Abstract
Background
Cisplatin is a cornerstone anticancer drug with pronounced ototoxicity, whereas oxaliplatin, a platinum derivative with a different clinical profile, is rarely ototoxic. This difference has not been explained.
Methods
In HCT116 cells, cisplatin (20 μM)-induced apoptosis was reduced by a calcium chelator from 9.9-fold induction (95% confidence interval [CI] = 8.1- to 11.7-fold), to 3.1-fold induction (95% CI = 2.0- to 4.2-fold) and by superoxide scavenging from 9.3-fold (95% CI = 8.8- to 9.8-fold), to 5.1-fold (95% CI = 4.4- to 5.8-fold). A guinea pig model (n = 23) was used to examine pharmacokinetics. Drug concentrations were determined by liquid chromatography with post-column derivatization. The total platinum concentration in cochlear tissue was determined by inductively coupled plasma mass spectrometry. Drug pharmacokinetics was assessed by determining the area under the concentration–time curve (AUC). Statistical tests were two-sided.
Results
In HCT116 cells, cisplatin (20 μM)-induced apoptosis was reduced by a calcium chelator from 9.9-fold induction (95% confidence interval [CI] = 8.1- to 11.7-fold to 3.1-fold induction) (95% CI = 2.0- to 4.2-fold) and by superoxide scavenging (from 9.3-fold, 95% CI = 8.8- to 9.8-fold, to 5.1-fold, 95% CI = 4.4- to 5.8-fold). Oxaliplatin (20 μM)-induced apoptosis was unaffected by calcium chelation (from 7.1- to 6.2-fold induction) and by superoxide scavenging (from 5.9- to 5.6-fold induction). In guinea pig cochlea, total platinum concentration (0.12 vs 0.63 μg/kg, respectively, P = .008) and perilymphatic drug concentrations (238 vs 515 μM × minute, respectively, P < .001) were lower after intravenous oxaliplatin treatment (16.6 mg/kg) than after equimolar cisplatin treatment (12.5 mg/kg). However, after a non-ototoxic cisplatin dose (5 mg/kg) or the same oxaliplatin dose (16.6 mg/kg), the AUC for perilymphatic concentrations was similar, indicating that the two drugs have different cochlear pharmacokinetics.
Conclusion
Cisplatin- but not oxaliplatin-induced apoptosis involved superoxide-related pathways. Lower cochlear uptake of oxaliplatin than cisplatin appears to be a major explanation for its lower ototoxicity.
CONTEXT AND CAVEATS
Prior knowledge
Chemotherapy with cisplatin is often associated with hearing loss (ie, ototoxicity), but treatment with oxaliplatin, another platinum-based chemotherapeutic agent, is rarely associated with ototoxic side effects.
Study design
A cancer cell line was used to investigate whether inhibitors of superoxide- and calcium-mediated signaling could alter cisplatin- or oxaliplatin-induced apoptosis. A guinea pig model was used to examine cisplatin- or oxaliplatin-induced ototoxicity and pharmacokinetics of both drugs in the cochlea.
Contribution
In cancer cells, cisplatin-induced apoptosis, but not oxaliplatin-induced apoptosis, was reduced by lowering the concentration of calcium ions or superoxide ions. In guinea pig cochlea, total and perilymphatic drug concentrations were lower after oxaliplatin treatment than after cisplatin treatment and appear to be a major explanation for its lower ototoxicity.
Implications
Additional research is warranted into the mechanisms of how treatment with cisplatin vs oxaliplatin leads to ototoxicity.
Limitations
It is not clear how cytotoxicity in cancer cells relates to ototoxicity in vivo. Drug concentrations in the perilymph over time could not be measured in an individual guinea pig because only one sample can be taken from each cochlea. Human subjects and guinea pigs may or may not have the same cochlear pharmacokinetics.
From the Editors
Cisplatin is a mainstay in the treatment of a variety of solid tumors, notably testicular cancer. The major toxic side effects include nephrotoxicity, peripheral neurotoxicity, and ototoxicity (1). Inner ear toxicity, which may result in disabling hearing loss and tinnitus, is often a dose-limiting side effect that hampers optimal cisplatin-based chemotherapy. Ototoxicity has a general dose dependence but with considerable interindividual variability. The main targets of the ototoxicity are the outer hair cells in the organ of Corti and the vascularized epithelium in the lateral wall of the cochlea, the stria vascularis (2). Cisplatin induces a caspase-dependent apoptotic pathway in sensitive cochlear cells (3). The molecular mechanisms that trigger apoptosis in the cochlea have not been elucidated, but several mechanisms have been proposed that appear to involve increased oxidative stress (2,4). The organ of Corti is hidden in the deep compartment of the inner ear and is protected by a blood–labyrinth barrier. This barrier restricts the entry of cisplatin to the perilymphatic compartment of the inner ear and can thereby dampen the effects of short peaks in the concentration of cisplatin in the systemic circulation (5).
Oxaliplatin is a third-generation platinum-based drug that is used predominantly for treatment of colorectal carcinoma. Although oxaliplatin has neurotoxic side effects, ototoxic side effects have rarely been observed (1). Oxaliplatin is rapidly and non-enzymatically biotransformed to other molecular species (6,7) and one or more of these species may contribute to its cytotoxicity (8). The terminal half-life of oxaliplatin in humans is approximately 14 minutes (9) and that of cisplatin is 25 minutes (10). Many studies (11,12) have demonstrated different cellular effects of cisplatin and oxaliplatin, with a main focus on differences in DNA damage responses and gene expression profiles that can be attributed to the more bulky oxaliplatin-derived DNA adducts.
Although platinum-based drugs are generally considered to be DNA-damaging agents, evidence is accumulating that nucleus-independent mechanisms of cytotoxicity are also important. Experiments with enucleated cytoplasts have clearly demonstrated that molecular events triggered by cisplatin, which are independent of nuclear DNA, lead to activation of caspases 3 and 7 (13,14) or other yet unidentified proteases (ie, DEVDases) that are specific for the amino acid sequence Asp-Glu-Val-Asp (ie, DEVD). Such events are likely to contribute to the therapeutic efficacy and side effects of platinum-based drugs. In particular, side effects that are independent of DNA damage may be more pronounced in terminally differentiated cells such as those in the organ of Corti because these cells no longer replicate and thereby have very low DNA turnover, which should minimize the risks and consequences of DNA damage.
The thioredoxin system plays a major role in redox regulatory and antioxidant cellular systems; the thioredoxin system consists of isoforms of the 12-kDa redox-active thioredoxin protein and the selenoprotein thioredoxin reductase. Thioredoxin reductases use β-nicotinamide adenine dinucleotide phosphate (NADPH) to reduce the active site disulfide of thioredoxin. The reduced thioredoxin is thereby enabled to support the activity of various thioredoxin-dependent antioxidant enzymes, including peroxiredoxins and methionine sulfoxide reductases (15–18). This enzymatic network is very important for health and disease in general (16,19) and for cancer development or treatment in particular (20,21). Indeed, expression of thioredoxin reductase is required for the formation of specific types of tumors, as demonstrated via xenograft experiments with lung adenocarcinoma cells (22). Because of its dependence on a highly reactive nucleophilic and solvent-accessible selenocysteine residue (23,24), thioredoxin reductase is inhibited by various electrophilic anticancer agents, including platinum-based drugs (25,26). Inhibition of this enzyme with a platinum-based drug may lead to cytotoxic effects that result from inhibition of the entire thioredoxin system (16,20,21) and, more specifically, from the gain of a cell-death–provoking pro-oxidant function in the cisplatin-derivatized enzyme, which forms so-called selenium-compromised thioredoxin reductase-derived apoptotic proteins (SecTRAPs) (27).
