Abstract
Background
A trial of neoadjuvant gemcitabine and pemetrexed (GP) chemotherapy in patients with resectable non-small cell lung cancer was conducted. The goal was to achieve a disease response rate of 50% and to determine if the expression levels of genes associated with gemcitabine and pemetrexed metabolism are predictive of response.
Methods
Patients had staging with a computed tomography (CT) scan, whole body F-18 fluorodeoxyglucose positron emission tomography (PET), and mediastinoscopy. Four biweekly cycles of GP were given. Patients were restaged, and those with resectable stage IB-III disease had thoracotomy. Fresh frozen tumor specimens were collected before and after chemotherapy and the mRNA levels of 14 target genes determined by real-time reverse transcriptase polymerase chain reaction (RTPCR).
Results
Fifty-two patients started therapy. The radiographic disease response rate was 35% (95% confidence interval [CI] 21.7 to 49.6%), and the progression rate was 6%. Forty-six patients had a thoracotomy. The complete tumor resection rate was 77% (40/52). There were no perioperative deaths or deaths related to chemotherapy. Tumor response to chemotherapy was inversely correlated with the level of expression of RRM1 (p ≤ 0.001; regulatory subunit of ribonucleotide reductase) and TS (p = 0.006; thymidylate synthase); i.e., the reduction in tumor size was greater in those with low levels of expression.
Conclusions
Neoadjuvant GP is well tolerated and produces an objective response rate of 35%. Tumoral RRM1 and TS mRNA levels are predictive of disease response and should be considered as parameters for treatment selection in future trials with this regimen.
Keywords: Gemcitabine, Pemetrexed, Ribonucleotide Reductase, Thymidylate Synthase, Non-Small Cell Lung Cancer
Introduction
Incorporation of platinum-containing chemotherapy into the management of resectable non-small-cell lung cancer (NSCLC) has become the standard of care for patients with metastatic disease in N1 or N2 lymph nodes.1–3 Neoadjuvant treatment results in response rates of approximately 33–64%,4–7 and adjuvant therapy increases absolute overall survival by approximately 5–15%.1–3 However, the approach of treating all patients with a platinum-containing regimen may have reached a plateau in terms of efficacy. In addition, there is significant toxicity associated with this approach including a treatment-related mortality of approximately 1–2%.1,7
We had reported a response rate of 34% in patients with operable stage I–III disease using a non-platinum regimen consisting of gemcitabine and vinorelbine in a phase II neoadjuvant trial.8 The combination of gemcitabine and pemetrexed (GP) had yielded a response rate of 31% in patients with previously untreated advanced-stage NSCLC if given on a 3-weekly schedule, and efficacy appeared to be dependent on the drug sequencing and scheduling.9 However, prior investigations of both drugs suggested that synergy as a result of sequencing may vary from one in vitro system to another.10–12 In a phase I trial of G immediately followed by P given biweekly, the maximum tolerated doses were 1500 mg/m2 and 500 mg/m2 respectively.13 The regimen produced a response rate of 21% in untreated patients with advanced NSCLC, and it was well tolerated.13
We conducted a single-institution trial of neoadjuvant gemcitabine and pemetrexed in patients with resectable NSCLC with the goal to describe the clinical efficacy and tolerability of the chosen regimen and to investigate the predictive utility of mRNA expression of genes involved in the metabolism of these drugs on therapeutic efficacy.
Materials and Methods
Clinical trial characteristics and study population
The study was approved by the University of South Florida’s Institutional Review Board (ClinicalTrials.gov #NCT00226577). Clinical staging was determined by physical examination, computed tomography of the chest and upper abdomen (CT), whole body FDG positron emission tomography (PET), magnetic resonance imaging of the brain (MRI), bronchoscopy, and mediastinoscopy. Histological confirmation of NSCLC; stage IB-IIIA and selected IIIB (2 lesions in one lobe, T4); age ≥18 years; a performance status (PS) of 0–1; measurable disease by RECIST; and no prior therapy for lung cancer were required for eligibility. These criteria were met by 52 patients.
Preoperative chemotherapy with G 1,500 mg/m2 immediately followed by P 500 mg/m2 was administered on days 1, 15, 29, and 43. Subsequent doses of chemotherapy were delayed or reduced for toxicity if appropriate. Patients received oral folate at a dose of 350–1,000 µg daily and subcutaneous vitamin B12 at a dose of 1,000 µg every 9 weeks starting one week before chemotherapy. Dexamethasone was given at a dose of 4 mg every 12 hours on the day before, the day of, and the day after chemotherapy. All toxicities were graded according to the common toxicity criteria (CTC, version 3.0).
