Abstract
Objectives
To compare the effectiveness of cranberry extract with low-dose trimethoprim in the prevention of recurrent urinary tract infections (UTIs) in older women.
Patients and methods
One hundred and thirty-seven women with two or more antibiotic-treated UTIs in the previous 12 months were randomized to receive either 500 mg of cranberry extract or 100 mg of trimethoprim for 6 months. Trial registration: ISRCTN80031108.
Results
Thirty-nine of 137 participants (28%) had an antibiotic-treated UTI (25 in the cranberry group and 14 in the trimethoprim group); difference in proportions relative risk 1.616 (95% CI: 0.93, 2.79) P = 0.084. The time to first recurrence of UTI was not significantly different between the groups (P = 0.100). The median time to recurrence of UTI was 84.5 days for the cranberry group and 91 days for the trimethoprim group (U = 166, P = 0.479). There were 17/137 (12%) withdrawals from the study, 6/69 (9%) from the cranberry group and 11/68 (16%) from the trimethoprim group (P = 0.205), with a relative risk of withdrawal from the cranberry group of 0.54 (95% CI: 0.19, 1.37).
Conclusions
Trimethoprim had a very limited advantage over cranberry extract in the prevention of recurrent UTIs in older women and had more adverse effects. Our findings will allow older women with recurrent UTIs to weigh up with their clinicians the inherent attractions of a cheap, natural product like cranberry extract whose use does not carry the risk of antimicrobial resistance or super-infection with Clostridium difficile or fungi.
Keywords: urinary infections, UTIs, antibiotics
Introduction
Urinary infection is the most common bacterial infection in older people and recurrent urinary tract infection (UTI) is particularly common in older women. The current management of recurrent UTI involves either repeated courses of antibiotics or low-dose long-term antibiotic prophylaxis.1 The evidence in support of antibiotic prophylaxis is strong, with 11 placebo controlled trials of which 10 show a significant treatment benefit.1 In these trials, antibiotic prophylaxis was highly effective: number needed to treat (NNT) to prevent one recurrence was 1.85, but side effects severe enough to stop treatment were equally common (NNT for severe side effects was 1.58). The main side effects measured in the trial were fungal super-infection (oral or vaginal thrush) and gastrointestinal infections. However, a growing reluctance to prescribe antibiotics is emerging because of concerns about antimicrobial resistance and other adverse effects on the normal bacterial flora, such as super-infection with Clostridium difficile. At the same time, there has been a resurgence of interest in the role of cranberry products, stimulated by the conclusion of a Cochrane review that ‘there is some evidence from two good quality RCTs that cranberry juice may decrease the number of symptomatic UTIs over a 12 month period in younger women’ (mean ages 32 and 43 years).2
The Cochrane review of antibiotic prophylaxis and SIGN guideline 88 stated that a head-to-head trial of cranberry versus low-dose antibiotic in the prevention of recurrent UTI was required because previous placebo controlled trials had demonstrated effectiveness for both, with the effectiveness of antibiotic therapy being considerably superior.2,3 While cranberry juice has been studied in an underpowered trial of UTI prevention in 376 hospitalized older people,4 there is a dearth of evidence concerning its effectiveness in recurrent urinary infections in old age. This is a surprising gap in the literature, given that UTI occurs more frequently in old age than at any other time of life. In contrast, the literature on antibiotic prophylaxis does suggest that this is likely to be effective in older women. Of the 11 trials identified in the Cochrane review of antibiotic prophylaxis for recurrent UTI, eight included post-menopausal women.1 We therefore designed a trial to compare the effectiveness and acceptability of low-dose trimethoprim with cranberry products in the prevention of recurrent UTI in older women. Trial registration: ISRCTN80031108.
Methods
Study population
Inclusions: community dwelling women aged ≥45 years with at least two antibiotic-treated UTIs or episodes of cystitis in the previous 12 months confirmed by their general practitioner (GP) but not necessarily confirmed by microbiological culture. Participants were recruited predominantly through the eastern node of the Scottish Primary Care Research Network and also from responses to an article in a local newspaper featuring the study.
