses were carried out to compare the functional effects of DA replacement therapy based on the re-introduction of DA synapses with either a wild-type profile of DA release and uptake, or a situation in which a disrupted uptake of DA by the implanted neurons induced high extra-synaptic DA levels in denervated, l-dopa-primed dyskinetic rats.
Material and Methods
Animals
Adult female Sprague–Dawley rats with unilateral 6-OHDA lesions (medial forebrain bundle injections; Charles River Breeding Laboratories. Wilmington, MA), were included in this study. All animal procedures were performed in accordance with the guidelines of the National Institute of Health and were approved by the Institutional Animal Care and Use Committee (IACUC) at McLean Hospital, Harvard Medical School. Animals were housed according to standard conditions, in a dark/light cycle of 12 h, with ad libitum access to food and water.
Behavioural analysis
Drug-induced rotations
Rotational behaviours in response to amphetamine (4 mg/kg i.p), and apomorphine (0.1 mg/kg i.p) were measured prior to transplantation and at different time-points post-transplantation (post-tx) (Fig. 1). Rats were randomly placed into plastic bowls for 90 or 40 min (amphetamine or apomorphine tests) and left and right full-body turns were quantified by a computerized system. The net rotational asymmetry score was expressed as full-body turns per minute (Galpern et al., 1996).
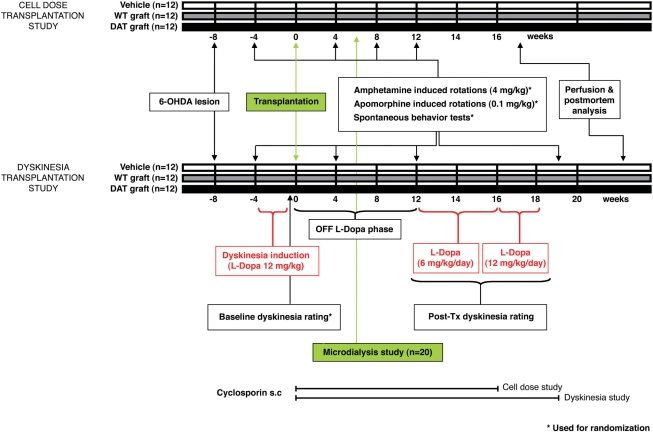
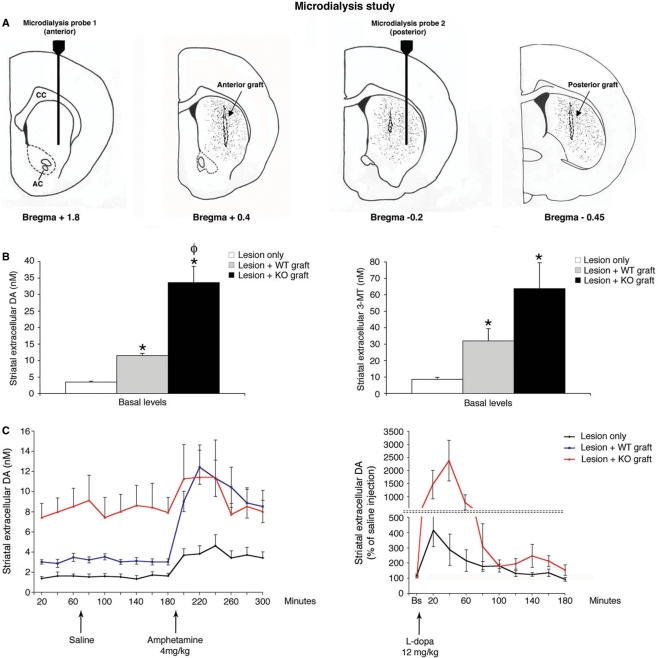
Fig. 1.
Experimental design. 6-OHDA rats, non-primed (cell dose study), or primed with l-dopa (dyskinesia study), were used. According to baseline behavioural evaluations, rats were randomly allocated into three groups to receive wild-type or DATKO grafts, or saline. The effects of transplantation in parkinsonism (cell dose and dyskinesia studies), and l-dopa induced dyskinesias (dyskinesia study), were studied beginning 4 weeks post-tx. In a parallel experiment, 6-OHDA-lesioned rats were used to analyse the effects of both graft types in striatal extra-cellular DA levels.
Cylinder test
Spontaneous forelimb use was assessed at baseline, 12 and 19 weeks post-tx, using the cylinder test (Schallert, 1999). Animals were placed in a plastic cylinder and the number of left and right forepaw contacts with the cylinder wall were counted. Left (-impaired) forepaw contacts were presented as percentage of total wall contacts. Lesioned rats showing >20% left forelimb use before transplantation were excluded.
Dyskinesia scale
Recordings and scoring of abnormal involuntary movements (AIMs) were performed independently by two trained observers, blinded to the experimental condition. Abnormal movements were videotaped at 15, 40, 80 and 120 min after l-dopa administration. For each time point, animal behaviour was recorded for 2 min with the rats in their home cage, and additional 2 min with each rat placed alone in a clean empty rat cage. AIMs were scored according to a rat dyskinesia scale (Lee et al., 2000). Briefly, four different dyskinetic behaviours; orolingual dyskinesias (choreo-athetoid movements of jaw and tongue), limb dyskinesias (choreo-dystonic movements of the forelimb), trunk-hindlimb dystonia (including loose of postural reflexes) and locomotive dyskinesias (small radius rotations contralateral to the lesion side) were scored from 0 to 4 for each subtype, according to the time and severity of each AIM (Lee et al., 2000). The maximum score for each test was 64. Enhancement of normal behaviours was not included in the rating.
Spontaneous dyskinesias
Spontaneous behaviours were observed daily in the home cages during 10 min periods by researchers blinded to the experimental conditions. At baseline and 3, 7 and 18 weeks post-tx, l-dopa-primed rats, were subjected to a stressful situation (hung from the tail for 20 s) and then placed in a transparent cylinder. Stress-induced behaviour was recorded for 3 min and spontaneously occurring dyskinetic behaviours were scored using the AIMs scale. Observations during l-dopa phase were made 72 h after l-dopa administration.
LIDs
During a pre-transplantation induction period of 21 days rats received daily intraperitoneal (IP) injections of l-dopa methyl ester (12 mg/kg, Sigma-Aldrich) combined with the peripheral dopa decarboxylase inhibitor benserazide–HCl (15 mg/kg, Sigma-Aldrich). Baseline dyskinetic behaviours were scored at Day 21 of l-dopa administration, and after a single l-dopa challenge 7 days later. Rats with AIM scores <20 were not used for transplantation. Twelve weeks post-tx l-dopa treatment was reassumed. From weeks 12–15 post-tx animals received a daily dose of 6 mg/kg of l-dopa and 15 mg/kg of benserazide (low dose), and at weeks 16 and 17 a daily dose of 12 mg/kg and 15 mg/kg of benserazide (high dose). Higher l-dopa doses were not tested as it has been shown that doses >12 mg do not elicit further dyskinesias in the 6-OHDA rat model of PD (Lee et al., 2000). AIMs were rated once a week during the low-dose phase and twice a week during the high-dose phase.
Apomorphine-induced dyskinesias
AIMs induced by apomorphine administration in l-dopa-primed dyskinetic rats, were scored at 4 and 19 weeks post-tx using the AIMs score. Two trained researchers, blinded to the experimental conditions, observed the rats over 2-min periods 5, 15, 30 and 45 min post injection. The first three observations were made with the rats in the plastic bowls used for apomorphine-induced rotations, and the last one was done in the home cage. Contraversive rotations were only scored at the home cage 45 min post injection.
VM dissection, cell suspension preparation and transplantation procedures
Homozygote breeding pairs (DATKO or littermate wild-type) were used for each experiment. E12.5 pregnant females were terminally anaesthetized by an IP injection of sodium pentobarbital (60 mg/kg, Sigma, MO), and VM dissection and cell suspension preparation were carried out as previously described (Haque et al., 1997). A confirmatory PCR was carried out with the remaining tissue from each embryo used for transplantation.
Rats were pre-anaesthetized with an i.m injection of acepromazine/atropine sulphate (3.3 mg/kg, Boehringer Ingelheim Vetmedica and 0.2 mg/kg, Phoenix Scientific, respectively), and anaesthetized with i.m injection of ketamine/xylazine (60 mg/kg, Fort Dodge and 3 mg/kg, Phoenix Scientific, respectively). Rats were placed in a Kopf stereotaxic frame (Kopf Instruments) and VM cell suspension or vehicle (HBSS-glucose 0.2% DNAse) was injected in four deposits distributed in 2 AP tracts, using a 22-gauge, 10 µl Hamilton syringe. From bregma: (i) AP + 0.4, L −3, V −5.5 and −4.5; (ii) AP − 0.5, L −3.6, V −5.5 and −4.5. In the cell dose transplantation study, a group of rats (n = 24) received a total of 135 000 DATKO or wild-type VM cells or vehicle (‘Low cell dose’). An additional group (n = 14) received 500 000 DATKO or wild-type VM cells (‘High cell dose’). In the dyskinesia transplantation study (n = 24) 6-OHDA lesioned rats, dyskinetic after chronic treatment with l-dopa, received 250 000 wild-type or DATKO VM cells or vehicle. Starting the day before surgery, rats were immunosuppressed with subcutaneous injections of cyclosporine A (15 mg/kg/day, Sandimmune; Sandoz, East Hannover, NJ). Eight weeks post-tx the dose was reduced to 12 mg/kg/day.