Following chemotherapy, CT and PET scans were repeated between days 50 and 63. Radiographic response was expressed as a continuous variable by calculating the percentage of change in the sum of all greatest tumor diameters comparing the post-treatment and pre-treatment CT scans (1-[sum post lesions/sum pre lesions] ×100) and also by RECIST as best overall response.
Patients with resectable disease had thoracotomy between days 64 and 77. The recommended surgery was lobectomy or pneumonectomy with mediastinal lymph node dissection. Segmentectomy or wedge resection was discouraged. Patients with unresectable disease and those with incomplete resections were treated at the discretion of their physician. All patients were followed at 3-monthly intervals for 2 years and then every 6 months with a CT scan.
Molecular investigations
Tumor samples were collected prior to and after therapy as frozen specimens. The standard operating procedure for collection included a recording of the time from biopsy or resection to freezing, and the time elapsed was 30 min or less in all cases. Frozen specimens were embedded in optimal cutting temperature (OCT) medium and cut in 5–7 µm sections.
Tumor cells were collected by laser capture microdissection (LCM) using the Arcturus system. Total RNA was extracted using a commercial method (Arcturus, Mountain View, CA), and cDNA was generated with oligo-dT and random primers. Real-time quantitative PCR analysis was performed in triplicate per sample (7900HT, ABI, Foster City, CA). The probe and primers for RRM1 were those previously described.14 Commercially available primers and probes were used for expression analysis of all other target genes (Table 2). The relative amount of RRM1 and TS mRNA in a sample was determined by comparing the threshold cycle with a standard curve as described.14 For the remaining genes, relative quantification was performed by comparison of the test samples to a single calibrator sample in a fluidic card assay. Negative controls without a cDNA template were included in all experiments.
Table 2.
mRNA Gene Expression Characteristics and Association With Radiographic Disease Response
| Gene Name | Probe set ID | N | Min | Max | Median | Mean | Spearman's rho | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical Response | 49 | 95% (increase) | −100% (decrease) | −19% | −19% | ||||
| RRM1 | regulatory subunit of ribonucleotide reductase | * | 35 | 0.37 | 7.93 | 1.68 | 2.72 | 0.649 | <0.001 |
| RRM2a | catalytic subunit of ribonucleotide reductase | Hs00367247-m1 | 32 | 0.00 | 5.42 | 0.90 | 1.38 | −0.017 | 0.924 |
| RRM2b | P53-inducible catalytic subunit of ribonucleotide reductase | Hs00153082-m1 | 32 | 0.00 | 12.24 | 1.34 | 1.99 | 0.276 | 0.125 |
| DCK | deoxycytidine kinase | Hs00176127-m1 | 32 | 0.46 | 40.39 | 3.82 | 5.52 | 0.130 | 0.476 |
| CDA | cytidine deaminase | Hs00156401-m1 | 32 | 0.00 | 118.66 | 2.42 | 10.27 | 0.028 | 0.878 |
| ENT1 (SLC29A1) | equilibration sensitive nucleoside transporter 1 | Hs00191940-m1 | 32 | 0.00 | 21.42 | 1.30 | 2.18 | 0.162 | 0.374 |
| 5'-NT (NT5C1A) | cytosolic 5’-nucleotidase | Hs00261369-m1 | 32 | 0.00 | 170843.81 | 0.00 | 10.89 | −0.081 | 0.657 |
| TS | thymidylate synthase | Hs00426591-m1 | 35 | 0.32 | 18.31 | 3.39 | 4.46 | 0.454 | 0.006 |
| DHFR | dihydrofolate reductase | Hs00758822-s1 | 32 | 0.00 | 9.83 | 0.85 | 1.29 | 0.166 | 0.362 |
| GARFT | phosphoribosylglycinamide formyl transferase | Hs00531926-m1 | 32 | 0.17 | 21.93 | 1.19 | 2.28 | 0.002 | 0.988 |
| FPGS | folylpolyglutamate synthase | Hs00191956- m1 | 32 | 0.36 | 32.75 | 2.23 | 3.50 | 0.222 | 0.220 |
| ENT2 (SLC29A2) | equilibration sensitive nucleoside transporter 2 | Hs00155426-m1 | 32 | 0.11 | 41.30 | 5.16 | 7.76 | 0.125 | 0.494 |
| RFC1 (SLC19A1) | reduced folate carrier | Hs00161870-m1 | 32 | 0.01 | 33.47 | 0.12 | 2.23 | 0.096 | 0.600 |
| γ-GH | gamma-glutamyl hydrolase | Hs00608257-m1 | 32 | 0.07 | 33.52 | 2.47 | 5.75 | −0.015 | 0.934 |
5'-FAM-TTTGC TCTTT GGATT CCGGA TCTCT TCA-TAMRA-3’
Statistical considerations
Correlation coefficients between the expression of 14 genes, 7 each potentially related to G and P efficacy, and the continuous variable tumor response were calculated according to Spearman.15 The statistical significance level was adjusted for multiple analyses according to Bonferroni to a p-value of 0.0036; i.e., 0.05 divided by 14, since 14 independent or dependent genes were tested on the same dataset.16 The 2-sided pooled t-test or Wilcoxon Rank Sum test was used to test for significance between dichotomous variables and gene expression. The Kruskal Wallis test was used to test for significance between non-continuous variables with more than 2 values and gene expression. Kaplan-Meier survival estimates were generated to describe the disease-free and overall survival (DFS and OS). OS was estimated from the date of diagnosis to the date of death or last observation, and DFS was estimated from the date of surgery to the date of recurrence, death, or last observation. Patients without an event were censored as of the date of last observation.
