Abstract
Lapatinib, a selective orally available inhibitor of epidermal growth factor receptor (EGFR) and ErbB2 receptor tyrosine kinases, is a promising agent for the treatment of breast cancer. We examined the effect of lapatinib on the development of mammary tumors in MMTV-erbB2 transgenic mice, which express wild-type ErbB2 under the control of the mouse mammary tumor virus promoter and spontaneously develop estrogen receptor (ER)–negative and ErbB2-positive mammary tumors by 14 months of age. Mice were treated from age 3 months to age 15 months with vehicle (n = 17) or lapatinib (30 or 75 mg/kg body weight; n = 16 mice per group) by oral gavage twice daily (6 d/wk). All statistical tests were two-sided. By 328 days after the start of treatment, all 17 (100%) of the vehicle-treated mice vs five (31%) of the 16 mice treated with high-dose lapatinib developed mammary tumors (P < .001). Among MMTV-erbB2 mice treated for 5 months (n = 20 mice per group), those treated with lapatinib had fewer premalignant lesions and noninvasive cancers in their mammary glands than those treated with vehicle (P = .02). Lapatinib also effectively blocked epidermal growth factor–induced signaling through the EGFR and ErbB2 receptors, suppressed cyclin D1 and epiregulin mRNA expression, and stimulated p27 mRNA expression in human mammary epithelial cells and in mammary epithelial cells from mice treated for 5 months with high-dose lapatinib. Thus, cyclin D1, epiregulin, and p27 may represent useful biomarkers of lapatinib response in patients. These data suggest that lapatinib is a promising agent for the prevention of ER-negative breast cancer.
CONTEXT AND CAVEATS
Prior knowledge
A small-molecule tyrosine kinase inhibitor specific for epidermal growth factor receptor (EGFR)—gefitinib—only partially prevents the development of estrogen receptor (ER)–negative mammary tumors in MMTV-erbB2 transgenic mice, which spontaneously develop ER-negative and ErbB2-positive mammary tumors by 14 months of age.
Study design
An examination of the effect of lapatinib, a dual kinase inhibitor that blocks the kinase activities of both EGFR and ErbB2, on mammary tumorigenesis in MMTV-erbB2 transgenic mice.
Contribution
Compared with vehicle-treated mice, lapatinib suppressed the development of ER-negative and ErbB2-positive invasive mammary tumors in MMTV-erbB2 mice treated for 12 months and the numbers of premalignant lesions and noninvasive cancers in the mammary glands of mice treated for 5 months.
Implications
Lapatinib may be useful for the prevention of ER-negative, ErbB2-positive breast cancer in humans.
Limitations
The effect of lapatinib was studied in only one mouse model. Lapatinib did not completely prevent mammary tumorigenesis in the MMTV-erbB2 mice.
From the Editors
The ErbB family of receptor tyrosine kinases consists of the epidermal growth factor receptor (EGFR; also known as ErbB1 or Her1), ErbB2 (Neu or Her2), ErbB3 (Her3), and ErbB4 (Her4). ErbB family members, particularly ErbB1 and ErbB2, are overexpressed or mutated in lung, colorectal, and breast cancers (1–3). The gene that encodes ErbB2 is amplified in approximately 30% of breast cancers (4), making this protein a potentially important target in the treatment of breast cancer. Overexpression of the ErbB2 protein is associated with aggressive disease and poor outcomes in patients with node-positive breast cancer (5) and in patients with node-negative breast cancer (6). All ErbB family members are characterized by an extracellular ligand-binding domain, a single transmembrane domain, and a cytoplasmic tyrosine kinase domain. Receptor-specific ligand binding to the extracellular domains of individual ErbB receptors results in homo- or heterodimerization of the receptor, phosphorylation of the tyrosine kinase domain, and activation of downstream signal transduction cascades (5,7–9).
MMTV-erbB2 transgenic mice that express wild-type ErbB2 under the control of the mouse mammary tumor virus promoter spontaneously develop estrogen receptor (ER)–negative and ErbB2-positive mammary tumors within 14 months of age (10–13). The ability of ErbB receptors to transform cells is mediated through two major signaling pathways: the mitogen-activated protein kinase (MAPK) pathway, which promotes DNA synthesis and cell cycle progression (14–17), and the phosphatidylinositol-3-kinase–Akt pathway, which enhances cell survival by inhibiting apoptosis (14,15,18).