In vivo microdialysis and HPLC
Twenty 6-OHDA lesioned rats were grafted with 250 000 wild-type or DATKO cell suspensions using the same coordinates described above. Eight weeks post-tx, graft survival was assessed by amphetamine and paw-reaching tests. Rats showing graft-mediated behavioural recovery, and 6-OHDA-lesioned controls were used for in vivo microdialysis. Ten weeks post-tx, two dialysis probes (3 mm membrane length, 0.24 mm external diameter, Cuprophane, 6 kDa cut-off, CMA-11; CMA/Microdialysis, Solna, Sweden) were attached to the stereotaxic frame with the tips separated by 2 mm and implanted into the grafted striatum. The posterior probe was implanted lateral and anterior to the posterior graft at coordinates AP 0, M-L −4, D-V −6.5 relative to bregma. The anterior probe was implanted 2 mm anterior to the posterior probe (1.6 mm anterior and 1 mm lateral to the anterior graft). Twenty-four hours after surgery, the dialysis probes were connected to a syringe pump and perfused with artificial cerebrospinal fluid (aCSF) (NaCl 147 mM, KCl 2.7 mM, CaCl2 1.2 mM, MgCl2 0.85 mM; CMA Microdialysis). To reliably determine the basal extra-cellular DA levels in the striatum of freely moving rats a quantitative ‘low perfusion’ rate microdialysis experiment was conducted (Gainetdinov et al., 2003). After an equilibration period for at least 1 h, the perfusate was collected at a perfusion rate of 0.1 µl/min every 60 min into collection tubes containing 2 µl of 0.5 N perchloric acid. To analyse the effects of amphetamine and l-dopa on the extra-cellular DA levels in striatum, a ‘conventional’ microdialysis method (perfusion flow rate 1 µl/min) in freely moving animals was employed 48 h after surgery (Gainetdinov et al., 2003). In these experiments, dialysis probes were equilibrated for 1 h using 1 µl/min CSF perfusion and samples were collected every 20 min into collection tubes containing 2 µl of 1 N perchloric acid. At this perfusion rate, three samples were collected as baseline, six samples after IP injection of saline solution and six samples after IP injection of amphetamine 4 mg/kg. The same procedures were repeated in a separate group (n = 8) of 6-OHDA-lesioned, DATKO grafted animals and 6-OHDA-lesioned controls and 48 h after probe implantation, the perfusate was collected every 20 min at 1 µl/min, for 1 h before and 3 h after IP injection of l-dopa 12 mg/kg + Benserazide 15 mg/kg.
Perfusate samples were assayed for DA and 3-methoxytyramine using HPLC with electrochemical detection. DA was separated on a microbore Unijet C18 reverse-phase column (C-18, 5 lm, 1 × 150 mm; BAS, West Lafayette, IN, USA) with a mobile phase consisting of 0.03 M citrate–phosphate buffer with 2.1 mM octyl sodium sulphate, 0.1 mM EDTA, 10 mM NaCl and 17% methanol (pH 3.6) at a flow rate of 90 μl/min and detected by a 3-mm glass carbon electrode (Unijet; BAS) set at + 0.8 V. The injection volume was 5 µl. The sensitivity of the method permitted detection of ∼3 fmol DA.
Post-mortem analyses
Rats were terminally anaesthetized by an IP injection of sodium pentobarbital (100 mg/kg) and intracardially perfused with ice-cold heparin saline (0.1% heparin in 0.9% saline) followed by 4% paraformaldehyde. Immunohistochemistry and stereological procedures were performed as in Redmond et al., 2008 (see Supplementary material). For the analysis of fosB/ΔfosB and pDARPP-32 (Thr34) expression, two sections were analysed (AP + 0.4 and −0.5) from 6-OHDA-lesioned, dyskinetic, grafted (wild-type and DATKO) and non-grafted animals. Two images form equivalent dorsolateral and dorsomedial striatal locations were taken from each section at either 20 × (pDARPP-32) or 40 × (fosB/ΔfosB), and equivalent images were taken from the contralateral, non-lesioned striatum. Quantification of fosB/ΔfosB and pDARPP-32 optical density (OD) was performed using NIH image 1.61 software.
In situ hybridization studies were performed as previously described (Henry et al., 1999) (see Supplementary material).
Receptor-binding studies
Fresh-frozen brains were cut into coronal sections (10 µm thick) using a cryostat (ThermoShandon) at −20°C onto Superfrost slides (Fisher Scientific, Hampton, NH). Slides were kept at −30°C for 1–3 months prior to use in autoradiography experiments. In the following experiments, three slides (nine sections) were used per animal from the striatal region containing the graft, for each binding assay. Experiments were performed as previously described (Cha et al., 1999) (see Supplementary material).
Results
Transplantation of foetal VM cell suspensions from wild-type and DATKO mice into non-l-dopa-primed 6-OHDA-lesioned rats
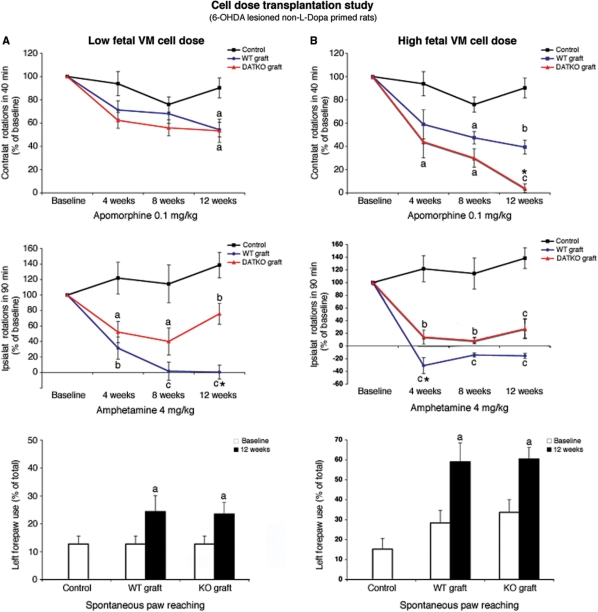
In the first experiment, 135 000 VM cells were grafted per rat. Both wild-type and DATKO transplantation groups showed a progressive recovery in apomorphine-induced contralateral rotations that reached significance versus controls at 12 weeks post-tx, without significant differences between wild-type and DATKO grafted rats (Fig. 2A, P > 0.05, see figure legends). Both wild-type and DATKO grafted rats showed significant recovery on amphetamine-induced ipsilateral rotations compared to controls, that started at 4 weeks post-tx and were maintained for the entire follow-up period (Fig. 2A, P < 0.05). Rats grafted with wild-type cells, but not those grafted with DATKO cells, showed a complete recovery of ipsilateral rotations at 8 and 12 weeks post-tx. After these ‘low cell dose’ transplantation, contralateral rotations were present in wild-type but not in DATKO grafted rats (Supplementary Fig. 1). The difference between transplantation groups was significant at 12 weeks post-tx (Fig. 2A, P < 0.05). Spontaneous motor activity evaluated with the paw-reaching test showed a significant improvement in the use of the contralateral forepaw in both the wild-type and the DATKO transplantation groups, 12 weeks post-tx (Fig. 2A, P < 0.05). No spontaneous dyskinesias were observed in any of the grafted rats during daily observations or in stress-induced tests (see Materials and methods section).
Fig. 2.
Behavioural effects of wild-type and DATKO grafts in 6-OHDA-lesioned rats. (A) Low cell dose (125 000 cells/host). Both graft types significantly reduced apomorphine-induced rotations at 12 weeks post-tx (one-way ANOVA and Tukey–Kramer multiple comparisons test). Both graft types significantly reduced amphetamine induced rotations at all time points tested, and wild-type grafts reduced rotations significantly more than DATKO grafts, 12 weeks post-tx (one-way ANOVA and Tukey–Kramer multiple comparisons test). Both grafts improved significantly the use of the contralateral forepaw in the cylinder test. (B) High cell dose (500 000 cells/host). Both graft types significantly reduced apomorphine induced rotations, and improvement of apomorphine-induced rotations was larger in DATKO grafted animals than in wild-type grafted animals 12 weeks post-tx (one-way ANOVA and Tukey–Kramer multiple comparisons test). Both graft types significantly reduced amphetamine induced rotations at all time points tested, but only wild-type grafted animals showed an on-average preference for turning contralateral to the lesioned hemisphere. Both grafts significantly improved the use of the contralateral forepaw in the cylinder test. Significance in grafted versus controls aP < 0.05; bP < 0.01; cP < 0.005. *P < 0.05 in wild-type versus DATKO grafted animals.
A second group of 6-OHDA-lesioned rats were grafted with 500 000 wild-type or DATKO cells, to simulate conditions with a larger number of DA neurons and presumably higher level of extra-cellular DA. DATKO grafted rats showed a significant improvement in apomorphine-induced contralateral rotations versus control rats that started 4 weeks post-tx, and became complete 12 weeks post-tx (Fig. 2B, P < 0.05). Wild-type grafted animals, on the other hand, did not show a significant recovery until 8 weeks post-tx, with a maximum improvement of ∼60%. The difference between transplantation groups was significant at 12 weeks post-tx (Fig. 2B, P < 0.05). Wild-type grafted animals showed a complete recovery of amphetamine-induced rotations, and on average a preference to rotate towards the contralateral unlesioned hemisphere, at all post-tx times tested (Fig. 2B, P < 0.05). In contrast, DATKO grafted rats significantly improved amphetamine-induced rotations but contralateral rotations were almost absent (Fig. 2B, P < 0.05, and Supplementary Fig. 1). Both wild-type and DATKO grafted rats normalized their use of the contralateral forepaw during the cylinder test performed 12 weeks post-tx (Fig. 2C, P < 0.05). Even after transplantation of 500 000 cells/rat, no spontaneous dyskinetic behaviours were observed in any of the groups at any of the observation time points.
