Abstract
Increasing evidence shows that there is an interaction between mitogen-activated protein kinase and Wnt signaling and that their interaction plays important roles in a variety of cellular processes. However, how the two signaling interacts is not clear. In this study, we found that β-catenin expression was strikingly increased in the intestinal normal mucosa and tumors of c-Jun N-terminal kinase (JNK) 1-deficient mice by immunohistochemical staining and that both β-catenin expression and transcriptional activity were significantly upregulated in JNK1-deficient mouse embryonic fibroblasts. However, active JNK1 significantly inhibited β-catenin expression and suppressed β-catenin-mediated transcriptional activity by enhancing glycogen synthase kinase 3β (GSK3β) activity. But β-catenin inhibition was significantly reduced by GSK3β RNA interference or GSK3β inhibitor lithium chloride and proteasome inhibitor MG132. Further, mutant β-catenin at the phosphorylation sites of Ser33 and Ser37 by GSK3β was resistant to activated JNK1-induced β-catenin degradation. Moreover, the physical interaction between JNK1 and β-catenin was detected by immunoprecipitation, and their colocalization was seen in cellular nuclei and cytoplasm. Taken together, our data provide direct evidence that JNK1 interacts with and negatively regulates β-catenin signaling through GSK3β pathway and that the β-catenin alteration is probably responsible for the intestinal tumor formation in JNK1-deficient mice.
Introduction
The c-Jun N-terminal kinases (JNKs) are members of the superfamily of mitogen-activated protein kinase (MAPK) family and play an important role in the regulation of a variety of cellular processes, including cell proliferation, differentiation and apoptosis (1,2). Numerous evidences demonstrated the distinctive functions of JNKs, but JNK1 is reported as a tumor suppressor. It was reported that targeted inactivation of JNK1 in mice increased the sensitivity to skin tumorigenesis induced by the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (3). In contrast, gain-of-function of JNK1 strongly inhibited the transformed growth of non-small cell lung cancer cells (4). Recently, our studies demonstrate a critical role of JNK1 in regulating intestinal homeostasis and in suppressing intestinal tumor formation in mouse (5). Further studies suggest that the suppressor role of JNK1 is linked to the altered expression of p21WAF/cip1. However, the inactivation of p21 alone is not sufficient to initiate tumor formation (6). Therefore, it was proposed that other potential signaling pathways be involved in tumor formation in JNK1-deficient mice.
Recently, an increasing body of literature reported the cross talk between MAPK and Wnt/β-catenin signaling in a variety of cellular processes (7–10). β-Catenin is a mediator in Wnt/β-catenin-signaling pathway. In the absence of Wnt ligands, β-catenin is phosphorylated by glycogen synthase kinase 3β (GSK3β) in a destruction complex of adenomatous polyposis coli (APC)–GSK3β–axin (11–14), ubiquitinated by β-transducin repeat-containing protein complex and degraded by proteasome system. In the presence of Wnt ligands, the destruction complex is inhibited. β-Catenin is stabilized and translocated into nucleus, where it acts as a coactivator of the T-cell factor (TCF)/lymphoid enhancer factor family of transcription factors, modulating expression of a broad range of target genes, such as c-myc and cyclin D1 (15). Although Wnt/β-catenin signaling is required for maintaining both stem cells and cell proliferation in the crypts of adult small intestine (16) and controls the differentiation in the villi (17,18), aberrant Wnt/β-catenin signaling, resulting from mutations in its components, disturbs the finely tuned equilibrium between stemness, proliferation and differentiation in intestinal epithelial cells along the crypt–villus axis, finally leading to tumor formation.
As an important substrate of JNK1, the cyclin-dependent kinase inhibitor p21WAF1 plays an important role in intestinal homeostasis and tumor formation. It was reported that c-myc, a downstream target of β-catenin/TCF4, directly represses p21 promoter. Disruption of β-catenin/TCF4 activity leads to decreased expression of c-myc, which releases p21 transcription and thus mediates G1 arrest and differentiation (19). In contrast, inactivation of p21 induces significant elevation in c-myc expression and cell proliferation (20). These observations suggest a possible interaction between JNK1 and Wnt/β-catenin-signaling pathway and that their interaction plays a role in intestinal tumorigenesis initiated by targeted JNK1 inactivation. Herein, we reported that JNK1 interacts with β-catenin and inhibits β-catenin signaling through GSK3β and proteasome system both in vivo and in vitro.
Materials and methods
Mouse tissues and immunohistochemical staining
Mouse intestinal normal mucosa and intestinal tumors were from JNK1+/+ and JNK1−/− mice, respectively, as described previously (5). Four micrometer formalin-fixed and paraffin-embedded sections were deparaffinized and rehydrated, quenched with 1.5% H2O2, blocked with 10% normal goat serum and probed with goat anti-β-catenin polyclonal antibody (sc-1496; Santa Cruz Biotechnology, Santa Cruz, CA). Detection was with biotinylated anti-goat IgG, followed by incubation with avidin–biotin complex (Vector Laboratories, Burlingame, CA) and substrate 3′,5′-diaminobenzidine, followed with hematoxylin counterstaining.
