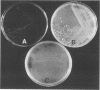
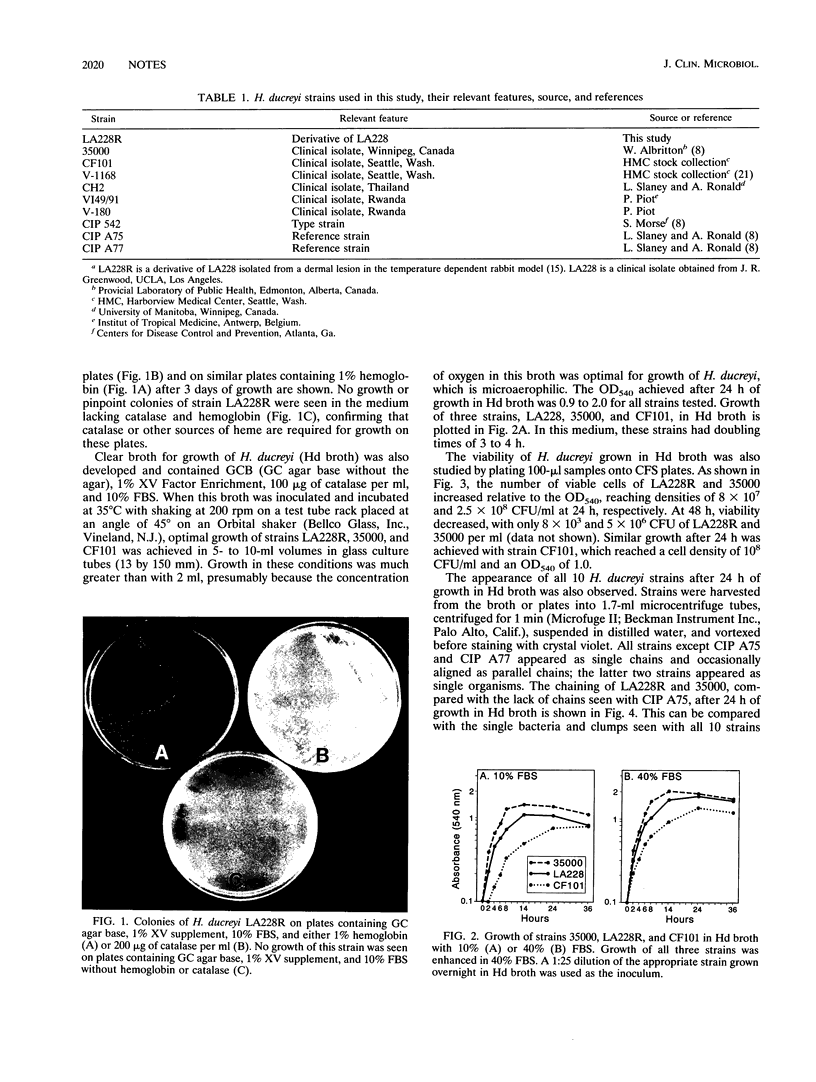
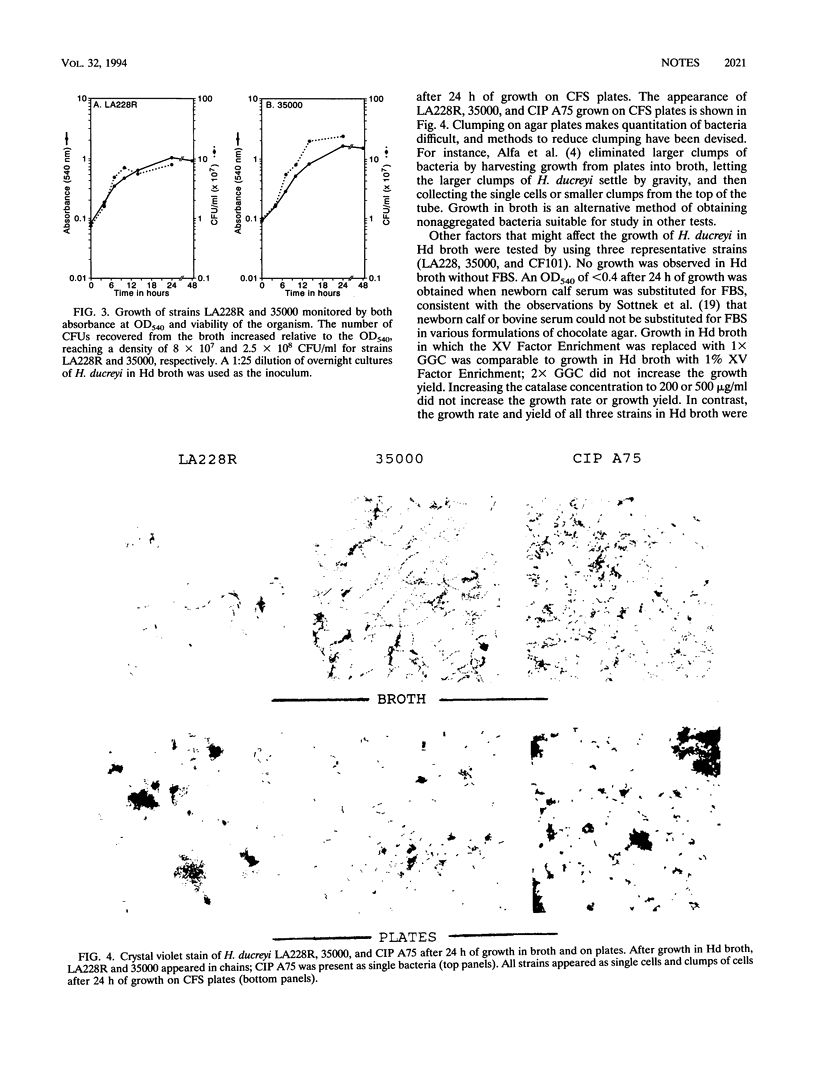
Abstract
Using catalase as a source of heme, we have developed both clear plate and broth media for the growth of Haemophilus ducreyi, the causative agent of chancroid. In the broth medium, the growth phase of the organism can be monitored and the organisms achieve a cell density of > 10(8) CFU/ml.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L. Biology of Haemophilus ducreyi. Microbiol Rev. 1989 Dec;53(4):377–389. doi: 10.1128/mr.53.4.377-389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa M. J., Degagne P., Hollyer T. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect Immun. 1993 May;61(5):1735–1742. doi: 10.1128/iai.61.5.1735-1742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Shillcock M., Jones P. Equilibrium and kinetic studies of the aggregation of porphyrins in aqueous solution. Biochem J. 1976 Feb 1;153(2):279–285. doi: 10.1042/bj1530279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Albritton W. L., Ronald A. R. Determination of the hemin requirement of Haemophilus ducreyi: evaluation of the porphyrin test and media used in the satellite growth test. J Clin Microbiol. 1978 Mar;7(3):243–246. doi: 10.1128/jcm.7.3.243-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G. W., Lian C. J., Wilt J. C., Ronald A. R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978 Apr;13(4):608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockett A. E., Dance D. A., Mabey D. C., Drasar B. S. Serum-free media for isolation of Haemophilus ducreyi. Lancet. 1991 Aug 3;338(8762):326–326. doi: 10.1016/0140-6736(91)90473-3. [DOI] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989 Apr;2(2):137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R., Back A. E. Isolation and cultivation of Haemophilus ducreyi. J Clin Microbiol. 1982 Apr;15(4):625–629. doi: 10.1128/jcm.15.4.625-629.