Exclusions: previous urological surgery, stones or anatomical abnormalities of the urinary tract; urinary catheter; diabetes mellitus; immunocompromised; pyelonephritis; severe renal impairment; blood dyscrasias; symptomatic UTI at baseline; cognitive impairment precluding informed consent; resident in institutional care; on long-term antibiotic therapy; on warfarin therapy; regular cranberry consumers; child bearing potential; unwilling to participate.
As a number of potential participants were occasional cranberry consumers, it was decided that such individuals could participate provided that there was a 2 week washout period prior to commencing the study.
Written informed consent was obtained from participants and the study was approved by the Tayside Committee on Medical Research Ethics (06/S1402/23) and the MHRA (Eudract no: 2006-001313-15).
Randomization
Participants were randomized to receive either one capsule of 500 mg of cranberry extract (Cran-Max™; Buckton Scott Health Products Ltd, UK) taken at bedtime for 6 months or one capsule of 100 mg of trimethoprim. Randomization was performed off-site by DHP pharma in Powys, UK, which is an MHRA-approved manufacturing site. Randomization was performed in blocks of four using Prisym PFW clin software to generate random numbers. Participants were given a study number sequentially by the research nurses. A copy of the treatment code was held by the Clinical Trials Pharmacist in Ninewells Hospital, Dundee.
Cranberry product and trimethoprim preparation
DHP pharma over-encapsulated 100 mg trimethoprim tablets into red size 00 capsules, and filled red size 00 capsules with 500 mg of cranberry extract (Cran-Max™). Both sets of capsules were identical in appearance.
Urine culture methods
A urine specimen was obtained at baseline from all participants and cultured in the Medical Microbiology Laboratory using standard protocols. Identification and susceptibility testing on positive cultures were performed by Vitek I (bioMérieux) or Stokes' susceptibility testing and chromogenic agar for speciation of Escherichia coli. Baseline results were not reported to clinical or research staff. Specimens from participants who developed symptoms of UTI during the trial were processed in the same way.
Outcome measures
Primary outcome
This was the proportion of participants in each group experiencing a recurrence of an antibiotic-treated UTI and the time to first recurrence.
Participants were censored (i.e. withdrawn from study participation) after their first UTI. UTI was defined as clinical symptoms of dysuria and frequency in the absence of vaginal discharge with or without microbiological confirmation.
Secondary outcomes
Adherence
The participants were provided with two sealed tubs at baseline each with 200 capsules containing either 100 mg of trimethoprim or 500 mg of cranberry extract. Adherence was assessed by capsule counting at 3 and 6 months and expressed as the number of capsules consumed divided by the number of capsules that should have been consumed during the duration of each individual's period of study participation.
Adverse events and follow-up
After the baseline visit, further home visits occurred at 3 and 6 months to re-check study eligibility, record adverse events, check adherence and to note the courses of antibiotics that had been prescribed for any indications. Participants were telephoned at 1, 2, 4 and 5 months to encourage participation and adherence, and to record any adverse events.
Statistical methods
Sample size
Based on the available literature, it was predicted that a final sample of 102 participants would be required to have 80% power at P = 0.05 of detecting a reduction in occurrences of urinary infection from 16% in the cranberry group to 1% in the trimethoprim group.5,6 In anticipation of a dropout rate of 15%, we intended to recruit at least 120 participants.
Statistical analysis
Data were entered onto an Excel database and then analysed using a Statistical Calculator v.2.06 (Mole Software, Alpes de Haute-Provence 04230, France). Full statistical analysis was completed prior to breaking the treatment code. Analysis was by intention to treat. Time to first recurrence of infection is presented as a Kaplan–Meier curve and differences between the groups were assessed using the log-rank test.