Cell lines and cell culture
Human embryonic kidney (HEK) 293T cell line, mouse L cells that secrete Wnt3a and human colon cancer cell lines SW480 were obtained from the American Type Culture Collection (Manassas, VA). JNK1+/+ and JNK1−/− mouse embryonic fibroblast (MEF) was derived from JNK1+/+ and JNK1−/− mice. HEK293T and MEF cells were maintained in Dulbecco’s modified Eagle’s medium, and the SW480 cells were grown in minimal essential medium and McCoy’s 5A, respectively. Medium was supplemented with 10% fetal bovine serum, 1× antibiotic/antimycotic solution (100 U/ml streptomycin, 100 U/ml penicillin and 0.25 μg/ml amphotericin B), 100 μmol/l non-essential amino acids and 100 mmol/l N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer solution (all from Invitrogen Corp., Carlsbad, CA). All cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Ultraviolet irradiation
Prior to ultraviolet (UV) irradiation (FB-UVXL-1000 UV cross-linker; Fisher Scientific, Pittsburgh, PA), the culture medium was removed to deliver as exact a UV dose as possible and then restored afterward. Two hours after UV irradiation, cells were harvested for immunoblotting analysis.
Plasmids and transfection
pcDNA3-Flag-MKK7-JNK1 (activated JNK1) and pcDNA3-hemagglutinin (HA)-β-catenin were constructed as described previously (21,22). GSk3β RNA interference (RNAi) plasmid pSuper-GSK3β and control pSuper-neo-GFP were kindly provided by Dr Christine Blattner (Forschungszentrum Karlsruhe, Institute for Toxicology and Genetics, Germany) (23). To construct the vectors expressing HA-tagged mutant β-catenin (HA-S33Y β-catenin, HA-S33F β-catenin and HA-S37A β-catenin), the coding sequences of β-catenin were amplified from the corresponding plasmids expressing Flag-tagged β-catenin (pcDNA3-Flag-S33Y β-catenin, pcDNA3-Flag-S33F β-catenin and pcDNA3-Flag-S37A β-catenin, kindly provided by Dr Zijie Sun, Stanford University School of Medicine and Dr Eric Fearon, University of Michigan, respectively) (24,25) and cloned into the plasmid pcDNA3-HA which encodes a HA epitope tag at the N-terminus. The primers used for β-catenin amplification were forward, 5′-CGGGATCCATGGCTACTCAAGCTGATTTGAT-3′ and reverse, 5′-GCTCTAGATTACAGGTCAGTATCAAACCAGGCC-3′. TCF-4 reporter plasmid TOPFLASH and the control inactive reporter FOPFLASH were from Upstate Biotechnology (Lake Placid, NY). Reporter plasmid pTK-Renilla was purchased from Promega (Madison, WI). For transfection, cells were plated to form a 50–70% confluent culture. The HEK293T and MEF cells were transfected using Lipofectamine 2000 (Invitrogen Corp.), whereas the SW480 cells were transfected with Amaxa nucleofection system (Gaithersburg, MD).
β-Catenin/TCF-4 reporter assay
Cells were plated in 24-well plates. After 24 h, cells were cotransfected with pcDNA-HA-β-catenin and pcDNA3-Flag-MKK7-JNK1, along with TOPFLASH or FOPFLASH using Lipofectamine 2000 according to the manufacturer’s instruction. Twenty-four or forty-eight hours after transfection, the cells were harvested. Firefly and Renilla luciferase activities were measured using a dual luciferase kit (Promega). The firefly luciferase data for each sample were normalized based on transfection efficiency as determined by Renilla luciferase activity. Each experiment was performed in triplicate and repeated at least three times.
Western blotting
Cells were harvested and suspended in radio immunoprecipitation assay (RIPA) lysis buffer (Upstate Biotechnology) containing protease inhibitor cocktail (Sigma–Aldrich Corp., St Louis, MO). After incubation on ice for 15 min, cell lysate was centrifuged at 12 000 r.p.m. for 15 min at 4°C. The protein content of the supernatant was determined using Bio-Rad Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA). Proteins were separated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories). Blots were probed with the antibodies specific for HA, p-JNK1, p-c-Jun, β-catenin (Santa Cruz Biotechnology), GSK3β, p-GSK3β (Cell Signaling Technology, Beverly, MA) and β-actin (Sigma–Aldrich Corp.). Signals were detected with enhanced chemiluminescence plus reagents (Amersham Pharmacia, Piscataway, NJ).
Coimmunoprecipitation assay
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and then incubated on ice for 15 min in RIPA lysis buffer supplemented with protease inhibitor cocktail. Total cell lysate was centrifuged at 12 000 r.p.m. for 15 min at 4°C. Three hundred micrograms of the supernatant was incubated with the anti-Flag antibody (GenScript Corporation, Scotch Plains, NJ) overnight at 4°C on a rotator, followed by addition of protein A/G plus-agarose (Santa Cruz Biotechnology) for 2 h at 4°C. Agaroses were washed five times in RIPA lysis buffer supplemented with protease inhibitor cocktail. Complexes were released from the protein A/G plus-agarose by boiling for 5 min in 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis loading buffer. The immunocomplex was separated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and western blotting was used to detect HA-β-catenin and GSK3β with anti-HA and anti-GSK3β antibody, respectively.