Transplantation of foetal VM cell suspensions from wild-type and DATKO mice into 6-OHDA-lesioned, l-dopa-primed dyskinetic rats
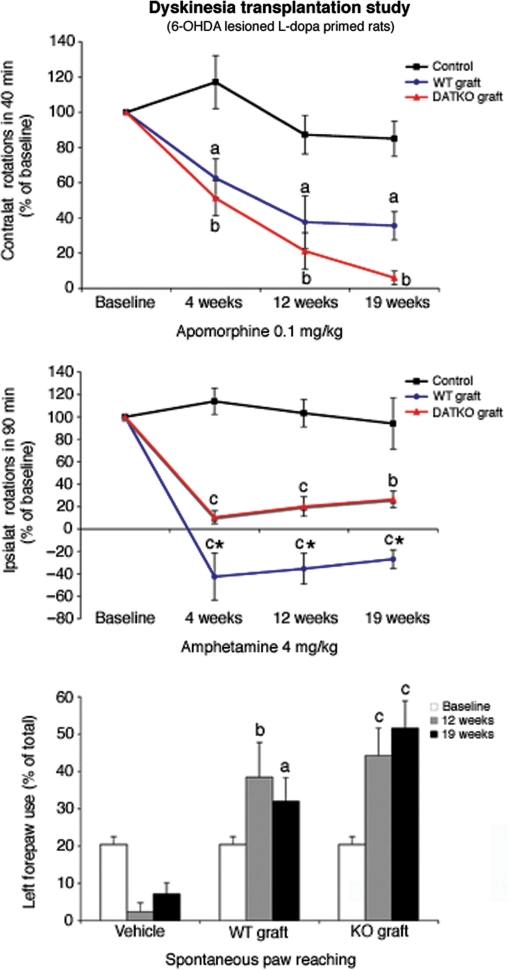
Based on the above studies, a cell dose of 250 000 cells/host was chosen as appropriate for the rest of the study. 6-OHDA-lesioned rats rendered dyskinetic and grafted with both wild-type and DATKO cells showed significant recovery in apomorphine and amphetamine-induced rotations compared to control rats, at all time points studied (Fig. 3, P < 0.05). Similar to the results in non-dyskinetic rats, DATKO grafts, but not wild-type grafts induced a full recovery of apomorphine-induced rotations at 12 weeks post-tx (Fig. 3, P < 0.05). Both wild-type and DATKO grafted animals showed significant reductions of amphetamine-induced rotations at all time points studied, but the reduction in wild-type grafted animals was significantly more pronounced than in DATKO grafted animals, including an on-average preference for turning contralateral to the lesioned hemisphere (Fig. 3, P < 0.05 and Supplementary Fig. 1). Evaluations 19 weeks post-tx showed that the recovery of motor asymmetries after apomorphine and amphetamine administration was not altered by post-tx l-dopa treatment (Fig. 3).
Fig. 3.
Behavioural effects of wild-type and DATKO grafts in 6-OHDA-lesioned, l-dopa-primed rats. Both graft types significantly reduced apomorphine induced rotations at all time points (one-way ANOVA and Tukey–Kramer multiple comparisons test). Both graft types significantly reduced amphetamine induced rotations at all time points, and the reduction in wild-type grafted animals was significantly larger than that in DATKO grafted animals (one-way ANOVA and Tukey–Kramer multiple comparisons test). Wild-type grafted animals showed a bias towards the contralateral unlesioned hemisphere in all time points. Both grafts improved significantly the use of the contralateral forepaw in the cylinder test.
Analysis of post-transplantation dyskinesias in 6-OHDA-lesioned, l-dopa-primed, dyskinetic rats
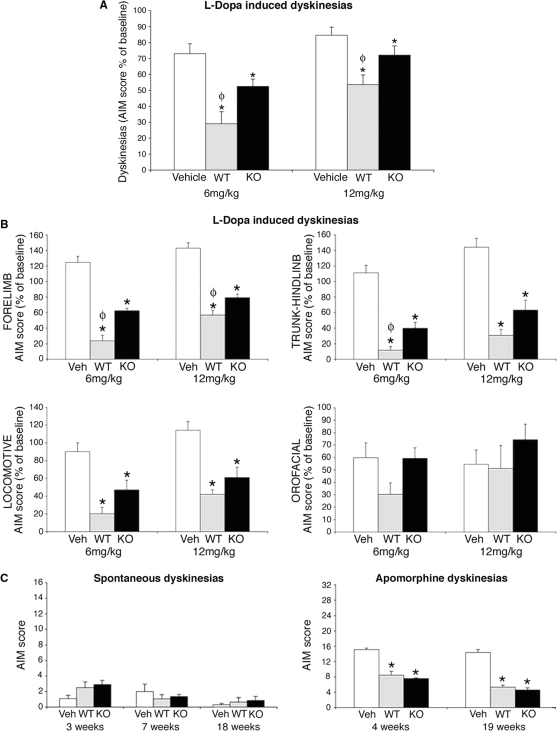
No spontaneous dyskinetic events were observed during daily observations. In the evaluation of spontaneous dyskinesias at 3, 7 and 18 weeks post-tx (see Materials and methods section), rats that received wild-type and DATKO cells showed, 3 weeks post-tx, very mild (<3/16 in AIM score) dyskinetic behaviours (mainly rotational behaviours) that were not different from those shown by vehicle injected rats at the same times of evaluation (Fig. 4C). During apomorphine tests 4, and 19 weeks post-tx, wild-type and DATKO grafted rats showed significantly fewer dyskinesias than surgical controls at both time points, without significant differences between the two transplantation groups (Fig. 4C).
Fig. 4.
Effects of wild-type and DATKO grafts in LID. (A) Both wild-type and DATKO grafts induced a significant recovery of LID at both l-dopa doses tested (one-way ANOVA and Tukey–Kramer multiple comparisons test. Wild-type and DATKO versus vehicle P < 0.001). Wild-type grafts reduced LID significantly more than DATKO grafts with both l-dopa doses tested (P < 0.05). (B) Both graft types significantly improved forepaw, trunk-hindlimb and locomotive dyskinesias but not orofacial dyskinesias compared to surgical controls (one-way ANOVA and Tukey–Kramer multiple comparisons test. Forelimb and findlimb P < 0.01 versus vehicle; Locomotive wild-type versus vehicle P < 0.001, DATKO versus vehicle P < 0.05 for 6 mg/kg and P < 0.01 for 12 mg/kg). Wild-type grafts improved forepaw dyskinesias (6 and 12 mg/kg doses) and trunk-hindlimb dyskinesias (6 mg/kg dose) significantly more than DATKO grafts (one-way ANOVA and Tukey–Kramer multiple comparisons test. Forelimb P < 0.001 for 6 mg/kg and P < 0.05 for 12 mg/kg. Trunk-hindlinb P < 0.05) (C) In both transplantation groups, very mild spontaneous dyskinesias were observed, that were not different from those seen in surgical controls. Dyskinetic behaviours observed during the apomorphine test (four time points. Locomotive dyskinesias evaluated only in the last time point with animals in their homecage. Scores 0–52), were milder in both graft groups compared with surgical controls (one-way ANOVA and Tukey–Kramer multiple comparisons test).
LIDs were studied using two doses: 6 and 12 mg/kg (the l-dopa dose used as pre-tx baseline). There was a significant recovery in LIDs in both transplantation groups compared to sham surgical control rats using both l-dopa doses, with improvements in all dyskinetic behaviours analysed except for orolingual dyskinesia (Fig. 4A and B). Similarly to what is observed in Parkinson's disease patients, there was a significantly different effect of the two doses in the generation of LIDs post-tx, shown by the significantly lower dyskinesias induced by 6 mg/kg of l-dopa compared to 12 mg/kg, in the three experimental groups (Fig. 4A, ‘dose effect’ t student P < 0.001). Interestingly, improvement in LIDs was larger in rats grafted with wild-type cell suspensions than in rats grafted with DATKO cell suspensions for both l-dopa doses tested (Fig. 4A). By symptoms, forelimb choreic movements after both l-dopa doses and trunk dystonic movements after 6 mg/kg of l-dopa were significantly more reduced in wild-type than in DATKO grafted rats (Fig. 4B).