We thus hypothesized that differential involvement and targeting of redox systems, in particular thioredoxin reductase, may be a mechanistically important difference in the effectiveness of cisplatin and oxaliplatin. We first tested this hypothesis in cultured human colon carcinoma cells by investigating whether cellular thioredoxin reductase is differentially targeted by cisplatin and oxaliplatin and whether redox-related events are equally involved with both drugs. Second, we assessed if thioredoxin reductase is present in the mammalian cochlea, so that it could be a possible cochlear target protein for cisplatin. Derivatization of thioredoxin reductase with cisplatin could clearly lead to increased oxidative stress, which has been demonstrated previously to be a causative factor for ototoxicity (see above). It should be noted that, in contrast to cultured colon carcinoma cells, the cellular targets in the cochlea for cisplatin (ie, the organ of Corti, stria vascularis, and spiral ganglion neurons) contain terminally differentiated cells, in which non-DNA–related cisplatin targets may be especially important mediators of the cisplatin-induced ototoxicity. Finally, the mechanisms responsible for ototoxicity are likely to be multifactorial and to include kinetics and cellular uptake in addition to cellular pathways of apoptosis and cell death induction. We therefore also examined whether differential pharmacokinetic profiles could explain the differences in ototoxicity between cisplatin and oxaliplatin in vivo. For these experiments, we used the well-characterized guinea pig model for inner ear studies (28) to sample fluid from the scala tympani of the cochlea. The samples were analyzed with a selective and sensitive method (liquid chromatography with post-column derivatization) to obtain concentrations of the active drugs (29). We reasoned that, with these experiments, we would be able to explain the considerably lower ototoxicity of oxaliplatin than of cisplatin.
Materials and Methods
Drugs, Chemicals, and Reagents
For all studies, cisplatin was from Bristol-Myers Squibb Pharmaceuticals (Platinol, New York, NY; 1 mg/mL). For in vitro studies, oxaliplatin was from Sigma-Aldrich Chemicals (Steinheim, Germany). For in vivo studies, oxaliplatin was from Sanofi-Aventis (Eloxatin, Paris, France; 5 mg/mL). All other chemicals used were of analytical grade or higher and were purchased from Sigma-Aldrich Chemicals, unless otherwise specified.
Cells and Cell Culture
HCT116 human colon carcinoma cells from Johns Hopkins University, Baltimore, MD, were cultured in McCoy’s 5A medium (Gibco/Invitrogen Ltd, Paisley, UK), supplemented with 10% fetal calf serum, penicillin (100 U/mL), streptomycin (100 μg/mL), and 2 mM L-glutamine. For all experiments involving monolayer cell culture, cells were seeded at 24 000 cells per cm2 in 200 μL of medium per well in microtiter plates or in 10 mL in tissue culture plates for flow cytometry. After overnight incubation, the supernatants were replaced with fresh medium with or without experimental drugs, as indicated.
Cytoplast Preparation and Apoptosis Measurement
Cytoplasts were prepared essentially as described previously (14). Briefly, cultured HCT116 human colon carcinoma cells were harvested, resuspended in 12.5% Ficoll containing complete medium and cytochalasin B at 10 μg/mL to destabilize cellular microfilaments, and incubated for 30 minutes at 37°C. A 1-mL suspension of these cells was then layered onto a density gradient prepared in ultracentrifuge tubes as follows: 1 mL of 25% Ficoll, 1 mL of 17% Ficoll, 0.25 mL of 16% Ficoll, and 0.25 mL of 15% Ficoll. The gradient was prepared in complete medium supplemented with cytochalasin B (10 μg/mL). After centrifugation in a prewarmed (32°C) Sorvall 90SE rotor for 60 minutes at 30 000 g, the enucleated cytoplasts were washed in medium and cultured for 16 hours in 10-cm tissue culture plates containing 8 mL of medium with or without cisplatin, as indicated. The cytoplasts were then stained with propidium iodide (5 μg/mL in phosphate-buffered saline [PBS] containing digitonin at 50 μg/mL) and fluorescein isothiocyanate-labeled antibody against caspase-3 (1:50 dilution) or against caspase-cleaved cytokeratin-18 (M30 antibody, 1:100 dilution), both courtesy of PEVIVA AB (Stockholm, Sweden), and analyzed by flow cytometry for the presence of these labeled antibodies in individual cells as a measure of apoptosis. Because cytoplasts have approximately 10% of the level of propidium iodide staining of intact cells, the populations of cytoplasts and contaminating intact cells could be identified by propidium iodide staining and separated by electronic gating. This procedure ensures that apoptosis was assessed separately in cytoplasts and intact cells.
Assessment of Cellular Apoptotic Signaling Pathways
The involvement of calcium in the apoptotic pathway induced by drug treatment was investigated by use of the Ca2+ chelator 1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid tetraacetyoxymethyl ester (BAPTA-AM; Molecular Probes Invitrogen, Paisley, UK). The involvement of reactive oxygen species was investigated by use of the superoxide anion scavenger Tiron (ie, 4,5-dihydroxy-1,3-benzenedisulfonic acid, disodium salt monohydrate; Sigma-Aldrich Chemicals). HCT116 cells were incubated with cisplatin or oxaliplatin in the presence or absence of BAPTA-AM at 10 μM or Tiron at 5 mM. After a 24-hour treatment, apoptosis was assessed in total cell culture lysates with Apoptosense (PEVIVA AB), an enzyme-linked immunosorbent-type assay with an antibody that specifically recognizes a neoepitope created by the caspase-specific cleavage of cytokeratin-18 to stable 43- and 17-kDa fragments (30).
Measurement of Cellular Thioredoxin Reductase Activity
Cultured HCT116 cells were used to investigate the effect of cisplatin or oxaliplatin on cellular thioredoxin reductase activity. Cells were preincubated for 30 minutes with the Ca2+ chelator BAPTA-AM (10 μM) or the superoxide anion scavenger Tiron (5 mM) before addition of cisplatin or oxaliplatin as indicated. After incubations for 2, 4, or 8 hours, cells were harvested, washed with PBS, and centrifuged at 800 g for 5 minutes at 4°C. The resulting cell pellet was resuspended in extraction buffer (50 mM Tris–HCl at pH 7.5, 2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, and 0.5% Nonidet P-40) and the cells were then lysed by three cycles of freezing and thawing. Cell extracts were cleared by centrifugation (16 000 g at 4°C for 5 minutes). Supernatants were collected and their protein concentrations were assessed with the Bradford method (Bio-Rad Laboratories, Inc, Hercules, CA) with bovine serum albumin as the protein standard. Thioredoxin reductase activity was subsequently determined in these supernatants as described previously (31) by use of the endpoint thioredoxin-catalyzed insulin reduction assay (slightly modified and applied to microtiter plates). In brief, cell extracts containing 15 μg of total protein in 10 μL of extraction buffer were incubated with 12 μg of recombinant mutant C62S and C73S human thioredoxin (32), 297 μM insulin, 1.3 mM NADPH, 85 mM HEPES buffer (pH 7.6), and 13 mM EDTA for 40 minutes at 37°C (in a total volume of 50 μL). The reaction was stopped by the addition of 200 μL of 8 M guanidine-HCl (dissolved in 0.2 M Tris–HCl, pH 8.0) containing 1 mM 5,5′-dithiobis(2-nitrobenzoic acid) and the absorbance at 412 nm was measured with a VersaMax microplate reader (Molecular Devices Corporation, Sunnyvale, CA).