Results
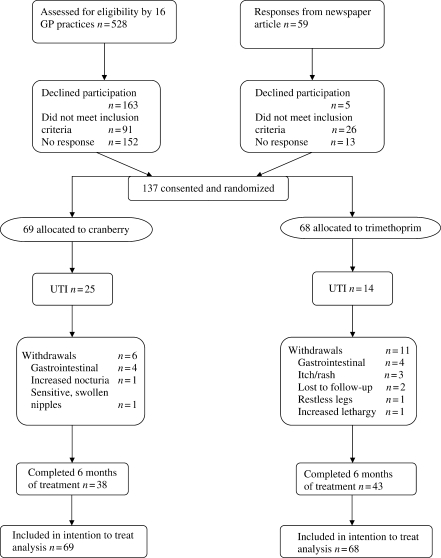
A total of 137 women were randomized, 69 to cranberry and 68 to trimethoprim (Figure 1).
Figure 1.
CONSORT flow chart.
There were no significant differences between the groups at baseline (Table 1).
Table 1.
Baseline characteristics
| Variables | Cranberry (n = 69) | Trimethoprim (n = 68) |
|---|---|---|
| Age (years) | ||
| mean (SD) | 62.6 (10.8) | 63.3 (10.1) |
| range | 45–93 | 46–88 |
| Living circumstances | ||
| living alone | 12 | 18 |
| sheltered housing | 1 | 7 |
| Number of medications | ||
| median (range) | 3 (0–13) | 4 (0–11) |
| Length of history of UTIs (years) | ||
| median (range) | 11 (1–50) | 18 (1–53) |
| Number of self-reported UTIs in past 12 months | ||
| median (range) | 3 (2–15) | 3 (2–8) |
| Number of antibiotic-treated UTIs in past 12 monthsa | ||
| median (range) | 3 (2–15) | 2 (2–8) |
| Bacteriuria at baseline | 5/69 (7.2%) | 7/68 (10.3%) |
| E. coli | 2 | 6 |
| K. pneumoniae | 1 | 0 |
| Streptococcus B | 1 | 1 |
| E. faecalis | 1 | 0 |
aMann–Whitney U-test (P = 0.72).
Primary outcome
A total of 39/137 (28%) of participants had a symptomatic antibiotic-treated UTI (25 in the cranberry group and 14 in the trimethoprim group); the difference in proportions was relative risk 1.616 (95% CI: 0.93, 2.79) P = 0.084.
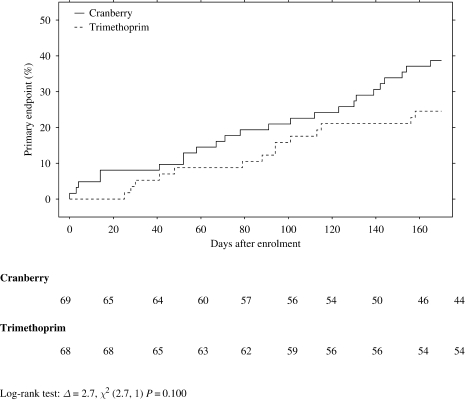
The time to first recurrence of UTI was not significantly different between the groups [log-rank test: Δ = 2.7, χ2 (2.7, 1) P = 0.100].
The median time to recurrence of UTI was 84.5 days for the cranberry group and 91 days for the trimethoprim group (U = 166, P = 0.479).
Secondary outcomes
Withdrawals
There were 17/137 (12%) withdrawals from the study, 6/69 (9%) from the cranberry group and 11/68 (16%) from the trimethoprim group (P = 0.205), with a relative risk of withdrawal from the cranberry group of 0.54 (95% CI: 0.19, 1.37).
The reasons were as follows: for the cranberry group, gastrointestinal upset n = 4; increased nocturia n = 1; sensitive swollen nipples n = 1 and the trimethoprim group, gastrointestinal upset n = 4; itch/rash n = 3; lost to follow-up n = 2; restless legs n = 1; increased lethargy n = 1. While gastrointestinal upsets were equally common in both groups, itch/rash and loss to follow-up occurred more commonly in the trimethoprim group.