Results
Induction chemotherapy and toxicity
A total of 52 eligible patients, 26 men and 26 women, between the ages 41 and 83 years (median 67 years), received at least one dose of chemotherapy. They were enrolled between April 2004 and April 2006, and their characteristics are described in table 1. Forty-two patients received all planned chemotherapy on time and without dose reduction. Three patients had dose delays without dose reductions. The reasons were a grade 3 lower extremity cellulitis, a grade 2 thrombocytopenia, and a grade 3 liver enzyme elevation. Seven patients had less than the intended 4 bi-weekly therapies. Three patients had only the first cycle because of grade 3 neutropenic fever with renal failure (stage T2N2M0), a grade 3 febrile drug reaction, and one patient requested immediate surgery without having experienced treatment-related side effects. Three patients only had cycles 1 and 2. The reasons were a grade 3 lower extremity phlebitis in one patient and grade 3 fatigue in two. One patient (stage T2N2M0) died after cycle 3 from pneumonitis of a likely viral etiology; however, a possibly treatment-related etiology could not be excluded.
Table 1.
Patient Characteristics, Disease Response, and Survival
| Radiographic Response Rate1 |
Pathologic Response Rate2 |
Median Overall Survival |
Median Disease-Free Survival3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All Patients | N=52 | 35% | (17/49) | 30% | (13/43) | >27.8 m | 33.7 m | ||
| Age median (range) 67 y (41–83) | |||||||||
| < 67 y | N=26 | 38% | (9/24) | 25% | (6/24) | >27.0 m | >21.1 m | ||
| ≥ 67 y | N=26 | 32% | (8/25) | 33% | (7/21) | >27.8 m | p=0.99 | 21.0 m | p=0.45 |
| Gender | |||||||||
| women | N=26 | 32% | (8/25) | 27% | (6/22) | >27.8 m | 21.1 m | ||
| men | N=26 | 38% | (9/25) | 33% | (7/21) | >27.4 m | p=0.80 | 33.7 m | p=0.75 |
| Performance Status | |||||||||
| zero | N=31 | 36% | (10/28) | 36% | (10/28) | >27.8 m | 33.7 m | ||
| one | N=21 | 33% | (7/21) | 20% | (3/15) | 16.8 m | p=0.006 | >11.6 m | p=0.96 |
| Weight Loss4 | |||||||||
| absent | N=47 | 34% | (15/44) | 31% | (12/39) | >27.4 m | 33.7 m | ||
| present | N= 5 | 40% | (2/5) | 25% | (1/4) | 16.1 m | p=0.03 | 9.1 m | p=0.31 |
| Smoking Status5 | |||||||||
| active | N=23 | 30% | (6/20) | 21% | (4/19) | >25.0 m | 21.0 m | ||
| quit (>1y) | N=27 | 41% | (11/27) | 36% | (8/22) | >27.8 m | p=0.42 | >20.7 m | p=0.37 |
| never smoker | N= 2 | NA6 | (0/2) | NA | (1/2) | ||||
| Histopathology7 | |||||||||