In Vivo Studies With Guinea Pigs
Forty-two guinea pigs from a commercially available wild-living strain (Duncan–Hartley) of both sexes and about 9 weeks of age were housed at the animal facility and kept in enrichment cages with nesting material, at a room temperature of 21°C with a 12-hour light–dark cycle and free access to food and tap water. The guinea pigs were anesthetized with an intramuscular injection of ketamine (Ketalar, Pfizer, Stockholm, Sweden) at 40 mg/kg and xylazine (Rompun vet, Bayer, Stockholm, Sweden) at 10 mg/kg before the surgical procedure and kept under anesthesia throughout the experiment by use of additional doses of ketamine at 25 mg/kg. Lidocaine (Xylocain, AstraZeneca, Södertälje, Sweden) was given locally before surgery. At the conclusion of the experiment, the animals were decapitated while still under anesthesia. For all surgical procedures, guinea pigs were placed on a Harward homeothermic surgical table and a constant rectal temperature of 38°C was maintained. Care and use of the animals reported in this study were approved by the local Animal Care and Use Committee, Stockholms Norra Djurförsöksetiska Nämnd (Ethical permits N423/04 and N213/05).
Immunohistochemistry
Thioredoxin reductase expression was investigated immunohistochemically in cochlea from four guinea pigs that were not treated with a platinum-based drug. Guinea pigs used for immunohistochemistry were deeply anesthetized as described above and decapitatated. The temporal bones were removed. The cochlea was opened at the round and oval windows, and the perilymphatic space was perfused with 4% paraformaldehyde in PBS. Cochleas were fixed in 4% paraformaldehyde in PBS for 1 hour at room temperature and stored in 0.5% paraformaldehyde in PBS at 4°C. After decalcification, tissues were embedded in paraffin and sectioned with a microtome. Four to six cochlear sections of 5 μm from every guinea pig were mounted per slide. The tissue sections were deparaffinated, boiled in diluted antigen-unmasking solution (Vector Laboratories, Burlingame, CA) for 10 minutes, and then cooled to room temperature for 20 minutes. After washing in PBS, sections were incubated with blocking serum (5% goat serum albumin in PBS) for 30 minutes at room temperature to minimize background staining. One separate slide and one section on each slide were used as negative controls. Rabbit anti-rat thioredoxin reductase 1 polyclonal antibodies (Upstate Technology, New York, NY; diluted 1:100 and 1:1000) were used as a primary antibody; all results shown used the 1:1000 dilution. Negative controls were incubated in PBS alone. To confirm the specificity, one section of each slide was incubated with primary antibody that had been preincubated with recombinant rat thioredoxin reductase 1 protein for 1 hour at 37°C. All sections overnight at 4°C. After washing with PBS, bound antibodies were visualized by use of indocarbocyanine (Cy3, red color)-conjugated goat anti-rabbit antibody (Jackson Immuno Research Laboratories Inc, West Grove, PA, diluted 1:1000) applied for 60 minutes at room temperature. After washing with PBS, the nuclei of all sections were counterstained with 4′,6-diamidino-2-phenylindole (blue color; diluted 1:10 000) for 1 minute and sections were then mounted. Slides from the whole cochleas were analyzed by fluorescence microscopy. Specificity was verified by use of sections not stained with primary antibody for negative control, as described previously for experiments in hamsters in which the same primary antibodies were used (33), as well as preabsorption of primary antibodies to the antigen (see above).
Assessment of Hair Cell Toxicity and Total Platinum Concentration
We used a total of 15 pigmented guinea pigs of both sexes with a weight of 313 ± 17 g (mean ± SD). The guinea pigs were divided into three groups: group I (n = 5) received a single dose of oxaliplatin at 16.6 mg/kg intravenously, group II (n = 5) received cisplatin at 12.5 mg/kg intravenously, and group III (n = 5) received cisplatin at 5 mg/kg intravenously. We used Tucker-Davis Technologies equipment (Gainesville, FL) to obtain thresholds for the auditory brain stem response from anesthetized animals before treatment with cisplatin or oxaliplatin and 96 hours after treatment. The shift in the auditory brain stem response threshold was calculated as the difference between electrophysiological hearing thresholds obtained before and after drug treatment and expressed in dB (34). After the final auditory brain stem response was measured, the animals were decapitated under deep anesthesia and the temporal bones were immediately removed from the rest of the skull. The perilymphatic space was opened, perfused with 4% paraformaldehyde in PBS, and fixed in 4% paraformaldehyde in PBS for 1 hour at room temperature, and then stored in 0.5% paraformaldehyde in PBS at 4°C.
The right cochlea from each guinea pig was rinsed in PBS, the basilar membrane with the organ of Corti was exposed by removing the bone and surrounding tissue, and the remaining cells were labeled with phalloidin (1:200 dilution) for 45 minutes in the dark at room temperature to visualize actin in the stereocilia of the hair cells. Inner and outer hair cells were counted in discrete steps from apex to base under a Zeiss fluorescence microscope. The criterion for hair cell loss was scar formation. The percentage of missing hair cells per 1 mm was calculated. The loss of hair cells was presented in cochleograms, which plot the percentage of missing hair cells against the distance from the round window (ie, one of two openings in the cochlear bone that connect the inner ear to the middle ear).
The left cochlea from each animal given cisplatin (12.5 mg/kg) or oxaliplatin (16.6 mg/kg) was used to determine the total platinum content in the cochlear tissue. The entire basilar membrane including the organ of Corti and stria vascularis was dissected. Analysis of total platinum in cochlear tissue was performed by use of inductively coupled plasma mass spectrometry by a certified method (Analytica AB, Luleå, Sweden).
Pharmacokinetics
A total of 23 albino guinea pigs of both sexes were used in the pharmacokinetic experiment. The guinea pigs were divided into two groups. Group I (n = 13), with a weight of 442 ± 61 g (mean ± SD), received a single intravenous dose of oxaliplatin (16.6 mg/kg at 5 mg/mL) and group II (n = 10), with a weight of 326 ± 30 g (mean ± SD), received a single intravenous dose of cisplatin (5 mg/kg at 1 mg/mL). From every guinea pig, four blood samples, two samples of cerebrospinal fluid, and one sample of scala tympani perilymph from the right and left cochlea, respectively, were drawn within a total time range of 1.5–90 minutes (at 1.5, 5, 10, 15, 20, 30, 40, 50, 60, and 90 minutes) after administration of the drug. Both internal jugular veins were exposed and a catheter was inserted on each side. To avoid blood clots, approximately 5 IU of sodium heparin in 0.1 mL of saline was administrated in each catheter. One catheter was used to deliver drug and the other was used to obtain blood samples. The bone surrounding the cochlea was resected on both sides by use of a dorsolateral approach, and a small hole was drilled in the basal turn of the cochlea to aspirate the scala tympani perilymph. The occipital bone was opened and the dura mater was exposed. A small hole was made in the dura mater so that clear cerebrospinal fluid from the subarachnoid space could be sampled. A small hook held up the dura to avoid mixing drain fluid and blood with cerebrospinal fluid. Oxaliplatin or cisplatin was given intravenously for 23 ± 10 seconds (mean ± SD). Every 0.35-mL blood sample removed was replaced with 0.35 mL of saline. A 10-μL syringe was attached to a micromanipulator, and clear cerebrospinal fluid (1–10 μL) was aspirated from the subarachnoid space, placed in an Eppendorf tube, and promptly frozen on dry ice.
A 1-μL syringe was placed in a Nagai micromanipulator and the tip of the syringe was inserted into the drilled hole in the basal turn of the cochlea. We quickly sampled the perilymph to reduce spillover of scala tympani perilymph fluid and thereby avoid contamination of cerebrospinal fluid through the cochlear aqueduct (35). One 1-μL sample of scala tympani perilymph was aspirated under careful inspection. Each sample was placed in a vial containing 25 or 50 μL of H2O (MilliQ grade) and then directly frozen on dry ice.