Effects of foetal VM cell transplantation on extra-cellular striatal DA levels
In 6-OHDA-lesioned rats that received sham surgery, basal extra-cellular striatal DA levels were, on average, 3.45 ± 0.28 nM (3.63 ± 0.38 nM in anterior probe and 3.26 ± 0.48 nM in posterior probe; Supplemantary Fig. 2) as determined by quantitative ‘low perfusion rate’ microdialysis approach (Fig. 5B). Basal extra-cellular DA levels in 6-OHDA-lesioned rats grafted with 250 000 wild-type cells were, on average, 11.5 ± 0.7 nM (10.38 ± 0.38 nM in anterior probe; 12.63 ± 0.94 nM in posterior probe; Supplementary Fig. 2), a 3-fold increase compared to basal levels of 6-OHDA-lesioned surgical controls (Fig. 5B). Transplantation of 250 000 DATKO cells induced a 10-fold increase in basal extra-cellular DA levels in the striatum compared with surgical controls (33.6 ± 5.2 nM. Fig. 5B, P < 0.001; 29.22 ± 8 in anterior probe and 38.06 ± 5.8 in posterior probe; Supplementary Fig. 2). These levels were 3-fold higher than basal extra-cellular DA levels measured in the striatum of wild-type grafted animals (Fig. 5B, P < 0.05). In conventional microdialysis experiments, injection of amphetamine 4 mg/kg caused an ∼2-fold increase in 6-OHDA-lesioned surgical controls (Fig. 5C), and a 4-fold increase in rats that received wild-type cells, whereas rats that received DATKO cells did not show a significant increment in extra-cellular DA compared to saline injection (1.2-fold, Fig. 5C). In a parallel experiment, administration of 12 mg/kg of l-dopa induced a 24-fold increase in extra-cellular DA levels of DATKO grafted rats that returned to baseline levels 180 min post-injection. 6-OHDA-lesioned surgical controls showed a 4-fold increase in extra-cellular DA levels that lasted for 120 min (Fig. 5C). Striatal transplantation of a mixture of wild-type and DATKO cells (35–65%, respectively), induced an increase in basal and amphetamine-induced DA levels, between those found in wild-type and DATKO grafted rats (data not shown), demonstrating a gene dose dependence of these graft-induced extra-cellular DA increases. Measures of the extra-cellular DA metabolite 3-methoxytyramine (3-MT) in striatal perfusates of all experimental groups showed similar changes to those seen in extra-cellular DA. Extracellular levels of 3-MT were on average 8.6 ± 1.2 nM in sham surgical controls, whereas animals grafted with wild-type and DATKO cells showed 3.5- (32 ± 7.5 nM) and 7-fold (63 ± 15.7 nM) increases in extra-cellular 3-MT, respectively (Fig. 5B). These levels of 3-MT were significantly higher in DATKO grafted animals than in sham surgical controls (Fig. 5B, P < 0.05). These results are in agreement with previous studies in DATKO mice that demonstrated that extra-cellular 3-MT levels reflect extra-cellular DA levels, and that both are increased in DATKO mice (Gainetdinov et al., 2003).
Fig. 5.
Changes in striatal extra-cellular DA measured by in-vivo microdialysis. (A) Schematic representation of probes and grafts placement in the microdialysis experiments. (B) Basal extra-cellular levels of DA and 3-methoxytyramine (3-MT) measured in striatal perfusates of freely moving rats. Data are expressed as mean ± SEM of samples from both anterior and posterior probes. Samples were obtained every 60 min during 6 h, at a 0.1 µl/min perfusion rate. *P < 0.05 wild-type versus controls, P < 0.001 DATKO versus controls, φP < 0.05 DATKO versus wild-type (Kruskal–Wallis and Dunn's multiple comparisons test). (C) DA extra-cellular levels after amphetamine injection (4 mg/kg i.p) were obtained every 20 min for 2 h at 1 µl/min perfusion rate. Amphetamine induced a significant increase in extra-cellular DA levels in wild-type grafted animals, whereas no increases were seen in DATKO grafted animals (repeated measures ANOVA and Tukey–Kramer multiple comparison's test). DA extra-cellular levels after l-dopa injection (12 mg/kg i.p), were obtained every 20 min for 3 h at 1 µl/min. l-dopa induced a significantly larger increase in extra-cellular DA levels in DATKO grafted animals than in 6-OHDA-lesioned surgical controls (Kruskal–Wallis and Dunn's multiple comparisons test).
Post-mortem analyses
Histological analysis of the grafts
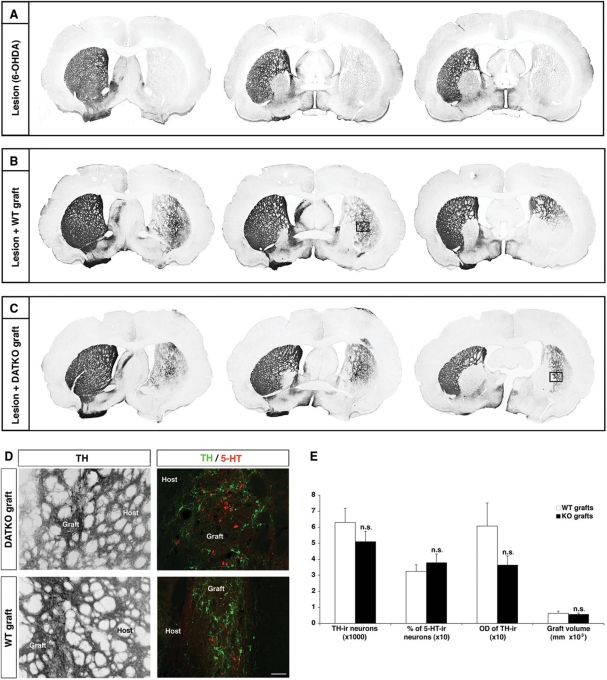
Rats from cell dose and dyskinesia transplantation studies were sacrificed 12 and 19 weeks post-tx, respectively (Fig. 1). Post-mortem analysis of both wild-type and DATKO grafts showed slender, well-integrated grafts extending dorso-ventrally and reinervating adjacent striatal areas (Fig. 6B and C). There were no differences in graft location analysed as distance from bregma, and according to its dorso-ventral and medio-lateral locations (Supplementary Fig. 3). Stereological estimations of graft volume, and optical density of TH-immunoreactive (-ir) striatal reinervation did not show significant differences between transplantation groups (Fig. 6E). Similarly, stereological estimations of the number of surviving TH-ir neurons in the grafts revealed no significant differences between wild-type and DATKO grafted animals (6320 ± 884 for wild-type versus 5121 ± 643 for DATKO, P = 0.28, Fig. 6E). Both DATKO and wild-type grafts had the typical morphology of foetal VM grafts, with TH-ir neurons preferentially distributed along the graft-host border, and some cell clusters located towards the centre of the graft (Fig. 6D). Cell type analysis with specific markers showed a similar distribution of dopaminergic cell subpopulations within both graft types, with A9-like dopaminergic neurons (TH+/Girk2+) preferentially located in the graft-host border and A10-like (TH+/Calb+) dopaminergic neurons preferentially located in the centre of the graft (data not shown). Serotoninergic neurons were present in both wild-type and DATKO grafts, with a tendency to be located in central areas of the graft. In both types of grafts serotoninergic neurons were ∼35% of all monoaminergic neurons (32.5 ± 0.41 for wild-type and 37.9 ± 0.53 for DATKO grafts. Fig. 6D and E).
Fig. 6.
Immunohistochemical analysis of wild-type and DATKO striatal grafts. (A–C) Low-magnification photomicrographs of coronal sections immunostained with TH at different AP levels, showing the location of surviving grafted dopaminergic neurons and DA striatal reinnervation. (D) Left panels show high-magnification photomicrographs of foetal grafts from boxes in B and C. In right panels, representative photomicrographs from double immunofluorescence stainings showing the distribution of dopaminergic and 5-HT neurons within the grafts. Scale bar = 100 µm. (E) Stereological analyses showed no difference in graft sizes, striatal innervation (measured by TH-OD), surviving dopaminergic neurons and percentage of 5-HT neurons (from total number of monoaminergic neurons in the grafts), between wild-type and DATKO grafts (NS = non-significant. Student t-test P > 0.05).
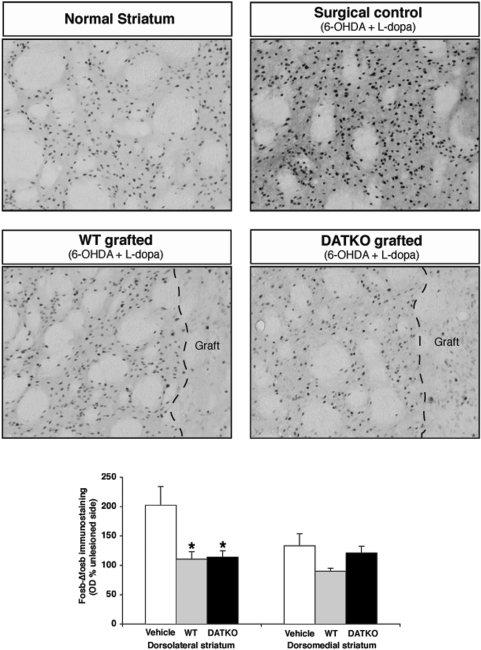
There was a regional specific increase in fosB/ΔfosB expression in the denervated striatum of 6-OHDA-lesioned, l-dopa-primed rats compared with the unlesioned striatum. In dorsolateral, but not in dorsomedial striatum of DA-depleted, l-dopa-primed, sham surgery controls there was a 2-fold increase in the OD of fosB/ΔfosB immunoreactivity compared to the unlesioned striatum (Fig. 7). Both graft types significantly reduced this increase in fosB/ΔfosB immunoreactivity in the dorsolateral striatum (one-way ANOVA and Tukey–Kramer post hoc test, P < 0.005, Fig. 7). There were no differences in the reduction of fosB/ΔfosB immunoreactivity between wild-type and DATKO grafted animals (Fig. 7).
Fig. 7.
Analysis of FosB/ΔfosB striatal expression. High-magnification photomicrographs of fosB/ΔfosB immunostaining from 6-OHDA-lesioned, dyskinetic animals, grafted with wild-type, DATKO and surgical controls. Two sections were analysed per animal (AP +0.4 and –0.5). Photomicrographs (40×) from dorsolateral and dorsomedial striatum were obtained form grafted and unlesioned sides and the OD of fosB/ΔfosB immunoreactivity was quantified. Values are expressed as percentage of contralateral unlesioned side. *P < 0.05. One-way ANOVA and Tukey–Kramer multiple comparisons test.
Analysis of p-DARPP-32 (Thr34) immunostaining also revealed an increase in the denervated striatum of 6-OHDA-lesioned, l-dopa-primed rats compared with the unlesioned striatum (+20%) (Supplementary Fig. 4). Both graft types significantly reduced this increase in the dorsolateral striatum (one-way ANOVA and Tukey–Kramer post hoc test, P < 0.01 wild-type graft, P < 0.05 DATKO graft). There was no difference in the reduction of p-DARPP-32 immunostaining between wild-type and DATKO grafted animals (Supplementary Fig. 4).