| squamous- | N=19 | 39% | (7/18) | 22% | (4/18) | >27.8 m | >20.7 m | ||
| non-squamous | N=33 | 32% | (10/31) | 36% | (9/25) | >27.4 m | p=0.93 | 21.1 m | p=0.73 |
| Stage | |||||||||
| I | N=16 | 31% | (5/16) | 27% | (4/15) | >27.8 m | 20.7 m | ||
| II | N=18 | 38% | (6/16) | 29% | (4/14) | >19.8 m | p=0.83 | 20.8 m | p=0.33 |
| III8 | N=18 | 35% | (6/17) | 36% | (5/14) | >25.0 m | >21.1 m | ||
| Tumor Resection | |||||||||
| complete | N=40 | 37% | (14/38) | 33% | (13/39) | >27.8 m | 33.7 m | ||
| incomp./not done | N=12 | 27% | (3/11) | 0% | (0/4) | 12.8 m | p=0.02 | NA | p=NA |
| Response to Chemotherapy9 | |||||||||
| CR/PR | N=17 | NA | (17/17) | 38% | (6/16) | >27.4 m | 18.2 m | ||
| SD | N=29 | NA | (0/29) | 24% | (6/25) | >27.8 m | p=0.71 | >33.7 m | p=0.39 |
| PD | N=3 | NA | (0/3) | 0% | (0/1) | >28.0 m | |||
not assessed in three patient
not assessed in nine patients
in the 40 patients with complete tumor resection
equal to or greater than 5% during the three months prior to diagnosis
a life-time never smoker was defined as a person that had smoked less than a total of 100 cigarettes
NA, not applicable
21 patients had adenocarcinoma including 5 with bronchioloalveolar features, 12 had other NSCLC subtypes including 2 adenosquamous carcinomas, 2 mixed adeno- and squamous carcinomas, and 1 large cell carcinoma with suggestive neuroendocrine features
two T4N0, T4 because of two separate tumor nodules in a single lobe of the lung
the pathologic disease response categories pPR and pNR were not significantly associated with OS (hazard ratio for pPR vs. pNR = 1.1, p = 0.90) or DFS (hazard ratio for pPR vs. pNR = 0.87, p = 0.78).
Radiographic response to chemotherapy
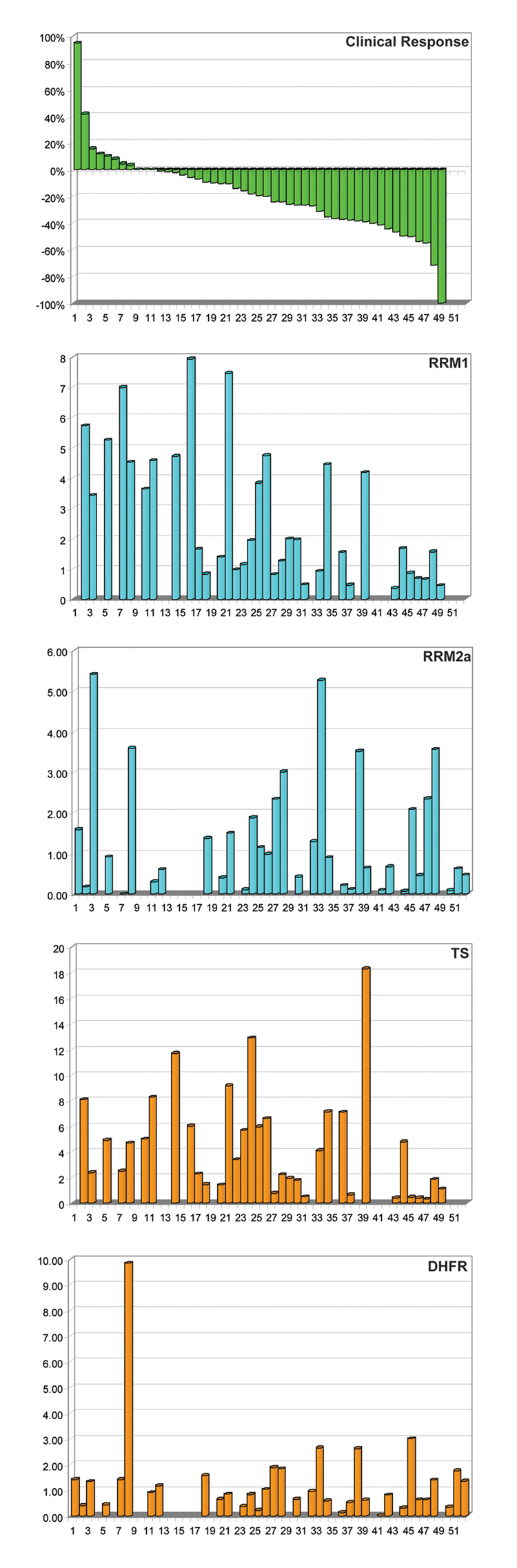
A radiographic response evaluation was possible in 49 patients. The best overall response was a complete remission (CR) in 1 (2%; 95% CI: 0.1–10.9%) patient; it was a partial remission (PR) in 16 (33%; 95% CI: 20.0–47.5%), stable disease (SD) in 29 (59%; 95% CI: 44.2–73.0%), and progressive disease (PD) in 3 (6%; 95% CI: 1.3–16.9%) patients. The response ranged from a 95% increase to a 100% decrease in the size of measurable lesions (Figure 1A). There were no statistically significant associations between radiographic disease response and the clinical parameters age (p = 0.62), gender (p = 0.96), performance status (p = 0.94), weight loss (p = 0.88), smoking status (p = 0.85), tumor histology (p = 0.95), and stage (p = 0.31) (Table 1).