Blood samples were collected in heparinized tubes (Microtainer, Becton Dickinson, Franklin Lakes, NJ), kept on ice, and subjected to centripetal ultrafiltration within 1 hour by use of a filter with a 10 000 molecular weight cutoff (Centrisart, Sartorius AG, Göttingen, Germany) for 20 minutes at 4000 g at 4°C. The ultrafiltrate was promptly frozen on dry ice in an Eppendorf tube. As internal controls, known amounts of cisplatin or oxaliplatin were added to blood samples from untreated guinea pigs and treated as outlined above. All samples were stored at −80°C until analysis, which occurred within 3 weeks. Liquid chromatography with post-column derivatization, as described previously (29), was used to determine the concentration of oxaliplatin. The cisplatin concentration was determined by following the same method but with modifications for the monohydrated complex of cisplatin, as described previously (36).
Pharmacokinetic Evaluation
We used the time–concentration data for calculations. The area under the concentration–time curve (AUC) was determined by the trapezoidal rule. Terminal elimination half-lives of oxaliplatin and cisplatin in blood were determined by use of WIN NONLIN version 1.5 SCI (Statistical Consulting Inc, Cary, NC).
Statistical Analysis
Statistical significance in the experiments with cultured tumor cells was analyzed with the Wilcoxon rank test. The variability of AUC (a measure of drug exposure) in the pharmacokinetic studies was determined according to the principles given by Yuan (37), with a subsequent posttest with the Tukey–Kramer multiple comparison test. Differences in terminal half-life after oxaliplatin (16.6 mg/kg) and cisplatin (5 mg/kg) treatment and concentrations of total platinum in cochlear tissues after oxaliplatin (16.6 mg/kg) and cisplatin (12.5 mg/kg) treatment were assessed with the Mann–Whitney U test. All statistical tests were two-sided. Differences for which P values were less than .05 were considered to be statistically significant.
Results
Apoptosis in Colon Carcinoma Cells Treated With Cisplatin and Oxaliplatin
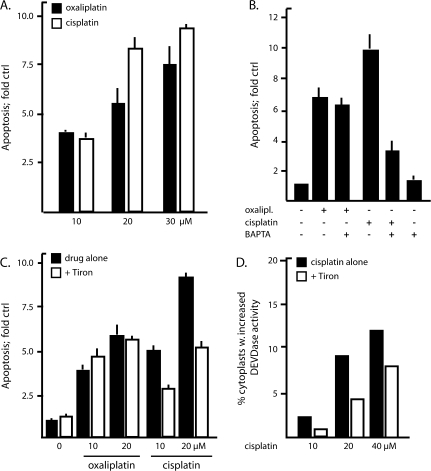
Because human HCT116 colon carcinoma cells show fairly similar growth inhibition responses to cisplatin and oxaliplatin, these cells were used to compare apoptosis induction by the two drugs. We treated these cells with cisplatin or oxaliplatin (each at 10–30 μM) for 24 hours and assessed apoptosis by determining the levels of a neoepitope on stable cytokeratin-18 fragments generated specifically by DEVDase cleavage (notably caspase-3 and -7) (30). We found that cisplatin and oxaliplatin induced fairly similar levels of apoptosis in human HCT116 colon carcinoma cells. Thus, 10, 20, and 30 μM oxaliplatin induced 3.9-fold (95% confidence interval [CI] = 3.7- to 4.1-fold), 5.9-fold (95% CI = 5.3- to 6.5-fold), and 7.5-fold (95% CI = 5.8- to 9.2-fold) increased levels of apoptosis, respectively, compared with background levels, whereas cisplatin at 10, 20, and 30 μM induced 3.7-fold (95% CI = 3.1- to 4.3-fold), 8.3-fold (95% CI = 7.2- to 9.4-fold), and 9.3-fold (95% CI = 8.6- to 10.0-fold), respectively (Figure 1, A). For comparison, after 24 hours of treatment, 20 μM cisplatin had induced approximately 50% nuclear fragmentation, which is typically indicative of apoptosis, as determined by fluorescence microscopy by counting of cells stained with ethidium bromide to visualize intact and fragmented nuclear DNA, respectively (data not shown).
Figure 1.
Apoptotic signaling induced by cisplatin and oxaliplatin. A) Comparison of apoptosis induced by cisplatin and oxaliplatin (each at 10, 20, or 30 μM). Cells were treated in triplicate samples (n = at least four separate experiments). B) Requirement for calcium in apoptosis induced by cisplatin but not by oxaliplatin (each at 20 μM). The calcium chelator 1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid tetraacetyoxymethyl ester (BAPTA) was added at 10 μM, as indicated. Triplicate samples were examined. Data from one representative experiment of three are shown; all results were similar. C) Requirement for reactive oxygen species in apoptosis induced by cisplatin but not by oxaliplatin (each at 10 or 20 μM). The superoxide anion scavenger Tiron was added at 5 mM, as indicated. Triplicate samples were examined (n = two separate experiments). A–C) HCT116 human colon cancer cells were incubated with drugs as indicated for 24 h, after which accumulated apoptosis levels were assessed with the Apoptosense assay of DEVDase activity, i.e., activity of proteases recognizing the amino acid sequence Asp-Glu-Val-Asp (DEVD). Error bars are 95% confidence intervals. Data are presented as fold increase in apoptosis compared with background levels of apoptosis in nontreated cells. Cisplatin induces superoxide-dependent apoptosis also in the absence of nuclear DNA damage. D) Cytoplasts (ie, enucleated HCT116 cells) were prepared, and duplicate cultures were treated with cisplatin (10, 20, or 40 μM) or with Tiron (5 mM), as indicated. Apoptosis in cytoplasts was measured as DEVDase activity by flow cytometry and data are presented as the percentage of DEVDase activity in untreated controls. Data are the mean of two experiments.
We have shown previously (38) with a melanoma cell line that cisplatin induced increased cytosolic Ca2+ levels within 1 hour after its addition and that apoptosis was inhibited by at least 50% by the addition of Ca2+ chelators. In the present study, we investigated this Ca2+ requirement in HCT116 cells and found that co-treatment with the Ca2+ chelator BAPTA-AM at 10 μM reduced 20 μM cisplatin-induced apoptosis from 9.9-fold above (95% CI = 8.1- to 11.7-fold) to 3.1-fold (95% CI = 2.0- to 4.2-fold) above background levels (Figure 1, B). By contrast, apoptosis induced by 20 μM oxaliplatin (7.1-fold above background, 95% CI = 6.0- to 8.2-fold) was not affected by co-treatment with the calcium chelator BAPTA-AM (6.2-fold above background, 95% CI = 5.4- to 7.0-fold) (Figure 1, B). Thus, cisplatin and oxaliplatin induce apoptosis through different pathways with differential involvement of Ca2+.
Cisplatin-induced ototoxicity involves the production of reactive oxygen species (2). We thus investigated the involvement of reactive oxygen species in cisplatin-induced and oxaliplatin-induced cytotoxicity in HCT116 cells by adding the superoxide anion scavenger Tiron (5 mM) to the cell cultures concomitantly with the platinum drug. We found that Tiron treatment reduced cisplatin- but not oxaliplatin-induced apoptosis (Figure 1, C). That is, Tiron reduced cisplatin-induced apoptosis from 9.3-fold above background (95% CI = 8.8- to 9.8-fold) to 5.1-fold (95% CI = 4.4- to 5.8-fold), whereas oxaliplatin-induced apoptosis was marginally reduced from 5.9-fold (95% CI = 4.7- to 7.1-fold) to 5.6-fold (95% CI = 5.3- to 5.9-fold). In addition, Tiron reduced cisplatin-induced apoptosis also in enucleated HCT116 cells (ie, cells from which nuclei were removed before drug treatment) (Figure 1, D). Thus, the superoxide-dependent apoptotic pathway induced by cisplatin appears to be, at least partially, independent of DNA damage.