Recently, several tyrosine kinase inhibitors (TKIs) have been tested for their efficacy as anticancer drugs. One of the small-molecule TKIs, gefitinib (ZD1839; Iressa), is highly specific for ErbB1 and exerts its antitumor effects by directly binding to the kinase domain, thereby inhibiting receptor signaling (10,11,14–16,18). Clinical trials have demonstrated the efficacy of gefitinib as a single agent in non–small-cell lung cancer (17–20) and small-cell lung cancer patients in whom chemotherapy had failed (21), particularly those with tumors that have an activating mutation in the ErbB1 receptor (22,23). We have previously shown that gefitinib only partially prevents the development of ER-negative mammary tumors in a preclinical mouse model (10,11). Given the fact that gefitinib does not completely prevent mammary tumorigenesis in the mouse model, we investigated whether lapatinib, a dual kinase inhibitor that blocks the kinase activities of both EGFR and ErbB2, would more effectively prevent ER-negative mammary tumors in MMTV-erbB2 transgenic mice.
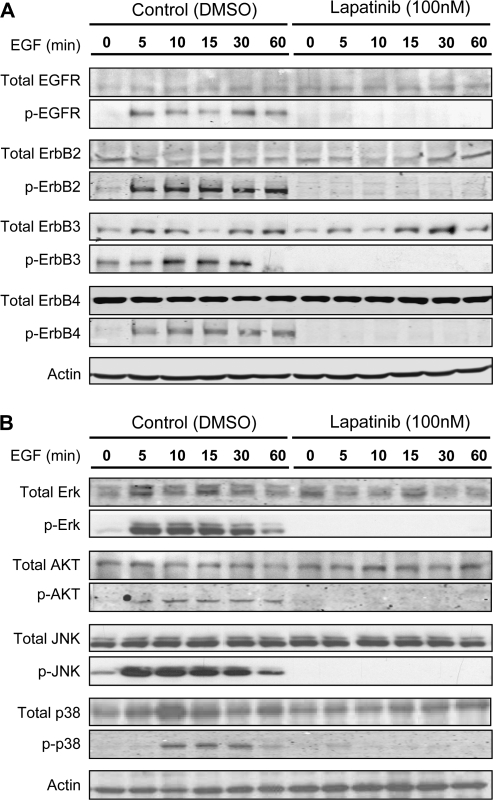
We first examined the effect of lapatinib on epidermal growth factor (EGF)–induced signaling in normal human mammary epithelial cells (HMECs). Immunoblot analysis of protein lysates from HMECs revealed that all ErbB family members underwent phosphorylation within 10 minutes after the addition of EGF to the culture medium (Figure 1). Pretreatment of HMECs with lapatinib (100 nM in dimethyl sulfoxide [DMSO], provided by GSK Pharmaceuticals, Research Triangle Park, NC) inhibited both the basal phosphorylation and EGF-induced phosphorylation of all ErbB receptor tyrosine kinases compared with cells treated with vehicle alone (Figure 1). We also observed phosphorylation of the intermediate signaling molecules Akt, extracellular signal-regulated kinase, c-Jun NH2-terminal kinase, and p38 MAPK within 10 minutes after EGF was added to the medium of HMECs, and lapatinib pretreatment of HMECs blocked the EGF-induced phosphorylation of these proteins (Figure 1). Lapatinib also blocked EGF-dependent signaling in human breast cancer BT474 cells (data not shown).
Figure 1.