Other adverse events
Other adverse events were similar between the groups (Table 2).
Table 2.
Adverse events other than those resulting in withdrawal
|
n (%) |
||
|---|---|---|
| Adverse event | cranberry (n = 69) | trimethoprim (n = 68) |
| Non-UTI urinary symptoms | 12 (17) | 9 (13) |
| Gastrointestinal upset | 9 (13) | 13 (19) |
| Thrush | 3 (4) | 3 (4) |
| Colds/flu | 4 (6) | 4 (6) |
| Difficulty swallowing capsules/aftertaste/dry mouth | 4 (6) | 1 (1) |
| Exacerbation of back pain | 4 (6) | 2 (3) |
| Tiredness/lethargy | 2 (3) | 3 (4) |
| Itch/rash | 2 (3) | 2 (3) |
| Abdominal abscess | 1 (1) | 0 |
| Breast carcinoma | 1 (1) | 0 |
| Deterioration in bilateral vision | 1 (1) | 0 |
| Vaginal dryness/atrophy | 1 (1) | 2 (3) |
| Falls | 1 (1) | 4 (6) |
| Shingles | 0 | 1 (1) |
| Excessive thirst | 0 | 1 (1) |
| Type II diabetes | 0 | 1 (1) |
| Routine surgery | 0 | 4 (6) |
| Migraine | 0 | 1 (1) |
Adherence
Adherence was good in both groups. Median (range) adherence was 99 (25–149)% and 100 (66–112)% in the cranberry and trimethoprim groups, respectively.
Antibiotic use
A total of 15/69 (22%) participants in the cranberry group and 17/68 (25%) in the trimethoprim group were prescribed antibiotics for indications other than UTI during their period of participation.
Causative organisms
For the 39 women who developed a symptomatic UTI during the trial, the urine culture results were as follows: E. coli, 16 (9 in the cranberry group and 7 in the trimethoprim group); Klebsiella pneumoniae, 3 (2 in the cranberry group and 1 in the trimethoprim group); no growth, 4 (2 in each group); mixed growth, 1 (in the cranberry group); no significant bacteriuria, 6 (4 in the cranberry group and 2 in the trimethoprim group). No urine specimen was obtained in 9 (7 in the cranberry group and 2 in the trimethoprim group).
Antibiotic resistance patterns
At baseline testing, 12 women had positive urine cultures with ≥104 cfu/mL. Of these, 8 were E. coli (6 susceptible to trimethoprim), 2 group B Streptococcus (not tested against trimethoprim) and one each of K. pneumoniae (trimethoprim-susceptible) and Enterococcus faecalis (trimethoprim-resistant). Overall, therefore, 7/9 (78%) subjects bacteriuric at baseline with Gram-negative bacteria had trimethoprim-susceptible organisms.
Nineteen out of 39 (49%) women had symptomatic recurrences with positive urine cultures of ≥104 cfu/mL. All were Gram-negative isolates. Of those with E. coli cultures, 11/16 were trimethoprim-susceptible, and of those with K. pneumoniae, 2/3 were trimethoprim-susceptible isolates. Thus, 13/19 of this subgroup of participants had trimethoprim-susceptible isolates.
Discussion
Our head-to-head trial has shown that for older women with recurrent urinary infections, the 6 month risk of developing a UTI on cranberry products is only 60% greater than that on low-dose trimethoprim; this difference was not statistically significant. Compared with cranberry extract, treatment with trimethoprim conferred fewer than 7 additional UTI-free days. Our primary endpoint was symptomatic UTI treated by the GP. However, recurrence rates for microbiologically confirmed symptomatic UTI were also similar (16% for cranberry versus 12% for trimethoprim).