Inhibition of Thioredoxin Reductase by Cisplatin and Oxaliplatin in HCT116 Colon Carcinoma Cells and Its Expression in the Cochlea
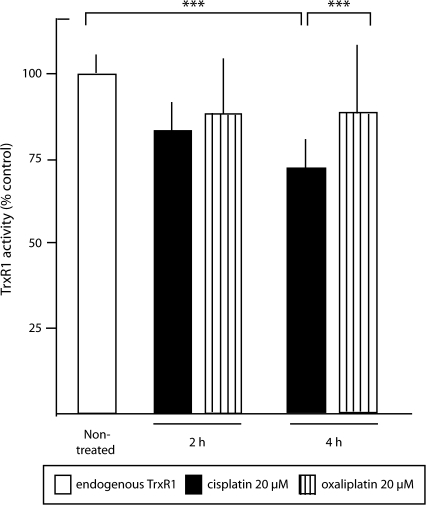
We investigated whether thioredoxin reductase inhibition by platinum-based drugs was associated with the induction of oxidative stress in HCT116 cells. We first determined that 8 hours of treatment with 80–100 μM cisplatin or oxaliplatin was required to inhibit more than 80% of total cellular thioredoxin reductase activity (data not shown). Because either 20 μM cisplatin or 20 μM oxaliplatin induced detectable apoptosis after 12 hours (data not shown), total inhibition of thioredoxin reductase activity is apparently not required for apoptosis induction. However, treatment with 20 μM cisplatin but not 20 μM oxaliplatin inhibited thioredoxin reductase activity (Figure 2). Cellular thioredoxin reductase activity was inhibited statistically significantly more by cisplatin than by oxaliplatin (P = .001). Co-treatment of cells with Tiron and cisplatin did not block the inhibition of thioredoxin reductase activity (data not shown) and, although not conclusive evidence, the result is in line with thioredoxin reductase being part of cisplatin-specific effects on cytosolic redox systems.
Figure 2.
Inhibition of cellular thioredoxin reductase 1 (TrxR1) activity by cisplatin and oxaliplatin. HCT116 human colon cancer cells were treated with 20 μM cisplatin or 20 μM oxaliplatin for 2 or 4 h. Thioredoxin reductase 1 activity was then determined in cell extracts with a thioredoxin-coupled endpoint insulin reduction assay (n = 12 replicates at 2 h; n = 15 replicates at 4 h). Enzyme activity is presented as relative to activity in the nontreated control. Error bars = 95% confidence intervals. ***P < .001, as determined with the two-sided Wilcoxon rank test.
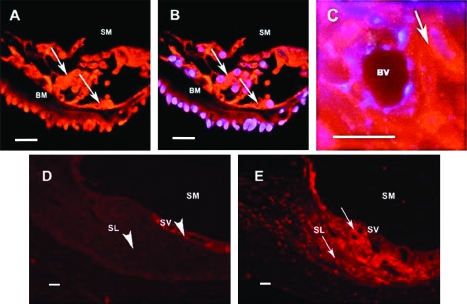
We used immunohistochemistry to determine whether thioredoxin reductase was expressed in the cochlea of guinea pigs. We found evidence of immunoreactivity for this protein in the cochlea of the guinea pig. The results show that thioredoxin reductase 1 was expressed in the organ of Corti (inner and outer hair cells), in the lateral wall (stria vascularis and fibrocytes of the spiral ligament), and in the neurons of spiral ganglion (Figure 3). The immunoreactivity was mainly localized to the cytosol or membranes and not the nuclei of the cells, as shown by the nuclear counterstaining. Negative controls of cochlea tissue sections stained with antibodies that had been preincubated with pure thioredoxin reductase showed markedly lower staining, supporting the specificity of the polyclonal antibodies against rat thioredoxin reductase 1 used in this study and the cross-reactivity of this antibody with the guinea pig orthologue of thioredoxin reductase.
Figure 3.
Thioredoxin reductase 1 immunoreactivity in the cochlea. Fluorescence photomicrographs of a guinea pig cochlea showing thioredoxin reductase 1 immunoreactivity (red) and superimposed images stained with 4’,6-diamidino-2-phenylindole (DAPI, blue) to show nuclei. Due to superimposed images the nuclei looks blue-purple. A) Organ of Corti with hair cells. SM = scala media; BM = basilar membrane. Arrows indicate cells that are positive for TrxR1. B) Organ of Corti with hair cells. SM = scala media; BM = basilar membrane. Arrows indicate cells that are positive for thioredoxin reductase 1. Blue-purple color indicates nuclear staining. C) Stria vascularis. BV = blood vessel. Arrow indicates a cell (intermediate cell) that is positive for thioredoxin reductase 1. Blue-purple color indicates DAPI nuclear staining. D) Negative control. Lateral wall of the cochlea. SM = scala media, SV = stria vascularis, SL = spiral ligament. Arrowheads indicate cells that are negative for thioredoxin reductase 1. E) Lateral wall of the cochlea. SM = scala media, SV = stria vascularis, SL = spiral ligament. Arrows indicate cells that are positive for thioredoxin reductase 1. Scale bars = 100 μm. Four animals (one cochlea from each animal) were used for this study.
Thus, apoptosis induced by cisplatin and oxaliplatin differed in regard to the involvement of Ca2+ cations and superoxide anions; cisplatin-induced apoptosis clearly involved both of these parameters, whereas oxaliplatin-induced apoptosis clearly showed less or no involvement of redox-dependent processes because it was unaffected by chelation or scavenging of either of these molecular species. The results also support the hypothesis that thioredoxin reductase targeting, at least in part, contributes to cisplatin cytotoxicity in tumor cells and in the cochlea.
Ototoxicity and Pharmacokinetics of Cisplatin and Oxaliplatin
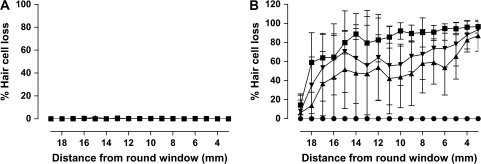
Because the ability of oxaliplatin to induce toxic effects on the cochlea and to enter cochlear fluids of guinea pigs had, to our knowledge, not been investigated, we studied the electrophysiological hearing thresholds and hair cell loss after oxaliplatin treatment. We also determined the uptake of oxaliplatin in scala tympani perilymph to compare these values with those of cisplatin. All 15 guinea pigs used to assess ototoxicity were tested for auditory sensitivity before and after drug treatment. An intravenous cisplatin dose of 12.5 mg/kg produced pronounced hair cell loss and total deafness (ie, no discernible electrophysiological wave response at the three tested frequencies 6.3, 12.5, and 20 kHz). An equimolar dose of intravenous oxaliplatin (16.6 mg/kg), however, did not produce any loss of inner or outer hair cells (Figure 4) and produced only minor changes of the pretreatment auditory brain stem thresholds. The change of the auditory brain stem threshold (the difference between the posttreatment threshold obtained 96 hours after drug administration and the pretreatment threshold measured before drug administration) was 11 dB at a frequency of 6.3 kHz (95% CI = 3 to 19 dB), 6 dB at 12.5 kHz (95% CI = 2 to 14 dB), and 14 dB at 20 kHz (95% CI = 3 to 25 dB), as measured 96 hours after drug administration. Guinea pigs treated with cisplatin at 5 mg/kg had normal hearing and no apparent loss of hair cells (data not shown).
Figure 4.
Drug effects on outer and inner hair cells. A) Oxaliplatin. B) Cisplatin. Data are presented as cochleograms that show the mean loss of hair cells from guinea pig cochlea after treatment for 96 h with intravenously administered oxaliplatin (16.6 mg/kg, n = 5) or cisplatin (12.5 mg/kg, n = 5). The percentage of missing inner and outer hair cells along the basilar membrane is shown relative to the distance from the round window (ie, one of two openings in the cochlear bone that connect the inner ear to the middle ear). Solid circles = inner hair cells; solid squares = outer hair cells in the first row; solid inverted triangles = outer hair cells in the second row; solid triangles = outer hair cells in the third row. Error bars = 95% confidence limits. Ten animals (one cochlea from each animal) were used for this study.