Figure 1.
Water fall blots of radiographic disease response and mRNA gene expression.
Pathologic response to chemotherapy
A pathologic response evaluation was performed by determination of the proportion of necrotic and/or fibrotic material on light microscopical evaluation of surgical resection specimens stained with H & E. This was possible in 43 patients, and it ranged from 0 – 90%. None of the patients had a pathological CR (≥95% necrosis/fibrosis), 13 (30%) had a pathological PR (50–94% necrosis/fibrosis), and 30 (70%) had no pathological response (pNR). There were no statistically significant associations between pathologic disease response and the clinical parameters age (p = 0.39), gender (p = 0.65), performance status (p = 0.18), weight loss (p = 0.69), smoking status (p = 0.96), tumor histology (p = 0.43), and stage (p = 0.66). Although there was a correlation between radiographic and pathologic response (Spearman’s rho = 0.23); i.e., better clinical response was associated with a higher proportion of necrosis and fibrosis, it was not statistically significant (p = 0.14).
Surgical treatment
A thoracotomy was performed in 46 patients, and it resulted in a complete resection in 40 patients. Thirty-two patients had a lobectomy, 2 had a bilobectomy, 8 had a pneumonectomy, 2 had a wedge resection, and 2 had no tumor resection. At the surgical resection, 17 patients were down-staged, 17 had no change, and 12 were up-staged compared to the initial staging. One patient died 2.7 months after a complete right upper and middle lobectomy for a T2N1 squamous cell carcinoma of a bronchopleural fistula.
Recurrence and Survival
As of the index date June 20, 2008, 32 patients were alive (20 without evidence for disease, 7 with recurrence, 3 with incomplete and 2 without resection), 14 had died from lung cancer (10 with recurrence, 1 with incomplete and 3 without resection), and 6 had died from causes other than lung cancer (4 from cardiovascular disease). The median follow-up for the 32 living patients was 36.5 months, and it was 30.7 months for the 20 patients with a complete resection and no event. In the 17 patients with disease recurrence after a complete resection, the first site of recurrence was within the region of resected disease in 2 patients, and it was in previously uninvolved hilar or mediastinal lymph nodes in 4 patients and in distant sites in 11 patients (5 brain, 3 bone, 2 lung, 1 adrenal). In one patient with local recurrence, a re-resection was successful; however, the disease recurred in the brain.
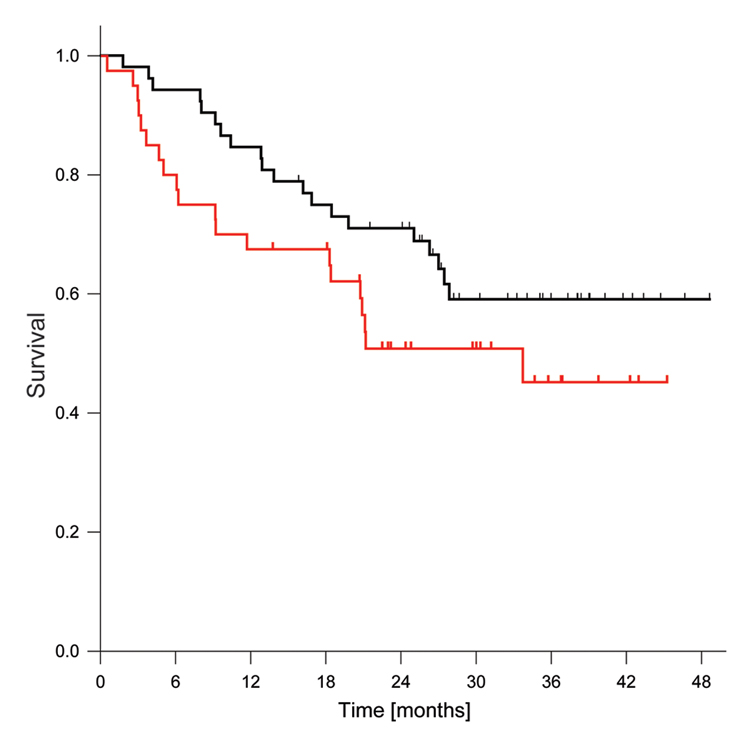
The median OS was >27.8 months, and the median DFS for patients with a complete surgical resection was 33.7 months (Figure 2). The 12-month and 24-month OS rates were 84.6% (95% CI: 71.6 – 92.0%) and 71.0% (95% CI: 56.5 – 81.4%), and the corresponding DFS rates were 67.5% (95% CI: 50.7 – 79.7%) and 51.3% (95% CI: 34.6 – 66.2%) respectively. The radiographic or pathologic response to chemotherapy was not significantly associated with OS or DFS (Table 1).