Immunofluorescence microscopy
Cells grown on a chamber slide (BD Biosciences, San Jose, CA) were cotransfected with pEGFP-β-catenin and pcDNA3-Flag-MKK7-JNK1. Twenty-four hours after transfection, cells were fixed with 4% paraformaldehyde in PBS for 10 min and rendered permeable by further treatment with 0.2% Triton X-100 for 5 min. Anti-Flag antibody (1:200; Genescript, Piscataway, NJ) was diluted in 1% bovine serum albumin in PBS and incubated with cells for 1 h. Cells were washed three times with PBS and then incubated with tetramethyl rhodamine isothiocyanate labeled anti-mouse immunoglobulin G (Santa Cruz Biotechnology) diluted in PBS for 1 h. Cells were washed, mounted with UltraCruz 4′-6-Diamidino-2-phenylindole containing mounting medium (Santa Cruz Biotechnology), viewed and photographed under a Zeiss LSM510 META confocal microscope (Zeiss, Jena, Germany).
Results and discussion
JNK1 deficiency caused striking elevation of β-catenin in the intestine of mice
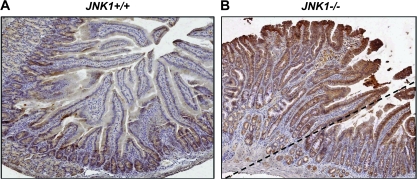
Our previous work demonstrated that mice with targeted inactivation of JNK1 spontaneously developed intestinal tumors (5). In contrast, there was no intestinal tumor formation in the mice that were wild-type or heterozygous for JNK1. Since Wnt/β-catenin signaling plays important role in intestinal tumorigenesis, we detected β-catenin expression in intestinal normal mucosa and intestinal tumors to elucidate the role of β-catenin in JNK1-initiated tumor formation and the potential correlation between JNK1 and Wnt/β-catenin signaling. As shown in Figure 1B, an adenoma was represented at the upside of dash line, with adjacent normal mucosa. Mouse tissues were evaluated immunohistochemically for β-catenin expression. Similar as reported earlier (19), β-catenin was highly expressed in the crypt of normal mouse small bowel, and its expression was decreased as cells extended the crypt–villus axis (Figure 1A). However, striking increase of β-catenin expression was seen in both tumor tissue and normal-appearing mucosa of JNK1 knockout mice compared with that in JNK1 wild-type mice (Figure 1B), suggesting that loss of JNK1 could cause β-catenin upregulation, the latter might be responsible for tumor formation in the intestine.
Fig. 1.
Genetic deficiency of JNK1 caused significant elevation of β-catenin in mouse intestine. Immunostaining of β-catenin was performed in normal-appearing intestinal normal mucosa and intestinal tumor tissues with anti-β-catenin antibody. Normal-appearing small intestinal tissues and tumor tissues were obtained from gender/age-matched JNK1+/+ (A) and JNK1−/− mice (B), respectively. In JNK1−/− mice (B), the tumor lesions were presented at the upside of dash line, whereas the normal-appearing tissues were presented at the downside.
Loss of JNK1 promotes β-catenin signaling
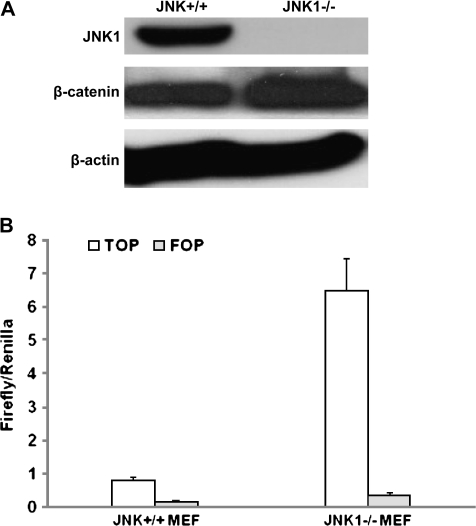
To validate the correlation of JNK1 and Wnt/β-catenin signaling, we determined whether JNK1 inactivation promoted β-catenin signaling in vitro. JNK+/+ and JNK1−/− MEFs were primary cultured and spontaneously immortalized following the NIH 3T3 protocol (26). Immunoblotting analysis confirmed the deficient status of JNK1 in JNK1−/− MEF (Figure 2A) and revealed that in consistent with alteration in mouse intestine, β-catenin protein level was also elevated in JNK1−/− MEFs (Figure 2A).
Fig. 2.
Loss of JNK1 promoted β-catenin protein expression and β-catenin-mediated transcriptional activity of TCF. (A) β-Catenin protein expression was upregulated in JNK1−/− MEFs. Both MEFs were lysed and subjected to immunoblotting analysis detecting the expression of β-catenin and JNK1. β-Actin was used as loading control. (B) β-Catenin–TCF4 reporter activity was upregulated in JNK1−/− MEFs. JNK1+/+ and JNK1−/− MEFs were cotransfected with Renilla and a construct of TOPFLASH or FOPFLASH. Twenty-four hours after transfection, cells were treated with Wnt3a condition medium of 1:4 dilution for 24 h and harvested for luciferase activity assay.
Wnt3a has been reported to induce phosphorylation of GSK3β at Ser-9, leading to accumulation of β-catenin and activation of Wnt pathway (27). Therefore, Wnt3a condition medium was prepared from the mouse L cells and used to treat MEFs transfected with the TCF-4 reporter plasmid TOPFLASH or the control inactive reporter FOPFLASH. TOPFLASH contains a luciferase reporter under the control of three copies of TCF-binding elements upstream of the thymidine kinase minimal promoter and is specifically regulated by Wnt/β-catenin signaling (28). Luciferase activity assay showed that Wnt3a condition medium strongly stimulated β-catenin-dependent transcription activity in JNK1+/+ MEF, and at a greater extent in JNK1−/− MEF (Figure 2B), indicating that loss of JNK1 led to enhanced β-catenin signaling activity.