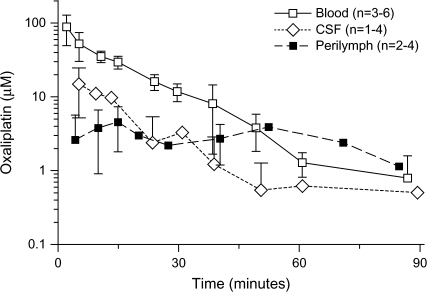
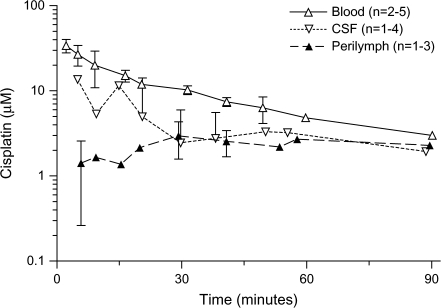
Concentrations of oxaliplatin and cisplatin in blood, cerebrospinal fluid, and scala tympani perilymph in the cochlea after administration of oxaliplatin at 16.6 mg/kg or cisplatin 5 mg/kg, respectively, were then determined (Figures 5 and 6). The AUC values for the drugs in the perilymph were similar (P > .05) for guinea pigs treated with low-dose cisplatin (5 mg/kg; 202 μM × minute, 95% CI = 167 to 228 μM × minute) or with higher dose oxaliplatin (16.6 mg/kg; 238 μM × minute, 95% CI = 202 to 262 μM × minute), although the molar cisplatin dose (16.7 μmol/kg) was only 40% of the molar dose of oxaliplatin (41.8 μmol/kg). The uptake of oxaliplatin into scala tympani perilymph was more rapid than the uptake of cisplatin, with the maximum oxaliplatin concentration of 4.57 μM (95% CI = 1.81 to 7.34 μM), which was reached after 15 minutes, compared with the maximum cisplatin concentration of 2.95 μM (95% CI = 1.58 to 4.33 μM), which was reached after 30 minutes. The peak concentrations of both oxaliplatin and cisplatin were found sooner in the cerebrospinal fluid than in the scala tympani perilymph fluid. The terminal half-life of oxaliplatin in guinea pig blood (12 minutes, 95% CI = 11 to 14 minutes) was shorter than that of cisplatin (33 minutes, 95% CI = 27 to 42 minutes).
Figure 5.
Pharmacokinetics of oxaliplatin. The concentration of oxaliplatin was measured in blood, cerebrospinal fluid (CSF), and scala tympani perilymph, as indicated, after intravenous administration of oxaliplatin (16.6 mg/kg) to guinea pigs. The number of samples per time point per treatment is indicated. Data are the mean; error bars are 95% confidence intervals when data from three or more guinea pigs were available. When the lower confidence limit was less than 0, no lower error bar is shown (a logarithmic concentration scale is used). Thirteen guinea pigs were used for this study.
Figure 6.
Pharmacokinetics of cisplatin. The concentration of cisplatin was measured in blood, cerebrospinal fluid (CSF), and scala tympani perilymph, as indicated, after intravenous administration of cisplatin (5 mg/kg) to guinea pigs (n = 10). The number of samples per time point per treatment is indicated. Data are the mean; error bars are 95% confidence intervals when data from three or more guinea pigs were available. When the lower confidence limit was less than 0, no lower error bar is shown (a logarithmic concentration scale is used). Ten guinea pigs were used for this study.
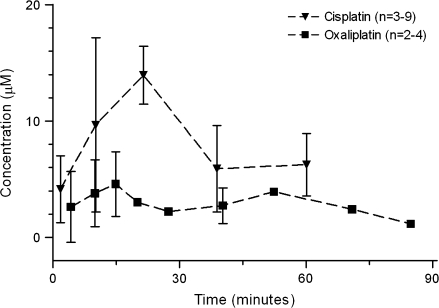
Concentrations in scala tympani perilymph were determined after treatment of guinea pigs with an intravenous equimolar dose of either cisplatin (12.5 mg/kg) or oxaliplatin (16.6 mg/kg); the concentration of cisplatin was recalculated from previously reported data from our laboratory (5). The AUC value was larger for cisplatin (515 μM × minute, 95% CI = 396 to 587 μM × minute) than that for oxaliplatin (238 μM × minute, 95% CI = 202 to 262 μM × minute; P < .001) (Figure 7). We also examined concentrations of platinum in cochlear tissues after oxaliplatin (16.6 mg/kg) and cisplatin (12.5 mg/kg) treatment. The total platinum content in the cochlear tissue was statistically significantly higher 96 hours after administration of cisplatin (total platinum = 0.63 μg/g, 95% CI = 0.54 to 0.73 μg/g, n = 5) than oxaliplatin (total platinum = 0.12 μg/g, 95% CI = 0.00 to 0.24 μg/g, n = 5) (P = .008). These results indicate that the pharmacokinetic differences between cisplatin and oxaliplatin may be sufficient to explain their different ototoxic profiles.
Figure 7.
Pharmacokinetics of cisplatin and oxaliplatin. Concentrations of the parent drugs were measured in scala tympani perilymph after intravenous administration of cisplatin (12.5 mg/kg) or oxaliplatin (16.6 mg/kg) to guinea pigs (n = 21 and n = 13, respectively). The concentration of cisplatin was recalculated from previously published data (5) (with kind permission of Springer Science and Business Media). Data are the mean; error bars are 95% confidence intervals when data from three or more guinea pigs were available.
Discussion
We found differences between the apoptosis signaling induced by cisplatin and oxaliplatin in proliferating HCT116 cancer cells, with redox-related effects being clearly associated with apoptosis induced by cisplatin but not by oxaliplatin under comparable conditions. Although these agents are sometimes regarded as interchangeable, these results show that they differ. We also identified thioredoxin reductase 1 as a potential target for cisplatin, in particular, in both HCT116 cells and cochlea. However, the differences in cochlear kinetics and cellular uptake that we found in the hearing end organ are sufficient to explain the difference in ototoxicity between cisplatin and oxaliplatin.
We have previously demonstrated that cisplatin has pronounced DNA damage- and nucleus-independent effects (13,14). In addition, levels of cytosolic Ca2+ and reactive oxygen species increase by 50% within 1 hour after cisplatin treatment (38), which is well before the onset of p53 accumulation, which occurs approximately 3 hours after treatment with either drug (M Berndtsson PhD, MSc E Hernlund MSc, Shoshan M, PhD; unpublished observations). In this study, we show that, although cisplatin and oxaliplatin induce similar levels of apoptosis in cultured HCT116 colon carcinoma cells, superoxide anion production and increased levels of intracellular calcium are required for cisplatin-induced but not oxaliplatin-induced apoptosis. Superoxide anion production was furthermore independent of DNA damage, as shown by the effects of cisplatin in enucleated cells. DNA-damaging drugs typically act by interfering with DNA replication (39). However, cells in the organ of Corti are terminally differentiated and do not divide. Therefore, the apoptotic redox signaling induced by cisplatin in enucleated cells may be a possible mechanism of cisplatin-induced ototoxicity. It should also be noted that the chloride concentrations in the McCoy's culture medium used for the tumor cells and in the mammalian perilymph were in the same range (120 and 110–140 mM, respectively). Different extracellular chloride concentrations are thereby not likely to affect the cisplatin toxicity differently in cell culture and in the outer hair cells in the organ of Corti.
