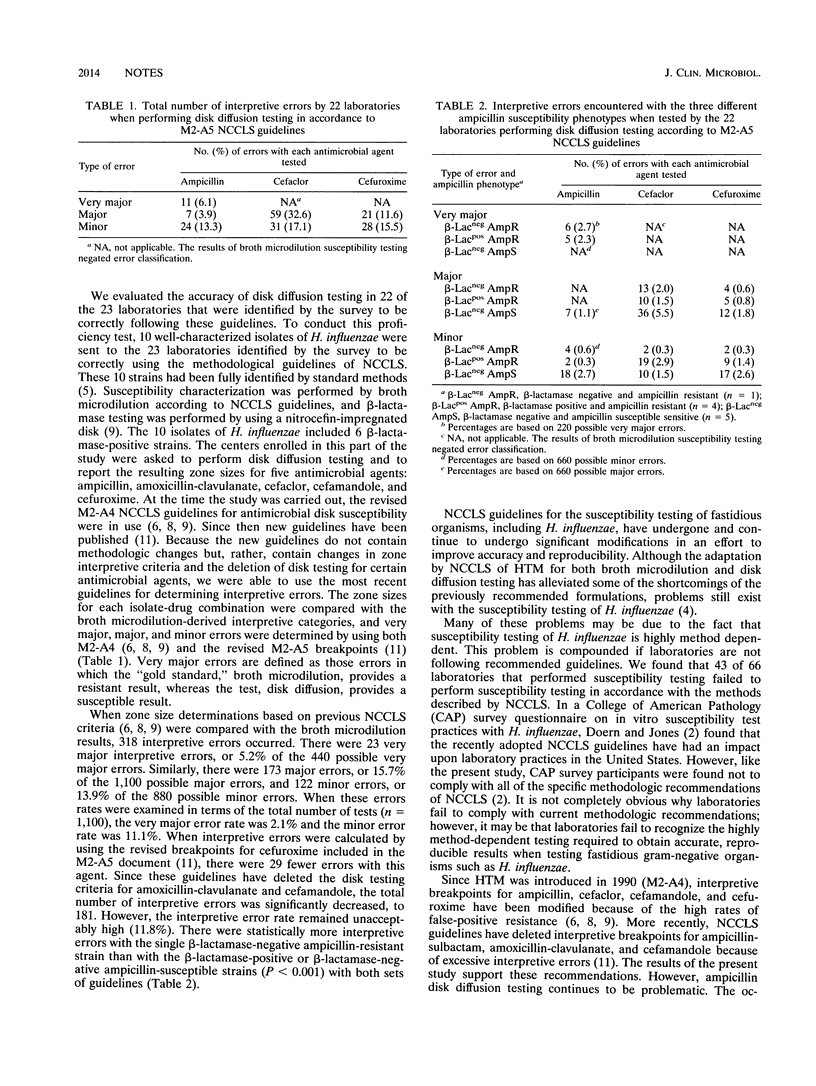
Abstract
We surveyed 75 clinical laboratories to determine if National Committee for Clinical Laboratory Standards (NCCLS) were being used for the susceptibility testing of Haemophilus influenzae. Of the 66 laboratories that performed susceptibility testing, all claimed to follow current NCCLS guidelines. However, upon further questioning, only 23, all of which used disk diffusion testing, accurately interpreted and followed the guidelines. Proficiency testing of 22 of these laboratories found that an unacceptable number of interpretive errors (> 10%) occurred. These results query the merit of routine disk diffusion susceptibility testing of H. influenzae to beta-lactam agents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doern G. V. In vitro susceptibility testing of Haemophilus influenzae: review of new National Committee for Clinical Laboratory Standards recommendations. J Clin Microbiol. 1992 Dec;30(12):3035–3038. doi: 10.1128/jcm.30.12.3035-3038.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern G. V., Jones R. N. In vitro susceptibility test practices with Haemophilus influenzae among College of American Pathologists survey participants in the United States. Diagn Microbiol Infect Dis. 1993 Jul;17(1):61–65. doi: 10.1016/0732-8893(93)90072-f. [DOI] [PubMed] [Google Scholar]
- Heelan J. S., Chesney D., Guadagno G. Investigation of ampicillin-intermediate strains of Haemophilus influenzae by using the disk diffusion procedure and current National Committee for Clinical Laboratory Standards guidelines. J Clin Microbiol. 1992 Jul;30(7):1674–1677. doi: 10.1128/jcm.30.7.1674-1677.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