Figure 2.
Kaplan-Meier overall and disease-free survival estimates. The black curve denotes OS and the red curve DFS. Tick marks indicate censored cases.
Pharmacogenomic variables predictive of disease response
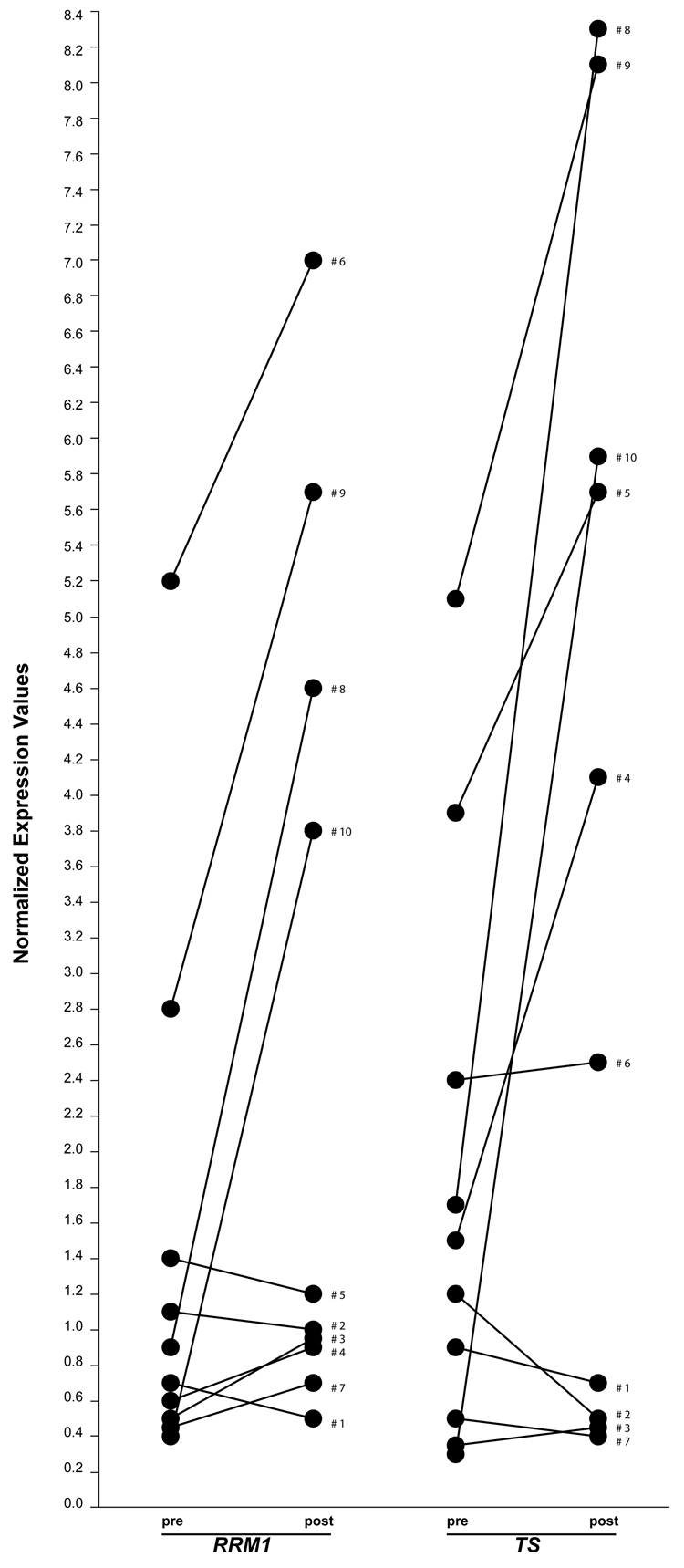
Pretreatment tumor specimens of sufficient quantity and quality for gene expression analysis by real-time RTPCR were available on 10 and post-treatment specimens on 35 patients. We evaluated if gemcitabine and pemetrexed therapy would alter the mRNA levels of RRM1 and TS. There was a significant correlation between pre- and post-treatment levels for both genes (Spearman’s rho = 0.786, p = 0.025) suggesting that post chemotherapy gene expression levels are representative of pretreatment levels. However, we observed that gene expression levels appear to increase with treatment (Figure 3). All subsequent analyses were performed on the 35 post-treatment specimens. The ranges of expression and other marker characteristics for all 14 genes are summarized in Table 2. The mRNA levels of RRM1 were significantly correlated with disease response (rho = 0.649, p = <0.001); i.e., low levels were predictive of tumor size reduction and high levels of tumor growth (Figure 1B). Of the 18 patients with RRM1 expression equal to or below the median of 1.68, 10 had a PR or CR, while only 2 of the 17 with high RRM1 expression responded.