Effect of lapatinib on signaling in normal human mammary epithelial cells (HMECs). Lapatinib was obtained from GlaxoSmithKline (Research Triangle Park, NC). HMECs (1 × 106 cells) from Clonetics (San Diego, CA) were treated with lapatinib (100 nM), dimethyl sulfoxide (0.1%), and epidermal growth factor from R&D Systems (Minneapolis, MN) (final concentration of 20 nM) and harvested at 0, 5, 10, 15, 30, and 60 minutes later. Protein lysates were generated and subjected to immunoblot analysis as previously described (24). A) Expression of total and activated tyrosine kinase receptors of the Her family. B) Expression of total and activated mitogen-activated protein kinases. The following primary antibodies were purchased from Cell Signaling Technology, Inc: phospho-epidermal growth factor receptor (p-EGFR) (rabbit polyclonal, 1:2000 dilution), total epidermal growth factor receptor (rabbit polyclonal, 1:2000 dilution), phospho-ErbB2 (rabbit polyclonal, 1:2000 dilution), phospho-ErbB3 (rabbit polyclonal, 1:1000 dilution), total ErbB4 (rabbit polyclonal, 1:1000 dilution), phospho-extracellular signal-regulated kinase I/II (rabbit polyclonal, 1:2000 dilution), total extracellular signal-regulated kinase I/II (rabbit polyclonal, 1:1000 dilution), phospho-Akt (rabbit polyclonal, 1:1000 dilution), total Akt (rabbit polyclonal, 1:2000 dilution), phospho-c-Jun NH2-terminal kinase (rabbit polyclonal, 1:1000 dilution), total c-Jun NH2-terminal kinase (JNK) (rabbit polyclonal, 1:1000 dilution), phospho-p38 (rabbit polyclonal, 1:1000 dilution), and total p38 (rabbit polyclonal, 1:1000 dilution). Other primary antibodies used were as follows: total ErbB2 (rabbit polyclonal, 1:2000 dilution, NeoMarkers/Labvision), total ErbB3 (rabbit polyclonal, 1:1000 dilution, Santa Cruz Biotech, Santa Cruz, CA), phospho-ErbB4 (rabbit polyclonal, 1:1000 dilution, Orbigen, San Diego, CA), and anti–β-actin (mouse monoclonal, 1:8000 dilution, Sigma-Aldrich, St Louis, MO). Secondary antibodies against mouse or rabbit were purchased from Amersham (Piscataway, NJ) and used at a 1:4000 dilution.
We next measured the effect of lapatinib on the growth of normal (HMECs), immortalized (MCF10A), and malignant (ER positive: BT474 and MCF7; ER negative: MDA-MB-468 and MDA-MB-231) human breast cell lines in vitro. Cells (1000–2000 cells) were treated for up to 10 days with various concentrations of lapatinib (0.1 nM to 10 μM in DMSO), and cell number was assessed by using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation assay (Promega, Madison, WI). HMECs (which require EGF for growth), BT474 cells (which overexpress ErbB2), and MDA-MB-468 cells (which overexpress EGF) were sensitive to lapatinib (as defined by having drug concentrations producing 50% growth inhibition [IC50] values less than 1 μM; HMECs: IC50 = 35 nM; MCF10A cells: IC50 = 800 nM; BT474 cells: IC50 = 2.5 nM; and MDA-MB-468 cells: IC50 = 90 nM). By contrast, MCF7 and MDA-MB-231 cells, which do not overexpress ErbB2 or EGFR, were relatively resistant to growth inhibition by lapatinib (IC50 for each ≥ 1 μM) (Supplementary Figure 1, available online).
We next examined the effect of lapatinib on the development of oncogene-induced mammary tumors in female MMTV-erbB2 transgenic mice (obtained from the Jackson Laboratory, Bar Harbor, ME). MMTV-erbB2 mice simulate oncogenic events seen in human breast cancers and develop focal tumors beginning at about 5 months of age [median time to tumor development is approximately 230 days (25)]. All MMTV-erbB2 mice develop ER-negative and ErbB2-positive mammary tumors by the age of 14 months (11–13). All mouse experiments were conducted under an institutional animal care and use committee–approved protocol. The mice were treated from age 3 months to age 15 months with vehicle (hydroxypropyl methylcellulose, DOW Chemical Company, Midland, MI; n = 17 mice) or lapatinib at 30 mg/kg body weight (n = 16 mice) or 75 mg/kg body weight (n = 16 mice) by oral gavage twice each day (6 d/wk). Mammary tumor development was monitored twice weekly, and tumor growth was measured with calipers. A mammary tumor was defined as a palpable mammary mass with a volume of at least 100 mm3. The tumor-free interval was defined from the start of treatment to the first appearance of a mammary tumor. Tumor-free interval curves were estimated by the Kaplan–Meier method and compared between vehicle and lapatinib treatment (low and high doses) using a generalized Wilcoxon test.