Receptor binding autoradiography
Analysis of DAT receptor binding demonstrated a complete reduction of specific binding in the lesioned striatum of 6-OHDA-lesioned rats (data not shown). Specific binding for DAT was observed in the grafted striatum of rats grafted with wild-type cells, but not in DATKO grafts, consistent with an absence of DAT expression in cells derived from DATKO mice (data not shown).
DA D1, D2 and NMDA-receptor binding sites were quantitatively analysed in the ipsilateral and contralateral striata in the following groups of rats: unlesioned, 6-OHDA-lesioned, 6-OHDA-lesioned receiving wild-type or DATKO grafts, l-dopa-treated 6-OHDA-lesioned dyskinetic rats and 6-OHDA-lesioned dyskinetic rats receiving wild-type or DATKO transplants. Densitometric analysis was performed in the whole striatum, dorso-lateral, medio-lateral, ventro-lateral and ventro-medial quadrants and at increasing distances away from the graft-host border. The results are summarized in Supplementary Table 1.
DA D1 receptors
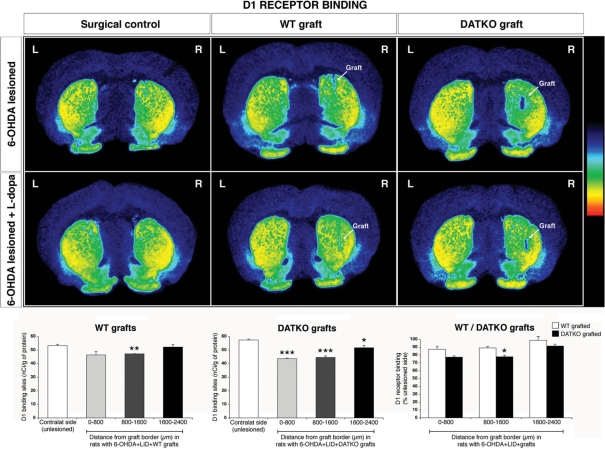
Following 6-OHDA-lesioning alone or 6-OHDA-lesioning and l-dopa priming, there was no change in DA D1 receptor-binding sites in the whole striatum compared with unlesioned animals. In dyskinetic 6-OHDA-lesioned rats that received either wild-type or DATKO grafts, DA D1 receptors in the whole striatum were significantly decreased compared to ungrafted dyskinetic 6-OHDA-lesioned animals (−17%, P < 0.001, wild-type graft; −19%, P < 0.001, DATKO graft; Fig. 8 and Supplementary Table 1A). Analysis of DA D1-binding sites adjacent to or further away from the graft border in dyskinetic rats grafted with DATKO cells showed that D1 receptor-binding sites were significantly reduced at all distances measured [0–800 μm (P < 0.001), 800–1600 μm (P < 0.001) and 1600–2400 μm (P < 0.05)] compared with the unlesioned contralateral side. In comparison, in the wild-type-grafted striatum, D1 receptor-binding sites were significantly reduced only at 800–1600 μm (P < 0.01) (Fig. 8 and Supplementary Table 1A).
Fig. 8.
Changes in DA D1 receptors in the grafted striatum. Pseudo-colour transformations of autoradiographs of DA D1 receptor binding, in 6-OHDA-lesioned animals and 6-OHDA-lesioned + l-dopa, following grafting with either wild-type or DATKO VM cells. Receptor-binding sites at distances adjacent to or further away from the graft border were quantified and each distance was compared with the contralateral unlesioned side (*P < 0.05, **P < 0.01, ***P < 0.0001, unpaired t-test).
A comparison of the degree of reduction of DA D1 receptor-binding sites between wild-type and DATKO-grafted striatum at distances adjacent to or further away from the graft border (data expressed as percentage of contralateral side) demonstrated that there was a significantly greater reduction in DATKO-grafted striatum compared to wild-type-grafted striatum at 800–1600 μm (P < 0.05) in dyskinetic grafted animals (Fig. 8 and Supplementary Table 1A). Similar changes were observed in 6-OHDA-lesioned, non-l-dopa-primed rats (see Supplementary material).
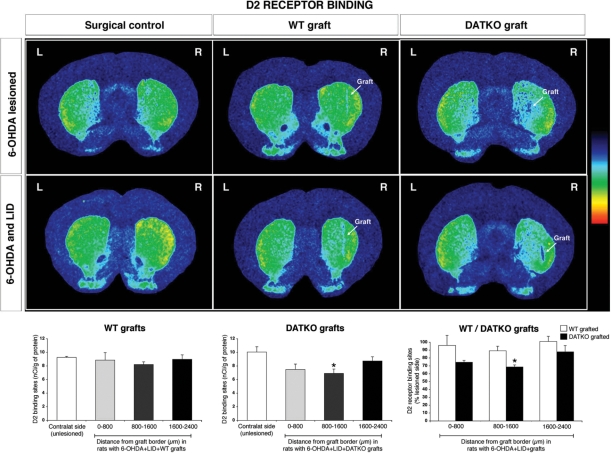
DA D2 receptors
Following 6-OHDA-lesioning alone or 6-OHDA lesion and l-dopa priming, there was no alteration in DA D2 receptor-binding sites in the whole striatum (Fig. 9 and Supplementary Table 1B). In dyskinetic l-dopa-treated 6-OHDA-lesioned rats receiving wild-type or DATKO grafts, DA D2 receptor-binding sites in the whole striatum were significantly decreased compared to ungrafted dyskinetic surgical controls (−19%, P < 0.01, wild-type graft; −26%, P < 0.001, DATKO graft) (Fig. 9 and Supplementary Table 1B). Analysis of DA D2-binding sites adjacent to and further away from the graft border in dyskinetic rats grafted with DATKO cells, showed that D2 receptor-binding sites were significantly reduced compared to the contralateral side of the same animals at 800–1600 μm (P < 0.05) (Fig. 9 and Supplementary Table 1B). In comparison, in the wild-type-grafted striatum, D2 receptor-binding sites were unchanged.
Fig. 9.
Changes in DA D2 receptors in the grafted striatum. Pseudo-color transformations of autoradiographs of DA D2 receptor binding in 6-OHDA-lesioned animals and 6-OHDA-lesioned + l-dopa, following grafting with either wild-type or DATKO VM cells. Receptor-binding sites at distances adjacent to or further away from the graft border were quantified and each distance was compared with the contralateral unlesioned side. (*P < 0.05, **P < 0.01, ***P < 0.0001, unpaired t-test).
A comparison of the reduction of DA D2 receptor-binding sites between wild-type and DATKO-grafted striatum (data expressed as percent of contralateral side), showed that there was a significantly greater reduction in DATKO than wild-type-grafted striatum at 800–1600 μm (P < 0.05) in dyskinetic animals (Fig. 9 and Supplementary Table 1B). Similar changes were observed in 6-OHDA lesioned, non- l-dopa-primed rats (see Supplementary material).
NMDA glutamate receptors
In 6-OHDA-lesioned animals, NMDA glutamate receptor-binding sites in the whole striatum were significantly increased (+18%, P < 0.05) compared to unlesioned animals (Supplementary Table 1C and Supplementary Fig. 5). In l-dopa-treated dyskinetic animals that received either wild-type or DATKO grafts, NMDA receptors in the whole striatum were significantly reduced compared to 6-OHDA-lesioned, dyskinetic surgical controls (−9.1%, P < 0.001, wild-type graft; −14%, P < 0.001, DATKO graft; Supplementary Table 1C and Supplementary Fig. 5). Analysis of NMDA receptor-binding sites adjacent to and further away from the graft border in dyskinetic rats grafted with wild-type or DATKO cells showed that NMDA receptor-binding sites were significantly reduced compared to 6-OHDA-lesioned, dyskinetic surgical controls at 0–800 μm and 800–1600 μm (P < 0.01 for 0–800 μm and 800–1600 μm in wild-type grafted animals, and P < 0.01 for 0–800 μm and P < 0.001 for 800–1600 μm in DATKO grafted animals; Supplementary Table 1C and Supplementary Fig. 5).
A comparison of NMDA receptor-binding sites between wild-type and DATKO-grafted striatum (data expressed as percentage of contralateral side) showed that there was no significant difference between wild-type and DATKO-grafted striatum in l-dopa-treated dyskinetic rats (Supplementary Table 1C and Supplementary Fig. 5).
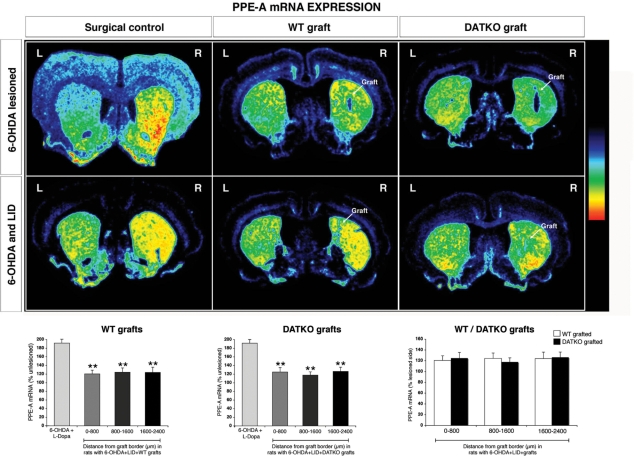
PEnk mRNA expression
Densitometric analysis of PEnk mRNA expression was performed in a similar manner to the receptor-binding studies. In l-dopa-primed 6-OHDA-lesioned rats grafted with either wild-type or DATKO-grafted animals, PEnk mRNA expression was significantly reduced compared to 6-OHDA-lesioned, l-dopa-primed, ungrafted animals (−55%, P < 0.01, wild-type-graft; −49%, P < 0.05, DATKO-graft) (Supplementary Table 1D). Analysis of PEnk mRNA expression adjacent to and further away from the graft border in l-dopa-treated dyskinetic rats grafted with either wild-type or DATKO cells showed that they were significantly reduced compared to 6-OHDA-lesioned, dyskinetic surgical controls at the three distances analysed (P < 0.01 for both wild-type and DATKO grafted animals; Fig. 10 and Supplementary Table 1D).