Figure 3.
Pre- and post-treatment mRNA levels of the genes RRM1 and TS in 10 patients.
The mRNA levels of TS were likewise correlated with disease response (rho = 0.454, p = 0.006) (Figure 1C); however, after using a Bonferroni adjustment for multiplicity of data analysis, the p-value was above the level of 0.0036. Of the 18 patients with TS expression equal to or below the median of 3.40, 7 had a PR or CR, while 5 of the 17 with high TS expression responded. The expression levels of all other genes were not significantly correlated with disease response (Figure 1D & E).
There was no significant difference in overall or disease-free survival between patients with high versus low RRM1 or TS mRNA expression when dichotomized by the gene expression medians of 1.68 for RRM1 or 3.39 for TS (Table 3).
Table 3.
Median Overall and Disease-Free Survival by RRM1 and TS mRNA Expression
| Median OS | Log-rank p-value |
Median DFS | Log-rank p-value |
|
|---|---|---|---|---|
| RRM1 high (>1.68) | >27.8 m | 0.083 | >20.7 m | 0.146 |
| RRM1 low (<1.68) | >27.4 m | 21.0 m | ||
| TS high (>3.39) | >27.8 m | 0.272 | >20.7 m | 0.187 |
| TS low (<3.39) | >27.0 m | 21.0 m | ||
Discussion
A major goal of current research efforts in NSCLC is to increase the efficacy of perioperative systemic therapy in patients with a complete surgical resection through incorporation of molecular parameters into clinical therapeutic decisions. Although most of the data demonstrating efficacy of perioperative systemic therapy has been generated with post-operative treatments, there is presently no evidence to suggest that pre-operative therapy is less efficacious. Assuming that both approaches result in similar clinical outcomes, pre-operative systemic therapy is substantially better suited for the prospective evaluation of the increasing number of molecular markers on treatment effects. In particular, drug targets can be studied pre and post therapy, disease response can be utilized as the primary outcomes variable, and dynamic parameters that may influence drug efficacy can be studied.
We investigated the pre-operative efficacy of a non-platinum doublet, namely gemcitabine and pemetrexed, in patients with surgically resectable NSCLC. The rationale for this combination was that both agents are antimetabolites with relatively well known mechanisms of action, both are well tolerated, both are efficacious and already integrated into patient care, and prior in vitro studies had suggested cytotoxic synergy between them. Platinum-based post-operative chemotherapy improves 5-year survival by 5–15% in patients with completely resected stage II and III disease.1–3 However, there is substantial toxicity associated with this therapy,1–3 and therapeutic benefit appears to be restricted to patients with low tumoral ERCC1 levels.17 Prior randomized phase III neoadjuvant studies that utilized platinum-based chemotherapy had reported response rates of 33%,5 41%,7 49%,6 and 64%.4 Approximately 75% of patients received all planned chemotherapy, and the chemotherapy-related mortality was approximately 2%. These response rates appear higher than our response rate of 35%; however, 18/52 patients in our study had stage III disease, while two of the referenced trials did not include stage III patients,5,7 and the proportion of stage III patients in the other two trials was 7%6 and 47%4 respectively. In two earlier randomized neoadjuvant trials with platinum-based therapy in patients with stage III disease, the reported response rates were 53% (16/30)18 and 35% (9/26).19 We had a priori set our expected response rate at 50%; a goal, which we did not achieve. We therefore conclude that the combination of gemcitabine and pemetrexed is unlikely to be superior to platinum-containing doublets if given to an unselected group of patients with resectable NSCLC.
Eighty-seven percent (45/52) of patients in our trial received the planned 4 cycles of therapy. Five patients had less than 4 cycles because of treatment-related grade 3 toxicities, and of these, four had surgery with a complete resection. One patient died prior to surgery from a presumed viral pneumonitis. We conclude that the combination of gemcitabine and pemetrexed is well tolerated and is deliverable as four bi-weekly treatments to a proportion of patients that is at least equal to the proportion of patients that can receive a platinum-containing doublet.