The upregulation of β-catenin by loss of JNK1 in vivo (Figure 1) and in vitro (Figure 2) could activate transcriptional factors and downstream targets such as c-myc, the latter directly suppresses p21 promoter activity and p21 expression, resulting in increase of cell proliferation and decrease of cell differentiation, which might be responsible for intestinal tumor formation in JNK1-deficient mice.
Activated JNK1 inhibits Wnt/β-catenin signaling
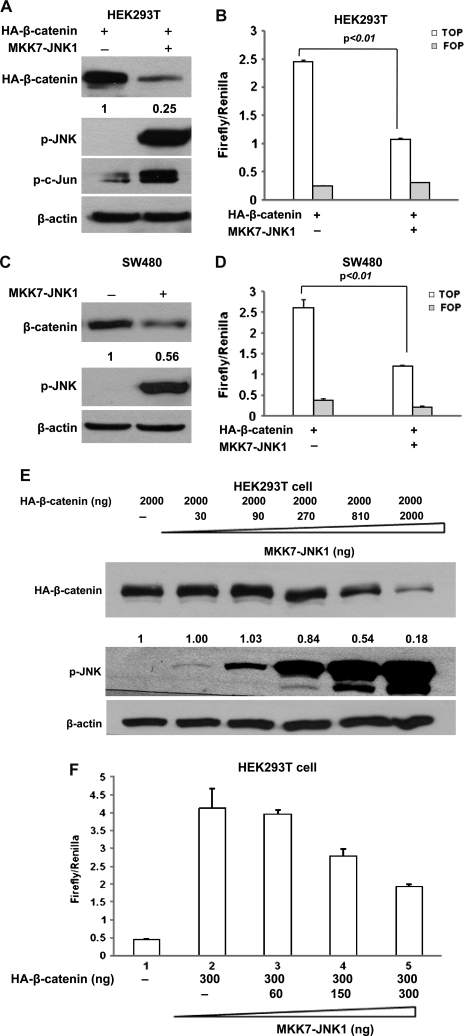
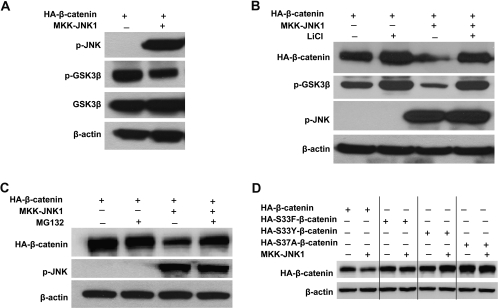
To identify the effect of JNK1 activation on Wnt/β-catenin signaling, we cotransfected constitutively activated JNK1 and β-catenin into HEK293T cells. The activated JNK1 is a fusion of JNK1 and MKK7, which causes constitutive JNK1 activation in cells without stimulation (22). As expected, immunoblotting analysis of the phosphorylation status of endogenous c-Jun with phospho-c-Jun-specific antibodies showed that the phosphorylation of c-Jun was greatly increased in HEK293T cells transfected with the constitutive active JNK1 construct (Figure 3A). Surprisingly, β-catenin protein level was reduced by 75% in HEK293T cells that exhibited JNK1 activation (Figure 3A), suggesting that activated JNK1 inhibited β-catenin expression.
Fig. 3.
Active JNK1 downregulated β-catenin expression and inhibited its transcriptional activity. (A) Active JNK1 reduced β-catenin protein level in HEK cell line HEK293T. HEK293T cells were cotransfected with pcDNA3-Flag-MKK7-JNK1 and pcDNA3-HA-β-catenin. Forty-eight hours after transfection, cells were harvested for immunoblotting analysis to detect the alterations of HA-β-catenin, p-JNK and p-c-Jun. β-Actin served as loading control. The density of band was quantified and the expression of β-catenin was normalized to β-actin. (B) Active JNK1 inhibited β-catenin-mediated transcriptional activity of TCF4. HEK293T cells were cotransfected with pcDNA3-Flag-MKK7-JNK1, pcDNA3-HA-β-catenin, TOPFLASH or FOPFLASH and a Renilla construct. Forty-eight hours after transfection, cells were harvested for luciferase activity assay. Each bar represents the mean ± SD for triplicate samples. Similar experiments were done in human colon cancer cell line SW480 (C and D). (E) Activated exogenous JNK1 reduced β-catenin protein level in a dose-dependent manner. HEK293T cells were cotransfected with pcDNA3-HA-β-catenin and different amounts of pcDNA3-Flag-MKK7-JNK1, as indicated. The protein was obtained for immunoblotting analysis to detect the alterations of HA-β-catenin and p-JNK. β-Actin served as loading control. (F) Activated exogenous JNK1 inhibited β-catenin-mediated transcriptional activity of TCF in a dose-dependent manner. HEK293T cells were cotransfected with pcDNA3-HA-β-catenin, TOPFLASH, Renilla, along with different amounts of pcDNA3-Flag-MKK7-JNK1, as indicated. Forty-eight hours after transfection, cells were harvested for luciferase activity assay. Each bar represents the mean ± SD for triplicate samples.