Fig. 10.
Changes in preproenkephalin in the grafted striatum. Pseudo-color transformations of in situ hybridization for preproenkephalin (PEnk; PPE-A), in 6-OHDA-lesioned animals and 6-OHDA-lesioned + l-dopa, following grafting with either wild-type or DATKO VM cells. mRNA PEnk expression at distances adjacent to or further away from the graft border was quantified and compared to the expression in 6-OHDA-lesioned, dyskinetic surgical controls. In addition, receptor-binding sites and mRNA expression, presented as a percentage of the unlesioned contralateral side, were compared between wild-type and DATKO grafted animals at distances near to and further away from the graft border (*P < 0.05, **P < 0.01, ***P < 0.0001, unpaired t-test).
A comparison of the reduction of mRNA expression between wild-type and DATKO-grafted striatum (data expressed as per cent of contralateral side) showed that there was no significant difference in l-dopa-treated dyskinetic rats receiving wild-type- and DATKO-grafts at any of the distances analysed.
Discussion
The study provides several major findings: (i) striatal transplantation of foetal VM tissue as a cell suspension improves both parkinsonism and LID in a 6-OHDA rat model of Parkinson's disease; (ii) drug induced behavioural tests showed a more complete normalization of DA receptor supersensitivity in DATKO grafted animals than in wild-type grafted animals, that was confirmed by receptor-binding studies; (iii) DATKO grafts produced large increases in extra-cellular DA levels throughout the denervated striatum that were 3-fold higher than those produced by wild-type grafts, and 10-fold higher than extra-cellular DA levels in denervated controls; (iv) DATKO grafts did not induce spontaneous dyskinesias and did not worsen the severity of previously present LID, demonstrating a lack of effect of high DA tone on LIDs. Both graft types reduced LID compared with sham surgical controls. Interestingly, grafts containing wild-type dopaminergic neurons reduced LID more than grafts containing DATKO dopaminergic neurons; and (v) Both wild-type and DATKO grafts induced a similar downregulation of PEnk, fosb/Δfosb and NMDA in striatal neurons of lesioned, l-dopa-primed dyskinetic rats. These conclusions and findings are discussed in detail below.
Integration of foetal VM grafts in the denervated striatum
Neural transplantation provides a new approach to Parkinson's disease therapy based on the idea that new neurons can provide functional neurotransmission and structural reconstruction of circuitry. DA replacement by neural transplantation is produced by newly generated terminals of grafted neurons into the denervated striatum, which contrast with the classic pharmacological approaches based on the unregulated effects of dopaminergic dugs in the striatum. Early work in the field demonstrated that foetal donor neurons can orient axonal growth towards their normal synaptic targets due to the presence of specific guidance cues in the adult host brain (Isacson et al., 1995; Isacson and Deacon, 1996). More specifically, recent studies in rats (Thompson et al., 2005), monkeys (Redmond et al., 2008) and Parkinson's disease patients (Mendez et al., 2005) have shown that different types of dopaminergic neurons (A9/SNc and A10/VTA neurons) with specific distribution within foetal grafts are able to reinervate their natural targets in the denervated striatum. Moreover ultrastructural studies of synapses formed by transplanted foetal dopaminergic neurons on host striatal medium spiny neurons indicate that these new synapses have a normal morphology and similar location within medium spiny neurons, although compared to normal striatum they show an increased tendency to be located on dendritic shafts rather than on dendritic spines (Freund et al., 1984, 1985; Bolam et al., 1987). Additionally, these new terminals provide a hyperinnervation of striatal cholinergic interneurons but it remains to be determined whether such changes are detrimental, or possibly result in a more efficacious synaptic transmission as postulated by Freund et al. (1984). Finally, microdialysis and electrophysiological studies have revealed the presence of normal pre- and post-synaptic control mechanisms in these newly generated synapses (Zetterstrom et al., 1986; Strecker et al., 1987; Fisher et al., 1991). As predicted by all these observations, foetal cell transplantation has been shown to reduce motor deficits in animal models (Perlow et al., 1979; Bjorklund et al., 1982; Redmond et al., 2008) and Parkinson's disease patients (Freed et al., 1992; Lindvall, 1998; Hauser et al., 1999; Piccini et al., 1999; Mendez et al., 2005). Unexpectedly, recent double-blind clinical trials in grafted Parkinson's disease patients showed cases of lack of efficacy and the occurrence of OFF-l-dopa dyskinesias (Freed et al., 2001; Olanow et al., 2003). These trials have revealed a need for in-depth studies of the physiological and molecular changes caused by dopaminergic denervation and chronic l-dopa therapy, and the effects of foetal grafts in this altered corticostriatal motor circuitry.
Striatal DATKO and wild-type foetal transplantation induce different improvements of parkinsonian signs
Both graft types induced a full recovery of spontaneous motor performance as demonstrated by the lack of side bias in the paw-reaching test, but pharmacological tests showed important differences in the functional impact of wild-type as compared to DATKO grafts. Amphetamine administration in animals that received wild-type grafts induced a marked reduction of the ipsilateral rotations seen pre-transplantation, and the appearance of contralateral rotations. In contrast DATKO grafted animals showed an almost complete recovery of amphetamine-induced rotations without the occurrence of overcompensatory contralateral rotations (Supplementary Fig. 1). Microdialysis experiments demonstrated a 4-fold increase in DA levels in the wild-type grafted striatum after amphetamine administration. This is in agreement with previous studies of in vivo microdialysis in grafted, 6-OHDA-lesioned rats (Zetterstrom et al., 1986; Herman et al., 1993). As expected, due to the need of DAT for amphetamine-mediated release (Jones et al., 1998b), microdialysis experiments confirmed that in DATKO grafted animals amphetamine administration induced a minimal increase of striatal DA. This increase was of similar magnitude to that seen in denervated controls and probably originated in the remaining host terminals. Thus, as extra-cellular DA levels prior to amphetamine were 3-fold higher in DATKO grafted striatum; extra-cellular DA levels after amphetamine administration were similar in both grafted groups (Fig. 6). The overcompensatory rotational response in wild-type grafted animals to amphetamine administration reflects an excessive activity of the grafted striatum, probably related to post-synaptic supersensitivity of non-reinervated striatal neurons, that are stimulated by DA released by the graft in response to amphetamine that diffuses to non-reinervated striatal areas. The lack of contralateral rotations in animals with DATKO grafted neurons despite the presence of similar extra-cellular DA levels compared to wild-type grafts, suggests that the high DA levels created by DATKO grafts also reached non-reinervated striatal areas producing a permanent reduction of DA receptor supersensitivity in these areas. This reduction in DA receptor supersensitivity seen in DATKO grafted animals is most likely due to the increase in the striatal volume covered by DA released by each terminal, resulting in volume transmission as described in DATKO mice (Jones et al., 1998a). Consistent with this, contralateral rotations in response to the full DA agonist apomorphine were significantly but not completely reversed by wild-type grafts (60% reduction), whereas animals grafted with DATKO cell suspensions showed an almost complete recovery of contralateral apomorphine-induced rotations. This indicates that wild-type VM grafts normalize DA transmission in reinervated but not in non-reinervated areas (as shown in Stromberg et al., 2000), whereas DATKO grafts (that reinervated ∼40% of the denervated striatum) normalized DA receptor activity beyond the reinervated areas.
These differences between the effects of wild-type and DATKO grafts in striatal DA receptors were in part explained by receptor binding analysis of striatal DA D1 and D2 receptors, which showed that while both graft types induced a significant downregulation of DA receptors compared with denervated controls, DATKO grafts had a more complete effect on DA receptors in areas around the graft, and a larger area of DA receptor downregulation. This effect on DA receptors was likely mediated both by DA reinervation and by the high extra-cellular levels of DA generated by the graft that reached non-reinnervated striatal areas by volume transmission, and normalized the activity of extrasynaptic DA receptors (Yung et al., 1995), and subsequently also improved drug-induced behaviours. The normalization of amphetamine- and apomorphine-induced responses with partial recovery of LID seen in DATKO-grafted animals (see below) indicates that this improvement, mediated by diffusion of extra-cellular DA to distant neurons, does not reflect a full structural and functional recovery of corticostriatal transmission. Supporting this idea, electrophysiological studies have shown partial normalizations in DA receptor activity in areas not reinervated by the grafts (Stromberg et al., 2000). Additionally, the current work and other studies (Fisher et al., 1991; Lane et al., 2006) show that foetal VM grafts can induce some improvements in amphetamine-induced rotational behaviours before grafted foetal neurons fully reinervate striatal neurons.
Both wild-type and DATKO grafts improved LIDs and did not induce OFF-l-dopa dyskinesias
OFF-l-dopa dyskinesias
6-OHDA-lesioned rats not primed with l-dopa and grafted with wild-type or DATKO cells did not show any sign of OFF- l-dopa dyskinesias, even when 500 000 cells were grafted per animal. In the rat model of LID, fully denervated animals show a high sensitivity to the fluctuations of extra-cellular DA induced by l-dopa treatment (Dethy et al., 1999), often showing dyskinetic behaviours even after the first l-dopa dose (Winkler et al., 2002 and Vinuela et al., unpublished data). This effect is even more dramatic when l-dopa is administered directly in the striatum of 6-OHDA-lesioned rats (Carta et al., 2006). In the present study, the permanently high extra-cellular DA levels produced by large DATKO grafts did not induce any type of spontaneous dyskinesias. Moreover, the lack of dyskinesias in animals grafted with large DATKO grafts, with the subsequent high extra-cellular DA levels in areas not reinervated by foetal DA fibres, supports the notion that tightly regulated DA release from foetal grafts does not produce spontaneous abnormal movements by itself.