The involvement of calcium cations and superoxide anions in cisplatin-induced apoptosis but not oxaliplatin-induced apoptosis is in agreement with the observation that inhibition of thioredoxin reductase may affect calcium homeostasis (40,41). Apoptosis was also induced by cisplatin despite only partial inhibition of thioredoxin reductase (Figure 2), which might reflect the fact that cisplatin-derivatized thioredoxin reductase has not only lost thioredoxin-reducing activity but also gained a dominant function as pro-oxidant inducer of cell death (27). This gain of function was associated with the conversion of thioredoxin reductase from an oxidoreductase with antioxidant activity to a SecTRAP with potent superoxide-producing NADPH oxidase capacity after its derivatization with cisplatin (27). If SecTRAPs are formed in cisplatin-treated cells, then this mechanism may be involved in intracellular superoxide production, in prooxidant cell death induction, and also in the antiapoptotic effect observed after treatment with the superoxide scavanger Tiron. Because of the importance of thioredoxin reductase as an enzymatic target contributing to cisplatin toxicity (20,25–27), because of the weaker inhibitory effect of oxaliplatin on its cellular activity, and because of the finding that thioredoxin reductase was expressed in the cochlea, we propose that the targeting of thioredoxin reductase by cisplatin could contribute to the ototoxicity of cisplatin, but this possibility must be investigated further.
We have also shown that the lack of major cochlear injury during treatment with oxaliplatin is likely explained by the pharmacokinetic profile of oxaliplatin. There was a striking difference between cisplatin and oxaliplatin in the uptakes into scala tympani perilymph fluid and in total platinum concentrations in cochlear cells. In the inner ear, transport of drugs between blood and receptor cells or neural tissue is hindered by the presence of barrier cell layers. In analogy with the blood–brain barrier, the concept of a blood–labyrinth barrier is generally accepted. This barrier can roughly be divided into the blood–perilymph barrier and the perilymph–endolymph barrier, although the functional division between these compartments is not fully understood. The blood–perilymph barrier, which consists mainly of endothelial cells sealed by tight junctions, is most likely the major barrier for pharmacologic substances in reaching the sensory epithelium of the hearing and balance organs. Cisplatin-induced ototoxicity is known to be mediated via injury to several terminally differentiated cellular targets in the cochlea (42), including the marginal and intermediate cells of the stria vascularis (43), which is a major component of the perilymph–endolymph barrier.
Our study had several limitations. First, we discovered more pronounced oxidative stress and calcium involvement in the cytotoxicity of cisplatin than of oxaliplatin in cell cultures, but it is not clear if these differences mediate differences in cytotoxicity also in vivo. Second, we identified thioredoxin reductase as a plausible cisplatin target in the cochlea but could present no absolute evidence for its involvement in cochlear ototoxicity. This task, which is highly demanding methodologically, is beyond the scope of the present work but is a major goal of ongoing endeavors. Third, it should be noted that it is technically not possible to evaluate the scala tympani perilymph concentration time curves of cisplatin and oxaliplatin for the individual guinea pigs, because only one sample can be taken from each cochlea by aspiration procedures. The statistical technique presented by Yuan (37) that we used in this analysis is a valuable tool for construction of concentration–time curves and for calculation of AUC from a group of guinea pigs in which only one sample was collected from each cochlea. The technique also allows estimation of the variance of AUC in such experiments. However, in this analysis, we determined the scala tympani perilymph concentrations separately in the left and right cochlea to reduce the number of experimental guinea pigs. The statistical test used for comparison of the AUC for cisplatin and oxaliplatin can thereby be viewed as an estimation procedure, as the number of observations was low. However, our conclusions were further supported by the observed fairly large concentration differences between cisplatin and oxaliplatin, as illustrated in Figure 7. Thus, limited cochlear uptake of oxaliplatin is a major explanation for the lower ototoxicity of oxaliplatin than cisplatin. These in vivo experiments were performed in guinea pigs, and it should not be taken for granted that human subjects have the same cochlear pharmacokinetics. For technical and ethical reasons, it is impossible to repeat our experiments in human subjects. We do believe, though, that the major aspects of our conclusions are valid for humans.
The AUC of oxaliplatin in the guinea pig perilymph was only half of that of cisplatin when equimolar doses of drugs were administered. It should also be noted that the therapeutic doses of oxaliplatin and cisplatin in humans are equimolar (ie, 135 mg/m2, or 0.34 mmol/m2, and 100 mg/m2, or 0.33 mmol/m2, respectively). Thus, our findings may represent differences in the transport of the two drugs from the blood to the extracellular compartments of the cochlea. We can hypothesize that four mechanisms may, in various possible combinations, be responsible for the higher cochlear concentration of cisplatin as reflected by perilymph kinetics. First, the elimination half-life of cisplatin was longer than that of oxaliplatin. One would thereby expect a higher concentration of cisplatin in the blood for a longer period, which would favor its uptake in scala tympani perilymph. Second, the permeability of the blood–labyrinth barrier might favor influx of cisplatin. Third, increased influx of cisplatin to the cochlear extracellular fluids might also be provided by a facilitated influx transport (eg, via a single or a group of organic cationic transporters). Fourth, oxaliplatin might also have a greater efflux from the scala tympani perilymph than cisplatin.
At equimolar doses, cisplatin (12.5 mg/kg), as expected, had severe ototoxic effects in the guinea pig model, whereas oxaliplatin (16.6 mg/kg) had minimal ototoxic effects. Reducing the dose of cisplatin to 5 mg/kg resulted in a low perilymph concentration of drug similar to that of oxaliplatin at 16.6 mg/kg and consequently did not induce the drug-induced auditory brain stem threshold shift and injury to the outer hair cells. These results strongly indicate that the pharmacokinetic differences between cisplatin and oxaliplatin may be sufficient to explain their different ototoxic profiles.
Funding
The authors would like to thank Karolinska Institutet Cancer Network (KICancer) Strategic Funds (to ESJA, MS, HE, and GL; 6231/03-722), Swedish Cancer Society (to ESJA; 3775, 5009, to MS; 4932), Cancerforeningen Stockholm (to MS; 061402), the foundations Tysta Skolan, Petrus and Augusta Hedlund Foundation (to ESJA), Alice and Knut Wallenberg Foundation (to ESJA; 2006.0102), and AFA Insurance (to GL; T-51:05) for their generous financial support. The funding agencies had no influence on the design of this study, collection of data, analysis or interpretation of the data, decision to submit the manuscript or writing of the manuscript, with the authors taking full responsibility for all of these activities.
Footnotes
Elias S. J. Arnér, Maria Shoshan, Hans Ehrsson, and Göran Laurell shared senior authorship.
Anette Franssson, BSc, Andreas Ekborn, MD, and Caroline Gahm, MD, are gratefully acknowledged for excellent technical support. The HCT116 cells were a kind gift from Dr Bert Vogelstein, and Dr Arne Holmgren kindly provided C63S C72S human thioredoxin.