To determine whether JNK1 activation can also affect the signaling activity of β-catenin, we cotransfected constitutively activated JNK1 and β-catenin into HEK293T cells together with TOPFLASH or the control FOPFLASH. Without Wnt signaling, the TOPFLASH reporter only had minimal activity in HEK293T cells (data not shown), but the transfection of β-catenin lead to a 10-fold increase in TOPFLASH reporter activity (Figure 3B), and the activated JNK1 strongly suppressed the β-catenin-mediated luciferase activity driven from TOPFLASH reporter, but have no effect on the control FOPFLASH reporter (Figure 3B). The inhibitory effect of activated JNK1 on β-catenin-dependent transcriptional activity is probably attributed to the specific downregulation of β-catenin. Since overexpressed β-catenin is mainly restricted into nucleus as shown in Figure 6B and reported previously (29–31), it is possible that β-catenin is expelled by active JNK1 from nucleus and targeted for degradation. In fact, the total level of exogenous of β-catenin seems to be reduced as well by UV irradiation (30). In addition, as described later, our studies further demonstrated that the degradation of β-catenin by active JNK1 requires GSK3β and proteasome system.
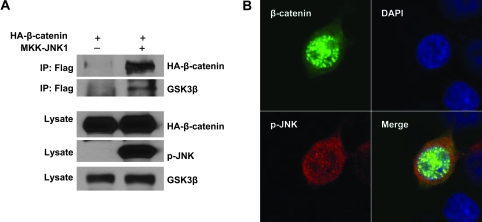
Fig. 6.
Activated JNK1 interacts with β-catenin and GSK3β. (A) Active JNK1 was binding to β-catenin and GSK3β by immunoprecipitation. β-Catenin (HA tagged) was cotransfected with empty vector or active JNK1 (Flag tagged) into HEK293T cells. Immunoprecipitation was performed with a Flag antibody. β-Catenin and GSK3β were analyzed with a HA antibody and anti-GSK3β antibody, respectively. (B) Active JNK1 and β-catenin were colocalized in the cell nucleus and cytoplasm. Active JNK1 (Flag tagged) and pEGFP-β-catenin were cotransfected into HEK293T cells. The cells were immunostained with a Flag antibody. Colocalization (yellow fluorescence) of active JNK1 (red fluorescence) and β-catenin (green fluorescence) was detected in the nucleus and cytoplasm.
In most colorectal cancers, the APC tumor suppressor gene is mutated. The mutated APC retains only partial activity for β-catenin phosphorylation (32). As a result, β-catenin is aberrantly accumulated in the nucleus and Wnt signaling is constitutively activated (28,33). To determine whether activated JNK1 inhibits Wnt/β-catenin in colorectal cancer cells, we used colon cancer cell line SW480 that contains a truncated form of APC (amino acids 1–1337) (34). The constitutively activated JNK1 was transfected into SW480 cells. Consistent with the observation in HEK293T (Figure 3A), endogenous β-catenin was inhibited ∼44% by active JNK1 (Figure 3C). β-Catenin-mediated transcriptional activity was also markedly suppressed by JNK1 activation (Figure 3D). Therefore, the negative regulation of JNK1 activation on the canonical Wnt pathway occurred in colon cancer cells.
To further confirm the regulation of JNK1 activation on Wnt/β-catenin signaling, a constant level of β-catenin was cotransfected with increasing amounts of constitutively activated JNK1 into HEK293T cells. We analyzed the level of exogenous β-catenin by immunoblotting assay using anti-HA tag antibody. As shown in Figure 3E, a decrease of β-catenin was seen with 810 ng of JNK1, and a dramatic reduction of β-catenin expression was seen with higher amounts of activated JNK1. Consistently, cotransfection of only 150 ng of active JNK1 repressed the β-catenin-mediated luciferase activity driven from TOPFLASH reporter by ∼33% and 300 ng repressed it by 55% (Figure 3F). Taken together, these results demonstrated the dose-dependent inhibition of JNK1 activation on Wnt/β-catenin signaling.
GSK3β and proteasome system were involved in the activated JNK1-mediated β-catenin downregulation
It is known that post-translational modifications play a critical role in the regulation of β-catenin turnover and that β-catenin is sequentially phosphorylated within its N-terminus by GSK3β (12). To investigate whether GSK3β activation is involved in the downregulation of β-catenin by activated JNK1, we examined the phosphorylation status of GSK3β by immunoblotting analysis. As shown in Figure 4A, Ser-9 phosphorylation of GSK3β was significantly decreased in HEK293T transfected with activated JNK1, indicating that GSK3β was activated and its activity was upregulated by JNK1 activation, promoting β-catenin degradation. This is the first time to report the regulation of GSK3β activity by JNK1 MAPK, although recent study showed that another MAPK member p38 directly phosphorylates and inactivates GSK3β, leading to an accumulation of β-catenin (35).
Fig. 4.