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer F. A., Simonsen J. N., Cameron D. W., Ndinya-Achola J. O., Kreiss J. K., Gakinya M. N., Waiyaki P., Cheang M., Piot P., Ronald A. R. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991 Feb;163(2):233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- Purcell B. K., Richardson J. A., Radolf J. D., Hansen E. J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991 Aug;164(2):359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- Schulte J. M., Martich F. A., Schmid G. P. Chancroid in the United States, 1981-1990: evidence for underreporting of cases. MMWR CDC Surveill Summ. 1992 May 29;41(3):57–61. [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985 Dec;49(4):398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottnek F. O., Biddle J. W., Kraus S. J., Weaver R. E., Stewart J. A. Isolation and identification of Haemophilus ducreyi in a clinical study. J Clin Microbiol. 1980 Aug;12(2):170–174. doi: 10.1128/jcm.12.2.170-174.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A. W., Zanen H. C. Characteristics of Haemophilus ducreyi in culture. J Clin Microbiol. 1984 May;19(5):672–674. doi: 10.1128/jcm.19.5.672-674.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Handsfield H. H., Peters D., Holmes K. K., Falkow S. Characterization of ampicillin resistance plasmids from Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Apr;21(4):622–627. doi: 10.1128/aac.21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Patton C. M., Tenover F. C., Barrett T. J., Stamm W. E., Steigerwalt A. G., Lin J. Y., Holmes K. K., Brenner D. J. Prevalence and characterization of hippurate-negative Campylobacter jejuni in King County, Washington. J Clin Microbiol. 1987 Sep;25(9):1747–1752. doi: 10.1128/jcm.25.9.1747-1752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserheit J. N. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992 Mar-Apr;19(2):61–77. [PubMed] [Google Scholar]