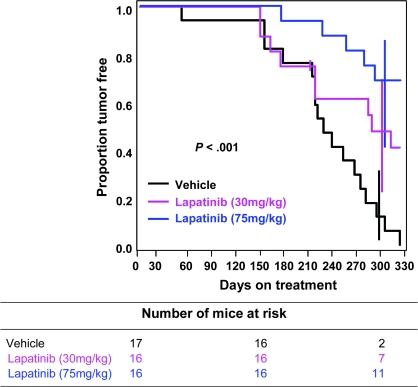
Figure 2 shows the proportion of mice that were free of mammary tumors vs the time on treatment. All vehicle-treated mice developed mammary tumors by 328 days on treatment (or by 418 days of age). Mice treated with low-dose (30 mg/kg) or high-dose (75 mg/kg) lapatinib had delayed development of mammary tumors compared with mice treated with vehicle. At the end of the experiment (day 328 of treatment), when all 17 (100%) of the vehicle-treated mice had developed tumors, nine of the original 16 mice treated with low-dose lapatinib developed mammary tumors (the Kaplan–Meier estimation of tumor incidence at 328 days of treatment was 59% because one mouse in the low-dose arm died at day 207 without a tumor and was censored). Five (31%) of the 16 mice treated with high-dose lapatinib had developed mammary tumors after 328 days of treatment. These delays in tumor development were statistically significant (P < .001, generalized Wilcoxon test). Thus, lapatinib treatment delayed mammary tumorigenesis and prevented the development of mammary tumors in most of the high-dose lapatinib-treated MMTV-erbB2 mice.
Figure 2.
Tumor-free interval in MMTV-erbB2 mice treated with lapatinib or vehicle. FVB strain female MMTV-erbB2 mice (3 months old) were housed in the institutional animal facilities, fed a controlled diet of AIN-76A Purified Diet (Harlan Teklad, Madison, WI), and randomly assigned to receive treatment with vehicle (hydroxylpropyl methylcellulose, Methocel K15M Premium, DOW Chemical Company) or lapatinib (at 30 or 75 mg/kg body weight; GW572016, GlaxoSmithKline) for 6 d/wk starting at 3 months of age (n = 16 for each of the lapatinib-treated groups and n = 17 for the vehicle group). Lapatinib was suspended in hydroxypropyl methylcellulose and administered by twice-daily oral gavage using a 20-gauge gavage needle in a volume of 0.1 mL. Tumor development was monitored twice weekly, and tumor growth was measured using calipers. Tumor volume was calculated by multiplying the square of the width (w) by the length (l) and dividing by two (V = w2l/2). The tumor-free interval was defined from the start of treatment to the first appearance of a palpable mammary tumor at least 100 mm3 in size. Tumor-free interval curves were estimated by the Kaplan–Meier method and compared between vehicle and lapatinib treatment (low and high doses) using the generalized Wilcoxon test (two-sided). The tick mark in the low-dose lapatinib arm represents a censored mouse that died at day 207 without a tumor. Error bars show 95% confidence intervals at 300 days.
We then compared the multiplicity of tumor development and tumor growth rate among the vehicle- and lapatinib-treated mice. Vehicle-treated mice had a mean of 1.24 tumors per mouse, compared with 0.56 tumors per mouse in the low-dose lapatinib group (difference = 0.673 tumors per mouse, 95% confidence interval [CI] = 0.269 to 1.086 tumors per mouse, P = .002, Dunnett multiple comparison test) and 0.31 tumors per mouse in the high-dose lapatinib group (difference = 0.923 tumors per mouse, 95% CI = 0.509 to 1.336 tumors per mouse, P = .001). Overall, the difference in tumor multiplicity among treatment groups was statistically significant (P < .001, analysis of variance). The tumor growth rates were not statistically different among vehicle, low-dose lapatinib, and high-dose lapatinib groups (P = .66, F test). We observed no toxic effects (ie, rash, dry skin, or diarrhea) in mice treated with either dose of lapatinib or weight loss in any of the treated mice (data not shown).
To ensure that the tumor-suppressive effect of lapatinib was not due to decreased expression of the erbB2 transgene, we measured the expression of the ErbB2 protein in normal and malignant mammary tissues from vehicle and high-dose lapatinib-treated mice by immunohistochemical staining. There was no difference in ErbB2 protein expression between vehicle and lapatinib-treated mice in either normal or malignant tissues, indicating that the cancer preventive effect of lapatinib is not through decreased expression of the erbB2 transgene (Supplementary Figure 2, available online). We also observed no reduction of ErbB2 protein in lysates of mammary glands or mammary tumors as measured by immunoblot analysis (data not shown).