Our pharmacogenomic studies indicate that the magnitude of tumor response to gemcitabine and pemetrexed is associated with tumoral expression of the genes RRM1 and TS. Patients whose tumors express these genes at low levels are more likely to experience a reduction in tumor size compared to those with high levels of gene expression. This association was significant for the gene RRM1, but failed to reach the significance level of 0.0036 for TS. Since we evaluated a total of 14 genes in these patients, we adjusted the significance level according to Bonferroni. This is a conservative approach to adjustment for multiplicity of data analysis, and most correlative investigations are conducted under less stringent conditions. It is thus our opinion, that the correlation coefficient of (−)0.454 seen for the association between TS expression and response to treatment is remarkable and requires further exploration.
In summary, the clinical correlative investigations described extend the previously known relationship between RRM1 expression and gemcitabine efficacy to the combination of gemcitabine and pemetrexed. They also provide reasonable evidence for an association between TS expression and efficacy of this combination. In an unselected group of patients with resectable NSCLC, gemcitabine and pemetrexed provide an objective radiographic response rate of 35% with good tolerability. We conclude that further investigation of this drug combination is warranted with selection of patients based on the expression of RRM1 and TS.
Acknowledgments
Supported in part by Grant No. R21 CA110487 from the National Cancer Institute and by Eli Lilly and Company
References
- 1.The International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 2.Winton TL, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin versus observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 4.Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative Chemotherapy Followed by Surgery Compared With Primary Surgery in Resectable Stage I (Except T1N0), II, and IIIa Non-Small-Cell Lung Cancer. J Clin Oncol. 2002;20:247–253. doi: 10.1200/JCO.2002.20.1.247. [DOI] [PubMed] [Google Scholar]
- 5.Scagliotti G. Preliminary results of Ch.E.S.T.: A phase III study of surgery alone or surgery plus preoperative gemcitabine-cisplatin in clinical early stages non-small cell lung cancer. Proc Am Soc Clin Oncol. 2005;23:626s. doi: 10.1200/JCO.2010.33.7089. [DOI] [PubMed] [Google Scholar]
- 6.Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT/EORTC 08012 multicentre randomized trial and update of systemic review. Lancet. 2007;369:1929–1937. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 7.Pisters KM, Vallieres E, Bunn PA, et al. S9900: Surgery alone or surgery plus induction paclitaxel/carboplatin chemotherapoy in early stage non-small cell lung cancer. Proc Am Soc Clin Oncol. 2007;25:389s. [Google Scholar]
- 8.Ramnath N, Sommers E, Robinson L, et al. Phase II study of neoadjuvant chemotherapy with gemcitabine and vinorelbine in resectable non-small cell lung cancer. Chest. 2005;128:3467–3474. doi: 10.1378/chest.128.5.3467. [DOI] [PubMed] [Google Scholar]
- 9.Ma C, Nair S, Thomas S, et al. Randomized phase II trial of three schedules of pemetrexed and gemcitabine as front-line therapy for advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5929–5937. doi: 10.1200/JCO.2005.13.953. [DOI] [PubMed] [Google Scholar]
- 10.Tonkinson JL, Worzalla JF, Teng CH, et al. Cell cycle modulation by a multitargeted antifolate, LY231514, increases the cytotoxicity and antitumor activity of gemcitabine in HT29 colon carcinoma. Cancer Res. 1999;59:3671–3976. [PubMed] [Google Scholar]
- 11.Adjei AA. Preclinical and clinical studies with combinations of pemetrexed and gemcitabine. Semin Oncol. 2002;29 Suppl 18:30–34. doi: 10.1053/sonc.2002.37468. [DOI] [PubMed] [Google Scholar]
- 12.Mey V, Giovanetti E, De Braud F, et al. In vitro synergistic cytotoxicity of gemcitabine and pemetrexed and pharmacogenetic evaluation of response to gemcitabine in bladder cancer patients. Br J Cancer. 2006;95:289–297. doi: 10.1038/sj.bjc.6603242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudek A, Larson T, McCleod M, et al. Phase 1/2 dose escalading study of twice-monthly pemetrexed and gemcitabine in patients with advanced cancer and non-small cell lung cancer. J Thorac Oncol. 2008;3:394–399. doi: 10.1097/JTO.0b013e318169cdc4. [DOI] [PubMed] [Google Scholar]
- 14.Bepler G, Sharma S, Cantor A, et al. RRM1 and PTEN as prognostic parameters for overall and disease-free survival in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:1878–1885. doi: 10.1200/JCO.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Spearman C. The proof and measurement of association between two things. Amer J Psychol. 1904;15:72–101. [PubMed] [Google Scholar]
- 16.Bonferroni C. Teoria statistica delle classi e calcolo delle probabilita. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali de Firenze. 1936;8:3–62. [Google Scholar]
- 17.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 18.Rosell R, Gomez-Codina J, Camps C, et al. Randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 19.Roth JA, Fosella F, Komake R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small cell lung cancer. J Natl Cancer Inst. 1994;86:673–680. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]