GSK3β and proteasomal degradation system are involved in the negative regulation of β-catenin by activated JNK1. (A) Active JNK1 suppressed phospho-Ser-9 GSK3β expression. pcDNA3-HA-β-catenin was transfected into HEK293T cells along with pcDNA3-Flag-MKK7-JNK1 or empty vector. Forty-eight hours after transfection, cells were harvested for immunoblotting analysis to detect the expression of GSK3β, p-GSK3β and p-JNK. (B) Blocking GSK3β activity by LiCl reduced β-catenin expression inhibition by activated JNK1. pcDNA3-HA-β-catenin was transfected into HEK293T cells along with pcDNA3-Flag-MKK7-JNK1 (lanes 3 and 4) or empty vector (lanes 1 and 2). Thirty-six hours after transfection, half of the cultures were treated overnight with 30 mM LiCl (lanes 2 and 4) and then harvested for immunoblotting analysis to detect the expression of HA-β-catenin, p-GSK3β and p-JNK. (C) Blocking proteasomal degradation by MG132 reduced the effect of active JNK1 on β-catenin degradation. HEK293T cells were cotransfected with pcDNA3-HA-β-catenin and pcDNA3-Flag-MKK7-JNK1 (lanes 3 and 4) or empty vector (lanes 1 and 2). Forty-four hours after transfection, 25 μM MG132 was added to the indicated samples (lanes 2 and 4). Cells were harvested 4 h later and subjected to immunoblotting analysis to detect the expression of HA-β-catenin, p-GSK3β and p-JNK. (D) Mutant β-catenin was resistant to activated JNK1-induced degradation. Wild-type β-catenin (HA-β-catenin) (lane 1 and 2) or various β-catenin mutants (HA-S33F β-catenin, lanes 3 and 4; HA-S33Y β-catenin, lanes 5 and 6 and HA-S37A β-catenin, lanes 7 and 8) were transfected into HEK293T cells along with pcDNA3-Flag-MKK7-JNK1 (lanes 2, 4, 6 and 8) or empty vector (lanes 1, 3, 5 and 7). Forty-eight hours after transfection, cells were harvested for immunoblotting analysis to determine the levels of HA-β-catenin. β-Actin served as loading control.
To further confirm the involvement of GSK3β activation in the regulation of β-catenin by JNK1 activation, lithium chloride (LiCl) was used to inhibit GSK3β activity, and the effect of activated JNK1 on β-catenin expression was determined. LiCl has been reported to induce the inhibitory Ser-9 phosphorylation of GSK3β while having no effect on other protein kinases (36,37). As shown in Figure 4B, the level of Ser-9 phosphorylation of GSK3β was elevated in the cells with LiCl treatment (lane 2 versus 1; lane 4 versus 3), whereas the JNK1 activity remained unchanged (lane 4 versus 3). Interestingly, the treatment with LiCl almost completely abrogated the ability of activated JNK1 to downregulate β-catenin expression (Figure 4B, lane 4 versus 3). Similarly, β-catenin inhibition by active JNK1 was also blocked in the presence of proteasome inhibitor MG132 (Figure 4C, lane 4 versus 3), demonstrating that activated JNK1 promotes β-catenin degradation through proteasome system.
It has been reported that phosphorylation of β-catenin at the Ser33 and Ser37 sites by GSK3β is essential for its degradation by proteasome system (38) and that various mutant β-catenin forms, such as S33Y, S33F and S37A, are relatively refractory to phosphorylation by GSK3β and hence to proteasomal degradation (39,40). Therefore, we constructed variant mutant β-catenin plasmids (as described in experimental procedure) and cotransfected them with active JNK1 plasmid into HEK293T cells, respectively. Immunoblotting analysis showed that mutant β-catenin, harboring S33Y, S33F or S37A, was much less susceptible to active JNK1-induced degradation (Figure 4D, lane 2 versus 1, lane 4 versus 3, lane 6 versus 5 and lane 8 versus 7), which further demonstrated the requirement of GSK3β for active JNK1 in targeting the degradation of β-catenin.
Activation of endogenous JNK1 downregulated β-catenin via GSK3β
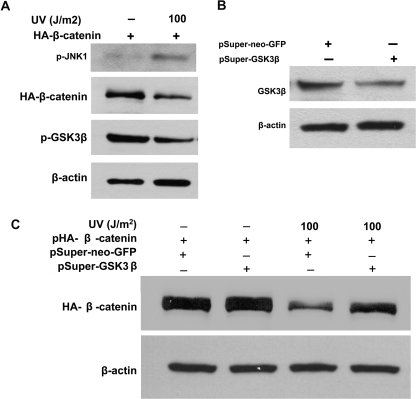
JNK1 is a stress-activated protein kinase that can be induced by various stimuli, including UV irradiation, inflammatory cytokines and chemotherapeutic drugs. In this study, UV irradiation was used to activate endogenous JNK1, and the subsequent effect on β-catenin expression was evaluated by immunoblotting. As shown in Figure 5A, indeed, UV irradiation induced activation of JNK1 (p-JNK1) in the HEK293T cells, corresponding with a significant reduction of β-catenin. Similar results were observed in the JNK1+/+ MEFs (data not shown). Reduced phosphorylation of GSK3β was also seen in UV-irradiated cells (Figure 5A), meaning activation of endogenous JNK1 enhanced GSK3β activity resulting from the reduction of GSK3β phosphorylation.
Fig. 5.