To examine whether lapatinib prevents premalignant lesions, we treated MMTV-erbB2 transgenic mice (at 3 months of age) with vehicle or lapatinib (75 mg/kg body weight) for 5 months (n = 20 mice per group). At the end of 5 months of treatment (or 8 months of age), the normal-appearing mammary glands from each mouse were removed and processed for histology and biomarker analysis (hematoxylin–eosin staining and immunohistochemical staining for ErbB2, Ki67 [a cell proliferation marker], and cleaved Caspase 3 [a marker of early apoptosis]), as previously described (26). Hematoxylin–eosin staining showed no difference in morphology between normal tissues derived from vehicle-treated vs lapatinib-treated mice or between hyperplastic tissues derived from vehicle-treated vs lapatinib-treated mice (Figure 3, A). We next examined the effect of lapatinib on the development of hyperplasia, noninvasive cancers (ie, mammary intraepithelial neoplasia [MIN], which is similar to human ductal carcinoma in situ [DCIS]), and microscopic mammary cancers. After 5 months of lapatinib treatment, 11 of 20 mice showed none of these lesions compared with three of 20 vehicle-treated mice (Figure 3, B). Hyperplasia was seen in 14 of 20 vehicle-treated mice compared with eight of 20 lapatinib-treated mice. We observed no difference between treatment groups in the number of mice that had a MIN lesion (one mouse in each group had a MIN lesion). However, two of 20 vehicle-treated mice developed microscopic invasive mammary tumors compared with none of the lapatinib-treated mice. This difference in the number of premalignant lesions, MIN lesions, and microscopic invasive tumors in the mammary glands of vehicle-treated and lapatinib-treated mice was statistically significant (P = .02, Fisher exact test, two-tailed) (Figure 3, B). These results indicate that lapatinib prevents mammary tumorigenesis by blocking the development of premalignant lesions and progression to invasive mammary tumors.
Figure 3.
Effect of lapatinib on the development of premalignant lesions and on biomarker expression in mammary glands. MMTV-erbB2 mice were randomly assigned to receive treatment 6 d/wk with vehicle or lapatinib (75 mg/kg body weight) for 5 months beginning at 3 months of age (n = 20 mice per group). The mice were killed by CO2 asphyxiation at 8 months of age, and their mammary glands were harvested. Mammary gland samples were fixed overnight in 4% phosphate-buffered formaldehyde and then embedded in paraffin for histological examination and biomarker measurement. A) Histology: Mammary tissue sections (4 μm thick) were stained with hematoxylin–eosin. Representative fields containing normal mammary gland, hyperplasia, mammary intraepithelial neoplasia (MIN), and invasive mammary tumors are shown. B) Premalignant lesions and microscopic invasive cancers. One #3 mammary gland was removed from each mouse and processed for histological assessment, and the number and types of premalignant lesions in one randomly chosen section of each mammary gland were assessed microscopically. Mice were categorized according to the most advanced lesion observed for each mouse. The data were analyzed using Fisher exact test (two-sided). C) Proliferation of mammary gland epithelial cells from MMTV-erbB2 mice treated with lapatinib or vehicle. Ki67 expression in mammary glands was examined by immunohistochemical staining using an anti-Ki67 antibody (rabbit monoclonal, undiluted, NeoMarkers). Data were generated from 20 mice from each experimental group. Representative fields are shown (left panels). The percentage of Ki67-positive cells was quantified by counting at least 1000 cells per one section per mouse (right panel). Error bars correspond to 95% confidence intervals. P value (two-sided) was calculated using the Wilcoxon rank sum test. D) Expression of cyclin D1, epiregulin, and p27 mRNAs in mouse mammary glands. The #4 mammary glands were collected from the MMTV-erbB2 mice treated with vehicle or lapatinib for 5 months. Enriched mammary epithelial cell pellets were collected, and total RNA was extracted from one #4 mammary gland from 15 of the 20 mice from each group (the #4 mammary glands from the remaining five mice were used for whole-mount preparations). These 15 mammary glands were used to prepare three pooled samples of five #4 mammary glands each. RNA was then extracted from these three pools of mammary glands as previously described (26). The relative mean mRNA expression level vs the level of cyclophilin mRNA from these three pooled samples as measured by quantitative real-time reverse transcription–polymerase chain reaction is plotted. Error bars correspond to 95% confidence intervals. The P values were from two-sided Student t tests. E) Immunoblot analysis of phospho-epidermal growth factor receptor (p-EGFR), phospho-ErbB2, and p27 in mouse mammary epithelial cells. The total cell lysate samples described in panel D (from 15 of the 20 mice per group) were used in this analysis. Antibodies against p-EGFR, phospho-ErbB2, and p27 were used (described in the legend to Figure 1). The blots were densitometrically quantified, and the mean values for the ratios of p-EGFR to actin, phospho-ErbB2 to total ErbB2, and p27 to actin were plotted. Error bars correspond to 95% confidence intervals. P values (two-sided) were obtained using Student t test.