A detailed study of spontaneous and stress-induced OFF- l-dopa behaviours only revealed mild dyskinetic behaviours during the first weeks post-tx and they were of the same magnitude and distribution in the transplantation groups and in l-dopa-primed sham surgical controls. Even during the l-dopa treatment phase no spontaneous dyskinesias were observed in any of the groups. In previous studies of OFF- l-dopa dyskinesias and foetal VM transplantation in this rat model, only one reported mild spontaneous dyskinetic behaviours in the first weeks post-tx (Lane et al., 2006), but after these early stages none of the studies reported spontaneous dyskinesias (Lee et al., 2000; Steece-Collier et al., 2003; Carlsson et al., 2006; Lane et al., 2006; Maries et al., 2006). The occurrence of these early dyskinetic behaviours is, most likely, related to processes of cell maturation and integration and with surgery-related events.
The possibility of OFF-l-dopa dyskinesias after striatal foetal VM cell transplantation has been underscored by two recent double-blind clinical trials testing the efficacy of fetal VM cell transplantation as solid pieces into the striatum of Parkinson's disease patients (Freed et al., 2001; Olanow et al., 2003). As a possible explanation for these dyskinesias, it has been suggested that small grafts would produce ‘hot spots’ of striatal DA reinervation, that would normalize striatal supersensitivity in a limited area and at the same time would give rise to a ‘spill-over’ of DA, released in a non-regulated neuro-humoral manner (Freed et al., 2001; Hagell and Cenci, 2005; Maries et al., 2006). This unregulated DA release would reach supersensitive receptors in striatal areas not reinervated by the graft and generate abnormal movements. This possibility has been also examined by transplantation studies in rodents that linked this graft-mediated effect on supersensitive post-synaptic terminals, with an incomplete focal pattern of striatal reinervation by these grafts. In contrast, such ‘hot spots’ described using PET (Ma et al., 2002), were not correlated with the occurrence of OFF- l-dopa dyskinesias in other clinical studies (Hagell et al., 2002). In the current study, microdialysis demonstrated that high DA levels released by DATKO grafts cover almost all striatum, also involving striatal areas not reinervated by the graft. Notably, this situation, which resembles a neurohumoral DA release, did not elicit OFF- l-dopa dyskinesias. The lack of dyskinesias and other behavioural changes in DATKO grafted rats, despite the presence of high striatal extra-cellular DA levels, contrast with the severe side-effects seen in Parkinson's disease patients after intraventricular injection of DA. These patients showed different combinations of dyskinesias, myoclonic jerks, severe depression and psychotic behaviours that disappeared when DA infusion was discontinued (Venna et al., 1984). A similar response has been reported in 6-OHDA-lesioned rats in which intrastriatal infusion of l-dopa elicited LID even in non- l-dopa-primed rats (Carta et al., 2006). In conclusion, our findings indicate high extra-cellular DA levels produced by striatal foetal grafts may not be responsible for the generation of OFF- l-dopa dyskinesias, even when they diffuse to non-reinervated striatal areas. Other aspects of these trials related with the surgical technique or cell preparation, and the striatal lesion and inflammation induced by them, are more likely to be related with this unexpected side-effect.
LIDs
Both graft types produced an overall reduction in LID compared to sham surgical controls. These results, and those of previous studies using wild-type rat cell suspensions (Lee et al., 2000; Carlsson et al., 2006; Lane et al., 2006; Maries et al., 2006) indicate that striatal foetal neural transplantation reduces LID when sufficient striatal DA reinervation and synaptic regeneration is achieved. In the presence of local mechanisms of regulation of DA release, presynaptic DA terminals from the graft take up l-dopa, decarboxylate it to DA and in the case of wild-type neurons, release it in a physiologically normal manner (see our microdialysis data and Zetterstrom et al., 1986). This contrasts with the situation in the denervated striatum without transplants, where l-dopa is decarboxylated in serotonin terminals, glial cells and blood vessels and DA is released in a non-regulated way primarily as a function of fluctuating levels of l-dopa, therefore exposing hypersensitive post-synaptic terminals to pulsatile DA levels (Djaldetti et al., 1996) leading to LID (Stocchi et al., 2005). Critical to understanding the neurobiology of dyskinesia in the context of DA transmission, dyskinesia reduction was also seen in DATKO grafted animals, which emphasizes the possibility that appropriate and complete striatal DA reinervation and not DA extra-cellular levels is the key factor for functional recovery and reduction of existing LID. New DATKO graft derived DA terminal networks in the host striatum re-establish synaptic function, yet DA levels are 10-fold higher than in the striatum of 6-OHDA lesion alone, and 3-fold higher than in the wild-type grafted striatum. In this situation of lack of reuptake, a dose of l-dopa induced a dramatic increase of extra-cellular DA, that was more pronounced than that seen in non-grafted denervated animals (see Results section and Dethy et al., 1999). Notably, wild-type grafts induced a significantly larger reduction of LID compared to DATKO grafted animals, which contrast with the broader and more complete down regulation of DA receptors, amphetamine and apomorphine tests by DATKO grafts. A possible explanation for this difference between wild-type and DATKO grafts would be that the neurotransmission provided by grafted wild-type dopaminergic neurons is likely to be more precise, and would have a more A9-(SNc-) like autoinhibition of DA levels, similar to normal or homeostatic DA levels at the terminals in the striatum, whereas DATKO neurons would mimic an A10- (VTA-) like neurotransmission, given that VTA dopaminergic neurons express both less DAT and D2 autoreceptors. Interestingly, dyskinesia reduction was linked in both graft types to similar reductions of the abnormally upregulated expressions of PEnk and fosB/ΔfosB (that parallels PDyn mRNA expression; Steiner and Gerfen, 1998; Andersson et al., 1999), markers of the activity of indirect and direct striatal output pathways, respectively. Both graft types induced similar reductions in NMDA receptors in the post-synaptic elements. This, and the fact that DATKO grafts induce only a partial improvement of LID, support the possibility that while DA alone is able to induce a downregulation of DA receptor supersensitivity, the presence of new functional DA presynaptic terminals may be necessary to revert the maladaptative synaptic plasticity processes that occur in medium spiny neurons as a result of dopaminergic denervation and chronic l-dopa treatment that are related to the development of LID (Cenci et al., 1998; Andersson et al., 1999; Calabresi et al., 2000; Nash and Brotchie, 2002; Oh and Chase, 2002; Lundblad et al., 2004; Pavon et al., 2006). Our results also emphasize the therapeutic importance of reducing the impact of pulsatile DA levels produced by l-dopa administration (Stocchi et al., 2005) by providing local and regulated DA transmission where available post-synaptic DA receptor terminal function can compensate and prevent abnormal transmission in the dendritic spines of striatal medium spiny neurons, even in the absence of pre-synaptic reuptake of DA. Notably, the presence in both graft types of ∼35% of serotoninergic neurons did not lead to the worsening of LID or to the occurrence of OFF-dyskinesia.
Based on these findings we propose that despite the capacity of extra-cellular DA to correct many aspects of striatal DA neurotransmission acting through extra-synaptic sites, the presence of terminals from the dopaminergic neurons that can regulate DA release locally provides a more complete correction of the changes in the circuitry in response to dopaminergic denervation and chronic l-dopa treatment. In addition, the use of DA reuptake deficient cells reveals that DA terminals from the grafts provide functional recovery even in the presence of abnormally high extra-cellular DA levels. These findings suggest that DA terminals, and not DA levels per se, restore function in an animal model of Parkinson's disease.
Supplementary material
Supplementary material is available at Brain online.
Funding
P50NS39793; The Michael Stern Foundation; The Orchard Foundation; The Consolidated Anti-Aging Foundation; The Harold and Ronna Cooper Family; Fondo de Investigaciones Sanitarias (FIS) Grant #CM06/0068.
Supplementary Material
Acknowledgements
We thank Dr Jang-Ho Cha and his laboratory, for providing facilities for performing receptor-binding studies and analysis of autoradiographs.