Activation of endogenous JNK1 downregulated β-catenin via GSK3β. (A) UV-mediated JNK1 activation promoted GSK3β activity and downregulated β-catenin expression. HEK293T cells were transfected with pcDNA3-HA-β-catenin plasmid. Twenty-four hours after transfection, cells were exposed to UV irradiation at 100 J/m2. The cells were harvested for immunoblotting analysis to detect the activation of JNK1 (p-JNK1) and GSK3β (p-GSK3β) and alterations of HA-β-catenin 2 h after UV exposure. (B) GSK3β was knocked down by RNAi. HEK293T cells were transfected with pSuper-neo-GSK3β targeting human GSK3β or empty vector pSuper-neo-GFP. Forty-eight hours after transfection, cells were harvested to determine the knockdown efficiency by immunoblotting analysis. (C) RNAi-mediated knockdown of GSK3β attenuated the reduction of β-catenin expression induced by UV irradiation. HEK293T cells were cotransfected with pcDNA3-HA-β-catenin and pSuper-neo-GSK3β or empty vector pSuper-neo-GFP, respectively. Forty-eight hours after transfection, cells were exposed to UV irradiation at 100 J/m2. The alterations of HA-β-catenin were analyzed by immunoblotting 2 h after UV exposure. β-Actin served as loading control.
To further confirm the role of GSK3β in the inhibition of β-catenin induced by UV-mediated JNK1 activation, RNAi was applied to knock down GSK3β expression. As shown in Figure 5B, GSK3β RNAi plasmid (pSuper-GSK3β) effectively reduced GSK3β protein level in comparison with the control cells that were transfected with control RNAi plasmid (pSuper-neo-GFP) (Figure 5B). Next, the β-catenin plasmid and pSuper-GSK3β plasmid were cotransfected into HEK293T cells and the cells were then exposed to UV irradiation to active endogenous JNK1 (Figure 5C). β-Catenin was reduced in the cells with control RNAi plasmid transfection and UV exposure (Figure 5C, lane 3 versus 1). However, the reduction of β-catenin was blocked by GSK3β RNAi (Figure 5C, lane 4).
Active JNK1 interacts with β-catenin
Our data provided strong evidence that JNK1 negatively regulated β-catenin through GSK3β. Thus, it is essential to elucidate whether there is an interaction between JNK1 and β-catenin and/or a complex of JNK1–GSK3β–β-catenin. Coimmunoprecipitation assay and confocal microscopy for subcellular localization were applied to examine the potential interaction and/or complex. Flag-tagged constitutive active JNK1 and HA-tagged β-catenin were cotransfected into HEK293T cells. When active JNK1 was immuoprecipitated with a Flag antibody, β-catenin was detected in the immunocomplex by immunoblotting analysis using a HA antibody (Figure 6A, lane 2). As a control, Flag antibody was not able to precipitate β-catenin in the cells without Flag-tagged constitutive-active JNK1 (Figure 6A, lane 1). Thus, we conclude that active JNK1 interacts with β-catenin. As expected, GSK3β was also detected in the precipitate by a Flag antibody (Figure 6A). In fact, as the phosphorylation substrate for GSK3β, β-catenin has been reported to interact with GSK3β in the cytoplasm (41,42). Therefore, JNK1, GSK3β and β-catenin might form a multiprotein complex.
The interaction between active JNK1 and β-catenin was confirmed by a colocalization assay. Flag-tagged constitutive-active JNK1 was transfected into HEK293T cells. The cells were fixed and immunostained with anti-Flag antibody for active JNK1 (red fluorescence). Indeed, active JNK1 and β-catenin proteins were colocalized in the nucleus and cytoplasm (Figure 6B), consisting with immunoprecipitation results, even though β-catenin was mainly localized in the nucleus and small portion in the cytoplasm (Figure 6B), as reported previously (29–31), but active JNK1 showed mixed nuclear–cytoplasmic but predominant cytoplasmic localization (Figure 6B).
Our results strongly demonstrated that activated JNK1 formed a complex with GSK3β and increased its activity and then degraded β-catenin. However, whether active JNK1 directly interacts with GSK3β or their interaction is mediated by other molecules is not clear and is under investigation.
Taken together, our present study showed that loss of JNK1 caused upregulation of β-catenin expression and transcription both in vivo and in vitro, but activated JNK1 interacted with and inhibited β-catenin signaling through GSK3β and proteasome system. We therefore concluded that the interaction between JNK1 and β-catenin might play an important role in the maintenance of intestinal homeostasis and in the modulation of intestinal tumorigenesis.
Funding
National Institutes of Health (R01 CA112081); American Institute for Cancer Research (05A121).