To examine the mechanism by which lapatinib prevents mammary tumorigenesis in this mouse model, we assessed cell proliferation and apoptosis by staining sections of mouse mammary glands from the mice treated for 5 months with lapatinib or vehicle with an antibody specific for the cell proliferation marker Ki67 (NeoMarkers/Labvision, Fremont, CA) (Figure 3, C) and an antibody specific for cleaved caspase 3 (Cell Signaling Technology, Inc., Danvers, MA) (data not shown). Mammary glands of mice treated with lapatinib had statistically significantly fewer Ki67-positive mammary epithelial cells than those of mice treated with vehicle (mean percentage of Ki67-positive cells, 3.28% vs 15.83%, difference = 12.55%, 95% CI = 1.77% to 23.33%; P = .02, Wilcoxon rank sum test). The percentage of cleaved caspase 3–positive cells in the mammary glands did not differ statistically significantly between vehicle- and lapatinib-treated mice, indicating that lapatinib did not induce apoptosis in normal-appearing mammary tissue (data not shown). These results suggest that lapatinib suppresses mammary tumorigenesis through inhibition of epithelial cell proliferation.
Previous studies have demonstrated that lapatinib decreases human breast cancer cell proliferation in vitro (27–29) and breast tumor growth in patients (28–30). Lapatinib also causes a G0/G1 cell cycle blockade by controlling the expression of cell cycle regulators such as cyclin D1 (27,31–34). To investigate the effect of lapatinib on the expression of cell cycle regulatory molecules (cyclin D1, p27) and growth regulatory molecules (EGFR, erbB2, epiregulin) in this mouse model, we measured the RNA levels of these molecules in mammary epithelial cells from the mice treated for 5 months with vehicle or lapatinib (Figure 3, D and E). Total RNA was obtained from pooled, enriched mammary epithelial cells as previously described (26). A quantitative real-time reverse transcription–polymerase chain reaction assay was performed to measure mRNA levels as previously described (35). Compared with mammary epithelial cells from vehicle-treated mice, those from lapatinib-treated mice expressed lower levels of mouse cyclin D1 mRNA (P = .156) and epiregulin mRNA (P = .029) and a higher level of mouse p27 mRNA (P = .052) (Figure 3, D). The mRNA levels of cyclin D1, epiregulin, and p27 were also statistically significantly decreased (in the case of cyclin D1 and epiregulin) or increased (in the case of p27) by lapatinib treatment compared with vehicle in EGF-stimulated HMECs (Supplementary Figure 3, available online). Lapatinib is a dual inhibitor of EGFR and ErbB2. Therefore, we next examined the effect of lapatinib on the activation of EGFR and ErbB2 in mammary epithelial cells from the mice treated with vehicle or lapatinib for 5 months by immunoblot analysis. Densitometric quantitation of the immunoblots revealed that phospho-EGFR and phospho-ErbB2 levels were decreased in mammary epithelial cells from lapatinib-treated mice compared with vehicle-treated mice (P = .023 and .003, respectively, Student t test,), whereas the level of p27 was increased (P = .006, Student t test) (Figure 3, E). The results in Figure 3, C and D, suggest that in this mouse model lapatinib suppresses mammary tumor development by reducing epithelial cell proliferation in normal and premalignant tissues of the mammary gland.
This study has several limitations. First, this cancer preventive effect of lapatinib has been shown in only one mouse model. For this finding to be generalizable, it will be important to test lapatinib in another model of ER-negative mammary tumors [such as the p53-null mouse model described by Medina et al. (36)]. Second, lapatinib did not absolutely prevent mammary tumorigenesis in all of the MMTV-erbB2 mice. Although tumorigenesis was delayed in the lapatinib-treated mice, 31% of the mice treated with the higher dose eventually developed mammary tumors. To overcome this limitation, it will likely be necessary to test lapatinib in combination with other cancer preventive drugs in the future.