References
- 1.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226(1–2):157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139(1–2):97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 4.Dehne N, Lautermann J, Petrat F, Rauen U, de Groot H. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol. 2001;174(1):27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- 5.Laurell G, Andersson A, Engström B, Ehrsson H. Distribution of cisplatin in perilymph and cerebrospinal fluid after intravenous administration in the guinea pig. Cancer Chemother Pharmacol. 1995;36(1):83–86. doi: 10.1007/BF00685738. [DOI] [PubMed] [Google Scholar]
- 6.Luo FR, Wyrick SD, Chaney SG. Pharmacokinetics and biotransformations of oxaliplatin in comparison with ormaplatin following a single bolus intravenous injection in rats. Cancer Chemother Pharmacol. 1999;44(1):19–28. doi: 10.1007/s002800050940. [DOI] [PubMed] [Google Scholar]
- 7.Jerremalm E, Wallin I, Yachnin J, Ehrsson H. Oxaliplatin degradation in the presence of important biological sulphur-containing compounds and plasma ultrafiltrate. Eur J Pharm Sci. 2006;28(4):278–283. doi: 10.1016/j.ejps.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Mani S, Graham MA, Bregman DB, Ivy P, Chaney SG. Oxaliplatin: a review of evolving concepts. Cancer Invest. 2002;20(2):246–263. doi: 10.1081/cnv-120001152. [DOI] [PubMed] [Google Scholar]
- 9.Ehrsson H, Wallin I, Yachnin J. Pharmacokinetics of oxaliplatin in humans. Med Oncol. 2002;19(4):261–265. doi: 10.1385/MO:19:4:261. [DOI] [PubMed] [Google Scholar]
- 10.Andersson A, Fagerberg J, Lewensohn R, Ehrsson H. Pharmacokinetics of cisplatin and its monohydrated complex in humans. J Pharm Sci. 1996;85(8):824–827. doi: 10.1021/js960037a. [DOI] [PubMed] [Google Scholar]
- 11.Arango D, Wilson AJ, Shi Q, et al. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br J Cancer. 2004;91(11):1931–1946. doi: 10.1038/sj.bjc.6602215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasetto LM, D’Andrea MR, Brandes AA, Rossi E, Monfardini S. The development of platinum compounds and their possible combination. Crit Rev Oncol Hematol. 2006;60(1):59–75. doi: 10.1016/j.critrevonc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Berndtsson M, Hägg M, Panaretakis T, Havelka AM, Shoshan MC, Linder S. Acute apoptosis by cisplatin requires induction of reactive oxygen species but is not associated with damage to nuclear DNA. Int J Cancer. 2007;120(1):175–180. doi: 10.1002/ijc.22132. [DOI] [PubMed] [Google Scholar]
- 14.Mandic A, Hansson J, Linder S, Shoshan MC. Cisplatin induces endoplasmic reticulum stress and nucleus-independent apoptotic signaling. J Biol Chem. 2003;278(11):9100–9106. doi: 10.1074/jbc.M210284200. [DOI] [PubMed] [Google Scholar]
- 15.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38(12):1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Gromer S, Urig S, Becker K. The thioredoxin system—from science to clinic. Med Res Rev. 2004;24(1):40–89. doi: 10.1002/med.10051. [DOI] [PubMed] [Google Scholar]
- 17.Nordberg J, Arnér ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 18.Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267(20):6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 19.Lillig CH, Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxid Redox Signal. 2007;9(1):25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 20.Urig S, Becker K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol. 2006;16(6):452–465. doi: 10.1016/j.semcancer.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Arner ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16(6):420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Yoo MH, Xu XM, Carlson BA, Gladyshev VN, Hatfield DL. Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J Biol Chem. 2006;281(19):13005–13008. doi: 10.1074/jbc.C600012200. [DOI] [PubMed] [Google Scholar]
- 23.Zhong L, Arnér ESJ, Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc Natl Acad Sci USA. 2000;97(11):5854–5849. doi: 10.1073/pnas.100114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SR, Bar-Noy S, Kwon J, Levine RL, Stadtman TC, Rhee SG. Mammalian thioredoxin reductase: oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci USA. 2000;97(6):2521–2526. doi: 10.1073/pnas.050579797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker K, Herold-Mende C, Park JJ, Lowe G, Schirmer RH. Human thioredoxin reductase is efficiently inhibited by (2,2’:6′,2’ ‘-terpyridine)platinum(II) complexes. Possible implications for a novel antitumor strategy. J Med Chem. 2001;44(17):2784–2792. doi: 10.1021/jm001014i. [DOI] [PubMed] [Google Scholar]
- 26.Witte AB, Anestål K, Jerremalm E, Ehrsson H, Arnér ESJ. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med. 2005;39(5):696–703. doi: 10.1016/j.freeradbiomed.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Anestål K, Prast-Nielsen S, Cenas N, Arner ES. Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells. PLoS ONE. 2008;3(4):e1846. doi: 10.1371/journal.pone.0001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salt AN, Plontke SK. Local inner-ear drug delivery and pharmacokinetics. Drug Discov Today. 2005;10(19):1299–1306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrsson H, Wallin I. Liquid chromatographic determination of oxaliplatin in blood using post-column derivatization in a microwave field followed by photometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795(2):291–294. doi: 10.1016/s1570-0232(03)00590-7. [DOI] [PubMed] [Google Scholar]
- 30.Hägg M, Biven K, Ueno T, et al. A novel high-through-put assay for screening of pro-apoptotic drugs. Invest New Drugs. 2002;20(3):253–259. doi: 10.1023/a:1016249728664. [DOI] [PubMed] [Google Scholar]
- 31.Arnér ESJ, Holmgren A. Measurement of thioredoxin and thioredoxin reductase. In: Maines M, Costa L, Reed D, Sassa S, editors. Current Protocols in Toxicology. New York, NY: John Wiley & Sons, Inc; 2000. pp. 7.4.1–7.4.14. [DOI] [PubMed] [Google Scholar]
- 32.Ren X, Bjornstedt M, Shen B, Ericson ML, Holmgren A. Mutagenesis of structural half-cystine residues in human thioredoxin and effects on the regulation of activity by selenodiglutathione. Biochemistry. 1993;32(37):9701–9708. doi: 10.1021/bi00088a023. [DOI] [PubMed] [Google Scholar]
- 33.Yoon BI, Kim DY, Jang JJ, Han JH. Altered expression of thioredoxin reductase-1 in dysplastic bile ducts and cholangiocarcinoma in a hamster model. J Vet Sci. 2006;7(3):211–216. doi: 10.4142/jvs.2006.7.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekborn A, Laurell G, Andersson A, Wallin I, Eksborg S, Ehrsson H. Cisplatin-induced hearing loss: influence of the mode of drug administration in the guinea pig. Hear Res. 2000;140(1–2):38–44. doi: 10.1016/s0378-5955(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 35.Salt AN, Kellner C, Hale S. Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hear Res. 2003;182(1–2):24–33. doi: 10.1016/s0378-5955(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 36.Videhult P, Laurell G, Wallin I, Ehrsson H. Kinetics of cisplatin and its monohydrated complex with sulfur-containing compounds designed for local otoprotective administration. Exp Biol Med (Maywood) 2006;231(10):1638–1645. doi: 10.1177/153537020623101009. [DOI] [PubMed] [Google Scholar]
- 37.Yuan J. Estimation of variance for AUC in animal studies. J Pharm Sci. 1993;82(7):761–763. doi: 10.1002/jps.2600820718. [DOI] [PubMed] [Google Scholar]
- 38.Mandic A, Viktorsson K, Strandberg L, et al. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22(9):3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12(9):440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Gitler C, Zarmi B, Kalef E, Meller R, Zor U, Goldman R. Calcium-dependent oxidation of thioredoxin during cellular growth initiation. Biochem Biophys Res Commun. 2002;290(2):624–628. doi: 10.1006/bbrc.2001.6214. [DOI] [PubMed] [Google Scholar]
- 41.Rigobello MP, Vianello F, Folda A, Roman C, Scutari G, Bindoli A. Differential effect of calcium ions on the cytosolic and mitochondrial thioredoxin reductase. Biochem Biophys Res Commun. 2006;343(3):873–878. doi: 10.1016/j.bbrc.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 42.van Ruijven MW, de Groot JC, Klis SF, Smoorenburg GF. The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear Res. 2005;205(1–2):241–248. doi: 10.1016/j.heares.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Laurell G, Ekborn A, Viberg A, Canlon B. Effects of a single high dose of cisplatin on the melanocytes of the stria vascularis in the guinea pig. Audiol Neurootol. 2007;12(3):170–178. doi: 10.1159/000099020. [DOI] [PubMed] [Google Scholar]