Validity of the trial
Our target of 120 participants for the trial was set to have 80% power to detect a difference in effectiveness of 15% in risk of recurrence between trimethoprim and cranberry, and assumed a 15% dropout rate. In fact we recruited 137 participants, of whom 17 (12%) withdrew but only two (1.5%) were lost to follow-up. The remaining withdrawals were because of side effects, which was one of the secondary outcomes for the trial. The participants represented 29% of the 470 people who were screened and met the inclusion criteria. Most participants were recruited by screening patient records from 16 GP practices, which is 20% of all the practices in Tayside. The primary outcome was objective (first recurrence of clinical UTI treated by the GP) and could not be influenced by the investigators. Moreover, participants and investigators were unaware of the participants' treatment group until the statistical analysis had been completed. Adherence to treatment in our study was very good in both groups, which together with the modest withdrawal rate lends further support to the acceptability of encapsulated cranberry extracts.2
The internal validity of the trial therefore seems good and we also believe that the results should be applicable to other primary care populations. Nonetheless, the trial result was not what we expected. The literature had led us to predict that trimethoprim would prove considerably more effective, but only at the expense of more adverse events. Withdrawals were indeed higher in the trimethoprim group, but other adverse event rates turned out to be low and remarkably similar between the groups.
Possible explanations
We have considered the possibility that neither treatment was effective. At the design stage, we considered the inclusion of a placebo group but rejected this option because Cochrane systematic reviews have concluded that both antibiotics and cranberry products are effective in preventing UTIs.1,2 There is uncertainty about how effective both treatments are in older women, especially for cranberry but we did not consider that this was sufficient justification for inclusion of a placebo group. Moreover our eligibility criteria required two or more antibiotic-treated UTIs in the previous 12 months so it was reasonable to expect that without prophylaxis most women would experience a recurrence within 6 months. It is therefore unlikely that totally ineffective prophylaxis would have allowed 81 (59%) of the 137 participants to have completed 6 months of treatment free of UTI recurrence.
We selected trimethoprim for antibiotic prophylaxis because it is as effective as co-trimoxazole for treatment of UTI but has fewer side effects.7 Trimethoprim was included in one of the placebo controlled clinical trials of antibiotic prophylaxis for UTI and proved as effective as co-trimoxazole and nitrofurantoin.6 Resistance to trimethoprim in bacteria causing UTIs has increased in Northern European and American countries from 10% to 15% in the 1970s to 15% to 20% in the 1980s.8 The prevalence of trimethoprim resistance in the E. coli isolates from our patients was 29%, which is only slightly higher than the average resistance for all primary care isolates from midstream urines in our laboratory (excluding catheter urine samples) of 24% in 2004. Resistance has yet to reach a level that should markedly reduce the effectiveness of trimethoprim in lower UTI. The recurrence rate after treatment of symptomatic lower UTI has been estimated for different levels of resistance to co-trimoxazole.9 At a resistance rate of zero, the recurrence rate was estimated to be 5%, rising to 12% at 20% resistance and 15% at 30% resistance.9 These calculations assumed that 60% of women would respond to co-trimoxazole if their infection was caused by a resistant organism. In a recent UK study, 61% of women with lower UTI caused by trimethoprim-resistant bacteria were symptom-free 1 week after trimethoprim treatment and 58% were free of bacteriuria 1 month after treatment.10 We believe that trimethoprim prophylaxis should be effective at the levels of resistance observed in our study and in the Tayside population. It is possible that nitrofurantoin might have proved more effective as resistance is less common; however, the evidence suggests that it has more side effects.1
We selected cranberry extract in preference to juice for our study because previous work has shown equivalent efficacy between cranberry capsules (containing at least 1:30 parts concentrated juice) and cranberry juice.11 Furthermore, cranberry capsules have potential advantages over juice; capsules are more convenient, cheaper (costs for 1 year of treatment are from £42 to £125 for cranberry tablets or capsules versus £175 to £257 for cranberry juice) and may overcome compliance issues for some individuals.12 The high rates of withdrawal from some previous studies suggest that cranberry juice may not be an acceptable therapy over a long period of time.