A clinical study (37) has demonstrated the anticancer activity of lapatinib in advanced breast cancer. Results of this study led to US Food and Drug Administration approval of lapatinib in combination with capecitabine for the treatment of women with metastatic breast cancer, and lapatinib is now being tested in clinical trials in the adjuvant setting for the treatment of early-stage breast cancer. Our results show that lapatinib suppresses the development of ER-negative ErbB2-positive invasive mammary tumors in MMTV-erbB2 mice. Thus, lapatinib may be useful for the prevention of ER-negative, ErbB2-positive breast cancer in humans. Our finding that lapatinib prevents the development of premalignant lesions in these mice suggests that it may also be useful for treating women with DCIS to prevent its progression to invasive breast cancer. These results have supported the development of a phase II trial at the Baylor College of Medicine (ClinicalTrials.gov identifier: NCT00570453 [ClinicalTrials.gov]), testing lapatinib as neoadjuvant therapy in women with either ErbB1- or ErbB2-positive DCIS. In this trial, the effect of lapatinib on DCIS cell proliferation will be assessed. If this trial shows that lapatinib suppresses the growth of DCIS cells, we will then conduct a phase III trial to determine whether lapatinib prevents the progression of DCIS to invasive breast cancer.
Funding
National Institutes of Health Specialized Program of Research Excellence (SPORE) Grant P50 CA58183 (to C.K.O.); the Breast Cancer Research Foundation (to P.H.B.).
Supplementary Material
Footnotes
T. M. Gilmer holds stock in GlaxoSmithKline (the maker of lapatinib), and C. K. Osborne is involved in a clinical trial that is partially funded by GlaxoSmithKline. We thank Dr Daniel Medina for assistance with pathology and for assistance with premalignant lesion assessment. We also thank Dr Sean Humphrey for critical review and helpful discussion of the manuscript. The study sponsors have no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007;19(2):124. doi: 10.1016/j.ceb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 3.Kallioniemi OP, Kallioniemi A, Kurisu W, et al. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci USA. 1992;89(12):5321–5325. doi: 10.1073/pnas.89.12.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Borg A, Tandon AK, Sigurdsson H, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50(14):4332–4337. [PubMed] [Google Scholar]
- 6.Joensuu H, Isola J, Lundin M, et al. Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: a nationwide population-based study. Clin Cancer Res. 2003;9(3):923–930. [PubMed] [Google Scholar]
- 7.Berger MB, Mendrola JM, Lemmon MA. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 2004;569(1–3):332–336. doi: 10.1016/j.febslet.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Hudelist G, Singer CF, Manavi M, Pischinger K, Kubista E, Czerwenka K. Co-expression of ErbB-family members in human breast cancer: Her-2/neu is the preferred dimerization candidate in nodal-positive tumors. Mammary Tumors Res Treat. 2003;80(3):353–361. doi: 10.1023/A:1024929522376. [DOI] [PubMed] [Google Scholar]
- 9.Nagy P, Jenei A, Damjanovich S, Jovin TM, Szolosi J. Complexity of signal transduction mediated by ErbB2: clues to the potential of receptor-targeted cancer therapy. Pathol Oncol Res. 1999;5(4):255–271. doi: 10.1053/paor.1999.0255. [DOI] [PubMed] [Google Scholar]
- 10.Lu C, Mohsin SK, Hilsenbeck S, Wakeling A, Brown PH. Response: Re: Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2004;96(9):715–716. doi: 10.1093/jnci/djh126. [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Speers C, Zhang Y, et al. Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2003;95(24):1825–1833. doi: 10.1093/jnci/djg117. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhang Y, Hill J, et al. The Rexinoid LG100268 prevents the development of preinvasive and invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin Cancer Res. 2007;13(20):6224–6231. doi: 10.1158/1078-0432.CCR-06-2681. [DOI] [PubMed] [Google Scholar]
- 13.Wu K, Zhang Y, Xu X-C, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002;62(22):6376–6380. [PubMed] [Google Scholar]
- 14.Spector NL, Xia W, Burris H, III, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23(11):2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 15.Xia W, Gerard CM, Liu L, Baudson NM, Ory TL, Spector NL. Combining lapatinib ( GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene. 2005;24(41):6213–6221. doi: 10.1038/sj.onc.1208774. [DOI] [PubMed] [Google Scholar]
- 16.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61(24):8887–8895. [PubMed] [Google Scholar]