Acknowledgments
We would like to thank Drs Zhinan Yin, Richard Flavell and Jian Tao (Yale University School of Medicine, New Heaven, CT) for providing JNK1−/− mouse and thank Dr Zijie Sun (Stanford University School of Medicine, Stanford, CA) and Dr Eric Fearon (University of Michigan, Ann Arbor, MI) for providing β-catenin plasmids and thank Dr Christine Blattner (Forschungszentrum Karlsruhe, Institute for Toxicology and Genetics, Germany) for providing GSK3β RNAi plasmids. We would also like to thank Drs Chang Tong and Xiuli Bi (University of Illinois at Chicago) for technical assistance and Ms Mei Ling Chen (Research Resources Center, University of Illinois at Chicago) for confocal microscopy image analysis.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- APC
adenomatous polyposis coli
- GSK3β
glycogen synthase kinase 3β
- HA
hemagglutinin
- HEK
human embryonic kidney
- JNK
c-Jun N-terminal kinase
- LiCl
lithium chloride
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- PBS
phosphate-buffered saline
- RNAi
RNA interference
- TCF
T-cell factor
- UV
ultraviolet
References
- 1.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 2.Johnson GL, et al. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 3.She QB, et al. Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 2002;62:1343–1348. [PubMed] [Google Scholar]
- 4.Winn RA, et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J. Biol. Chem. 2005;280:19625–19634. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- 5.Tong C, et al. c-Jun NH2-terminal kinase 1 plays a critical role in intestinal homeostasis and tumor suppression. Am. J. Pathol. 2007;171:297–303. doi: 10.2353/ajpath.2007.061036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng C, et al. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 7.Nateri AS, et al. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 8.Liao G, et al. Jun NH2-terminal kinase (JNK) prevents nuclear beta-catenin accumulation and regulates axis formation in Xenopus embryos. Proc. Natl Acad. Sci. USA. 2006;103:16313–16318. doi: 10.1073/pnas.0602557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rui Y, et al. A beta-catenin-independent dorsalization pathway activated by Axin/JNK signaling and antagonized by aida. Dev. Cell. 2007;13:268–282. doi: 10.1016/j.devcel.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, et al. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens J, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda S, et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishida S, et al. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J. Biol. Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 15.Bienz M, et al. Armadillo/beta-catenin signals in the nucleus—proof beyond a reasonable doubt? Nat. Cell Biol. 2003;5:179–182. doi: 10.1038/ncb0303-179. [DOI] [PubMed] [Google Scholar]
- 16.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 17.Pinto D, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Es JH, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat. Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 19.van de Wetering M, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 20.Yang W, et al. Inactivation of p21WAF1/cip1 enhances intestinal tumor formation in Muc2-/- mice. Am. J. Pathol. 2005;166:1239–1246. doi: 10.1016/S0002-9440(10)62342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han A, et al. A direct protein-protein interaction is involved in the suppression of beta-catenin transcription by retinoid X receptor alpha in colorectal cancer cells. Cancer Biol. Ther. 2008;7:454–459. doi: 10.4161/cbt.7.3.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei K, et al. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol. Cell. Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulikov R, et al. Glycogen synthase kinase 3-dependent phosphorylation of Mdm2 regulates p53 abundance. Mol. Cell. Biol. 2005;25:7170–7180. doi: 10.1128/MCB.25.16.7170-7180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolligs FT, et al. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma M, et al. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J. Biol. Chem. 2002;277:30935–30941. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- 26.Wadhwa R, et al. Spontaneous immortalization of mouse fibroblasts involves structural changes in senescence inducing protein, mortalin. Biochem. Biophys. Res. Commun. 1993;197:202–206. doi: 10.1006/bbrc.1993.2461. [DOI] [PubMed] [Google Scholar]
- 27.Shi C-S, et al. The mitogen-activated protein kinase kinase kinase kinase GCKR positively regulates canonical and noncanonical Wnt signaling in B lymphocytes. Mol. Cell. Biol. 2006;26:6511–6521. doi: 10.1128/MCB.00209-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korinek V, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 29.Kim K, et al. Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol. Biol. Cell. 2000;11:3509–3523. doi: 10.1091/mbc.11.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao G, et al. Jun NH2-terminal kinase (JNK) prevents nuclear beta-catenin accumulation and regulates axis formation in Xenopus embryos. Proc. Natl Acad. Sci. USA. 2006;103:16313–16318. doi: 10.1073/pnas.0602557103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thyssen G, et al. LZTS2 is a novel beta-catenin-interacting protein and regulates the nuclear export of beta-catenin. Mol. Cell. Biol. 2006;26:8857–8867. doi: 10.1128/MCB.01031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, et al. Adenomatous polyposis coli (APC) differentially regulates beta-catenin phosphorylation and ubiquitination in colon cancer cells. J. Biol. Chem. 2006;281:17751–17757. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 33.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 34.Nishisho I, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 35.Thornton TM, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F, et al. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J. Biol. Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 37.Tan J, et al. Pharmacologic modulation of glycogen synthase kinase-3beta promotes p53-dependent apoptosis through a direct Bax-mediated mitochondrial pathway in colorectal cancer cells. Cancer Res. 2005;65:9012–9020. doi: 10.1158/0008-5472.CAN-05-1226. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs SY, et al. Oncogenic beta-catenin signaling networks in colorectal cancer. Cell Cycle. 2005;4:1522–1539. doi: 10.4161/cc.4.11.2129. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs SY, et al. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene. 1999;18:2039–2046. doi: 10.1038/sj.onc.1202760. [DOI] [PubMed] [Google Scholar]
- 40.Hart M, et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 41.Davies G, et al. The interaction between beta-catenin, GSK3beta and APC after motogen induced cell-cell dissociation, and their involvement in signal transduction pathways in prostate cancer. Int. J. Oncol. 2001;18:843–847. doi: 10.3892/ijo.18.4.843. [DOI] [PubMed] [Google Scholar]
- 42.Provost E, et al. Functional correlates of mutation of the Asp32 and Gly34 residues of beta-catenin. Oncogene. 2005;24:2667–2676. doi: 10.1038/sj.onc.1208346. [DOI] [PubMed] [Google Scholar]