Glossary
Abbreviations:
- AIM
abnormal involuntary movement
- DA
dopamine
- DAT
dopamine transporter
- LID
l-dopa-induced dyskinesias
- LTP
long-term potentiation
- PDyn
prodynorphin
- PEnk
preproenkephalin
- 3-MT
3-methoxytyramine
- VM
ventral mesencephalic
References
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6:461–74. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- Bjorklund LM, Isacson O. Regulation of dopamine cell type and transmitter function in fetal and stem cell transplantation for Parkinson's disease. Prog Brain Res. 2002;138:411–20. doi: 10.1016/S0079-6123(02)38090-7. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U, Dunnett SB, Gage FH. Cross-species neural grafting in a rat model of Parkinson's disease. Nature. 1982;298:652–4. doi: 10.1038/298652a0. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Freund TF, Bjorklund A, Dunnett SB, Smith AD. Synaptic input and local output of dopaminergic neurons in grafts that functionally reinnervate the host neostriatum. Exp Brain Res. 1987;68:131–46. doi: 10.1007/BF00255240. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Bernardi G. Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci. 2000;23:S57–63. doi: 10.1016/s1471-1931(00)00017-3. [DOI] [PubMed] [Google Scholar]
- Carlsson T, Winkler C, Lundblad M, Cenci MA, Bjorklund A, Kirik D. Graft placement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol Dis. 2006;21:657–68. doi: 10.1016/j.nbd.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. J Neurochem. 2006;96:1718–27. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–706. [PubMed] [Google Scholar]
- Cha JH, Frey AS, Alsdorf SA, Kerner JA, Kosinski CM, Mangiarini L, et al. Altered neurotransmitter receptor expression in transgenic mouse models of Huntington's disease. Philos Trans R Soc Lond B Biol Sci. 1999;354:981–9. doi: 10.1098/rstb.1999.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase TN, Oh JD. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 2000;23:S86–91. doi: 10.1016/s1471-1931(00)00018-5. [DOI] [PubMed] [Google Scholar]
- Dethy S, Laute MA, Damhaut P, Goldman S. Pergolide potentiates L-DOPA-induced dopamine release in rat striatum after lesioning with 6-hydroxydopamine. J Neural Transm. 1999;106:145–58. doi: 10.1007/s007020050147. [DOI] [PubMed] [Google Scholar]
- Di Loreto S, Florio T, Capozzo A, Napolitano A, Adorno D, Scarnati E. Transplantation of mesencephalic cell suspension in dopamine-denervated striatum of the rat. Exp Neurol. 1996;138:318–26. [PubMed] [Google Scholar]
- Djaldetti R, Atlas D, Melamed E. Effect of subcutaneous administration of levodopa ethyl ester, a soluble prodrug of levodopa, on dopamine metabolism in rodent striatum: implication for treatment of Parkinson's disease. Clin Neuropharmacol. 1996;19:65–71. doi: 10.1097/00002826-199619010-00005. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fisher LJ, Young SJ, Tepper JM, Groves PM, Gage FH. Electrophysiological characteristics of cells within mesencephalon suspension grafts. Neuroscience. 1991;40:109–22. doi: 10.1016/0306-4522(91)90178-q. [DOI] [PubMed] [Google Scholar]
- Forni C, Brundin P, Strecker RE, el Ganouni S, Bjorklund A, Nieoullon A. Time-course of recovery of dopamine neuron activity during reinnervation of the denervated striatum by fetal mesencephalic grafts as assessed by in vivo voltammetry. Exp Brain Res. 1989;76:75–87. doi: 10.1007/BF00253625. [DOI] [PubMed] [Google Scholar]
- Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N Engl J Med. 1992;327:1549–55. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- Freund TF, Bolam JP, Bjorklund A, Stenevi U, Dunnett SB, Powell JF, et al. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: a tyrosine hydroxylase immunocytochemical study. J Neurosci. 1985;5:603–16. doi: 10.1523/JNEUROSCI.05-03-00603.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- Galpern WR, Burns LH, Deacon TW, Dinsmore J, Isacson O. Xenotransplantation of porcine fetal ventral mesencephalon in a rat model of Parkinson's disease: functional recovery and graft morphology. Exp Neurol. 1996;140:1–13. doi: 10.1006/exnr.1996.0109. [DOI] [PubMed] [Google Scholar]
- Hagell P, Cenci MA. Dyskinesias and dopamine cell replacement in Parkinson's disease: a clinical perspective. Brain Res Bull. 2005;68:4–15. doi: 10.1016/j.brainresbull.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Hagell P, Piccini P, Bjorklund A, Brundin P, Rehncrona S, Widner H, et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5:627–8. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- Haque NS, LeBlanc CJ, Isacson O. Differential dissection of the rat E16 ventral mesencephalon and survival and reinnervation of the 6-OHDA-lesioned striatum by a subset of aldehyde dehydrogenase-positive TH neurons. Cell Transplant. 1997;6:239–48. doi: 10.1177/096368979700600307. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, Kordower JH, et al. Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol. 1999;56:179–87. doi: 10.1001/archneur.56.2.179. [DOI] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Effect of repeated L-DOPA, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol. 1999;155:204–20. doi: 10.1006/exnr.1998.6996. [DOI] [PubMed] [Google Scholar]
- Herman JP, Rouge-Pont F, Le Moal M, Abrous DN. Mechanisms of amphetamine-induced rotation in rats with unilateral intrastriatal grafts of embryonic dopaminergic neurons: a pharmacological and biochemical analysis. Neuroscience. 1993;53:1083–95. doi: 10.1016/0306-4522(93)90491-w. [DOI] [PubMed] [Google Scholar]
- Isacson O, Deacon TW. Specific axon guidance factors persist in the adult brain as demonstrated by pig neuroblasts transplanted to the rat. Neuroscience. 1996;75:827–37. doi: 10.1016/0306-4522(96)00305-3. [DOI] [PubMed] [Google Scholar]
- Isacson O, Deacon TW, Pakzaban P, Galpern WR, Dinsmore J, Burns LH. Transplanted xenogeneic neural cells in neurodegenerative disease models exhibit remarkable axonal target specificity and distinct growth patterns of glial and axonal fibres. Nat Med. 1995;1:1189–94. doi: 10.1038/nm1195-1189. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998a;95:4029–34. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998b;18:1979–86. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane EL, Winkler C, Brundin P, Cenci MA. The impact of graft size on the development of dyskinesia following intrastriatal grafting of embryonic dopamine neurons in the rat. Neurobiol Dis. 2006;22:334–45. doi: 10.1016/j.nbd.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Lee CS, Cenci MA, Schulzer M, Bjorklund A. Embryonic ventral mesencephalic grafts improve levodopa-induced dyskinesia in a rat model of Parkinson's disease. Brain. 2000;123(Pt 7):1365–79. doi: 10.1093/brain/123.7.1365. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Update on fetal transplantation: the Swedish experience. Mov Disord. 1998;13(Suppl 1):83–7. [PubMed] [Google Scholar]
- Lindvall O, Sawle G, Widner H, Rothwell JC, Bjorklund A, Brooks D, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson's disease. Ann Neurol. 1994;35:172–80. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–23. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol. 2002;52:628–34. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- Maries E, Kordower JH, Chu Y, Collier TJ, Sortwell CE, Olaru E, et al. Focal not widespread grafts induce novel dyskinetic behavior in parkinsonian rats. Neurobiol Dis. 2006;21:165–80. doi: 10.1016/j.nbd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498–510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JE, Brotchie JM. Characterisation of striatal NMDA receptors involved in the generation of parkinsonian symptoms: intrastriatal microinjection studies in the 6-OHDA-lesioned rat. Mov Disord. 2002;17:455–66. doi: 10.1002/mds.10107. [DOI] [PubMed] [Google Scholar]
- Oh JD, Chase TN. Glutamate-mediated striatal dysregulation and the pathogenesis of motor response complications in Parkinson's disease. Amino Acids. 2002;23:133–9. doi: 10.1007/s00726-001-0118-2. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–14. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Obeso JA, Stocchi F. Drug insight: continuous dopaminergic stimulation in the treatment of Parkinson's disease. Nat Clin Pract Neurol. 2006;2:382–92. doi: 10.1038/ncpneuro0222. [DOI] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204:643–7. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- Piccini P, Brooks DJ, Bjorklund A, Gunn RN, Grasby PM, Rimoldi O, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2:1137–40. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–6. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Vinuela A, Kordower JH, Isacson O. Influence of cell preparation and target location on the behavioral recovery after striatal transplantation of fetal dopaminergic neurons in a primate model of Parkinson's disease. Neurobiol Dis. 2008;29:103–16. doi: 10.1016/j.nbd.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: optimizing behavioral assessment of outcome. In: In: Emerich DF, Dean RL III, Sanberg PR, editors. Totowa, NJ: Humana Press; 2000. pp. 131–51. Central nervous system diseases: innovative models of CNS diseases from molecule to therapy. [Google Scholar]
- Steece-Collier K, Collier TJ, Danielson PD, Kurlan R, Yurek DM, Sladek JR Embryonic mesencephalic grafts increase levodopa-induced forelimb hyperkinesia in parkinsonian rats. Mov Disord. 2003;18:1442–54. doi: 10.1002/mds.10588. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Vacca L, Ruggieri S, Olanow CW. Intermittent vs continuous levodopa administration in patients with advanced Parkinson disease: a clinical and pharmacokinetic study. Arch Neurol. 2005;62:905–10. doi: 10.1001/archneur.62.6.905. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Sharp T, Brundin P, Zetterstrom T, Ungerstedt U, Bjorklund A. Autoregulation of dopamine release and metabolism by intrastriatal nigral grafts as revealed by intracerebral dialysis. Neuroscience. 1987;22:169–78. doi: 10.1016/0306-4522(87)90207-7. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Kehr J, Andbjer B, Fuxe K. Fetal ventral mesencephalic grafts functionally reduce the dopamine D2 receptor supersensitivity in partially dopamine reinnervated host striatum. Exp Neurol. 2000;164:154–65. doi: 10.1006/exnr.2000.7421. [DOI] [PubMed] [Google Scholar]
- Thompson L, Barraud P, Andersson E, Kirik D, Bjorklund A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J Neurosci. 2005;25:6467–77. doi: 10.1523/JNEUROSCI.1676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venna N, Sabin TD, Ordia JI, Mark VH. Treatment of severe Parkinson's disease by intraventricular injection of dopamine. Appl Neurophysiol. 1984;47:62–4. doi: 10.1159/000101204. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of Parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–86. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–30. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T, Brundin P, Gage FH, Sharp T, Isacson O, Dunnett SB, et al. In vivo measurement of spontaneous release and metabolism of dopamine from intrastriatal nigral grafts using intracerebral dialysis. Brain Res. 1986;362:344–9. doi: 10.1016/0006-8993(86)90460-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.