- 17.Cappuzzo F, Ligorio C, Toschi L, et al. EGFR and HER2 gene copy number and response to first-line chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2(5):423–429. doi: 10.1097/01.JTO.0000268676.79872.9b. [DOI] [PubMed] [Google Scholar]
- 18.Asanuma H, Torigoe T, Kamiguchi K, et al. Survivin expression is regulated by coexpression of human epidermal growth factor receptor 2 and epidermal growth factor receptor via phosphatidylinositol 3-kinase/AKT signaling pathway in breast cancer cells. Cancer Res. 2005;65(23):11018–11025. doi: 10.1158/0008-5472.CAN-05-0491. [DOI] [PubMed] [Google Scholar]
- 19.Yokouchi H, Yamazaki K, Kinoshita I, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer. 2007;7(March 20):51. doi: 10.1186/1471-2407-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida K, Yatabe Y, Park JY, et al. Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer. J Thorac Oncol. 2007;2(1):22–28. [PubMed] [Google Scholar]
- 21.Dincer M, Aksoy S, Kilickap S, Altundag K. Gefitinib efficacy in relapsed small-cell lung cancer. Lung Cancer. 2006;53(2):255. doi: 10.1016/j.lungcan.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 23.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 24.Shen Q, Uray IP, Li Y, et al. The AP-1 transcription factor regulates breast cancer cell growth via cyclins and E2F factors. Oncogene. 2008;27(3):366–377. doi: 10.1038/sj.onc.1210643. [DOI] [PubMed] [Google Scholar]
- 25.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 26.Shen Q, Zhang Y, Uray IP, et al. The AP-1 transcription factor regulates postnatal mammary gland development. Dev Biol. 2006;295(2):589–603. doi: 10.1016/j.ydbio.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 27.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1(2):85–94. [PubMed] [Google Scholar]
- 28.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 29.Xia W, Bisi J, Strum J, et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancer. Cancer Res. 2006;66(3):1640–1647. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- 30.Burris HA, III, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib ( GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23(23):5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 31.Hegde PS, Rusnak D, Bertiaux M, et al. Delineation of molecular mechanisms of sensitivity to lapatinib in breast cancer cell lines using global gene expression profiles. Mol Cancer Ther. 2007;6(5):1629–1640. doi: 10.1158/1535-7163.MCT-05-0399. [DOI] [PubMed] [Google Scholar]
- 32.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib ( GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66(3):1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 33.Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67(3):1170–1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 34.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 35.Kim HT, Kong G, Denardo D, et al. Identification of biomarkers modulated by the rexinoid LGD1069 (bexarotene) in human breast cells using oligonucleotide arrays. Cancer Res. 2006;66(24):12009–12018. doi: 10.1158/0008-5472.CAN-05-2515. [DOI] [PubMed] [Google Scholar]
- 36.Medina D, Kittrell FS, Shepard A, et al. Biological and genetic properties of the p53 null preneoplastic mammary epithelium. FASEB J. 2002;16(8):881–883. doi: 10.1096/fj.01-0885fje. [DOI] [PubMed] [Google Scholar]
- 37.Geyer CE, Forster J, Lindquist D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 38.DeNardo DG, Cuba VL, Kim H, Wu K, Lee AV, Brown PH. Estrogen receptor DNA binding is not required for estrogen-induced breast cell growth. Mol Cell Endocrinol. 2007;277(1–2):13–25. doi: 10.1016/j.mce.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 39.DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogen-induced/activator protein-1-dependent genes. Mol Endocrinol. 2005;19(2):362–378. doi: 10.1210/me.2004-0267. [DOI] [PubMed] [Google Scholar]
- 40.Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 41.Niu G, Carter WB. Human epidermal growth factor receptor 2 regulates angiopoietin-2 expression in breast cancer via AKT and mitogen-activated protein kinase pathways. Cancer Res. 2007;67(4):1487–1493. doi: 10.1158/0008-5472.CAN-06-3155. [DOI] [PubMed] [Google Scholar]
- 42.Trost TM, Lausch EU, Fees SA, et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 2005;65(3):840–849. [PubMed] [Google Scholar]
- 43.Wu CJ, Qian X, O’Rourke DM. Sustained mitogen-activated protein kinase activation is induced by transforming erbB receptor complexes. DNA Cell Biol. 1999;18(10):731–741. doi: 10.1089/104454999314872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.