Our power calculation estimated the difference in effect size to be 15%. In our trial, the difference in effect size was 15% (40% for cranberry versus 25% for trimethoprim), which was not statistically significant because the efficacy of both treatments was lower than we had predicted. We estimated that recurrence with cranberry would be 16% to 20% and 1% to 5% with antibiotics.1,5,11 Our data regarding time to first recurrence suggest that the added benefit to patients from antibiotics is likely to be modest (Figure 2) and therefore that the value of information from a larger trial in older women is unlikely to justify the cost.13
Figure 2.
Time to first recurrence of UTI.
Conclusions
Our trial is the first to evaluate cranberry in the prevention of recurrent UTIs specifically in older women, and the first head-to-head double-blind comparison of cranberry versus antibiotic prophylaxis.
Trimethoprim had a very limited advantage over cranberry extract in the prevention of recurrent UTIs in older women and had more adverse effects. Our findings will allow older women with recurrent UTIs to weigh up with their clinicians the inherent attractions of a cheap, natural product like cranberry extract whose use does not carry the risk of antimicrobial resistance or super-infection with C. difficile or fungi.
Further research is now required to discover if our findings might apply to younger individuals with recurrent urinary infections.
Funding
Moulton Charitable Foundation. Buckton Scott Health Products Ltd, UK supplied the Cran-Max™ free of charge. Neither the funder nor the supplier had any role in the concept, design, running, analysis, interpretation or reporting of the study.
Transparency declarations
No conflicts of interest to declare.
Contributions: M. E. T. M., P. D. and G. P. participated in study design, I. A. participated in recruitment and data collection, F. D. participated in the analysis, and all participated in the interpretation of the data, drafting and revising the paper and approving the final version. M. E. T. M. is the guarantor for the paper.
References
- 1.Albert X, Huertas I, Pereiro II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004:CD001209. doi: 10.1002/14651858.CD001209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008:CD001321. doi: 10.1002/14651858.CD001321.pub4. [DOI] [PubMed] [Google Scholar]
- 3.Scottish Intercollegiate Guidelines Network. Management of Suspected Bacterial Urinary Tract Infection in Adults. SIGN 88. Edinburgh: 2006. pp. 1–46. [Google Scholar]
- 4.McMurdo ME, Bissett LY, Price RJ, et al. Does ingestion of cranberry juice reduce symptomatic urinary tract infections in older people in hospital? A double-blind, placebo-controlled trial. Age Ageing. 2005;34:256–61. doi: 10.1093/ageing/afi101. [DOI] [PubMed] [Google Scholar]
- 5.Kontiokari T, Sundqvist K, Nuutinen M, et al. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322:1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamm WE, Counts GW, Wagner KF, et al. Antimicrobial prophylaxis of recurrent urinary tract infections: a double-blind, placebo-controlled trial. Ann Intern Med. 1980;92:770–5. doi: 10.7326/0003-4819-92-6-770. [DOI] [PubMed] [Google Scholar]
- 7.Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA) Clin Infect Dis. 1999;29:745–58. doi: 10.1086/520427. [DOI] [PubMed] [Google Scholar]
- 8.Huovinen P, Sundstrom L, Swedberg G, et al. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother. 1995;39:279–89. doi: 10.1128/aac.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta K, Hooton TM, Stamm WE. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann Intern Med. 2001;135:41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 10.McNulty CA, Richards J, Livermore DM, et al. Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J Antimicrob Chemother. 2006;58:1000–8. doi: 10.1093/jac/dkl368. [DOI] [PubMed] [Google Scholar]
- 11.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558–62. [PubMed] [Google Scholar]
- 12.Foda MM, Middlebrook PF, Gatfield CT, et al. Efficacy of cranberry in prevention of urinary tract infection in a susceptible pediatric population. Can J Urol. 1995;2:98–102. [PubMed] [Google Scholar]
- 13.Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ. 1996;5:513–24. doi: 10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]