Abstract
Aims
The ŒDIPE trial examined the safety and efficacy of an abbreviated hospitalization after implantation or replacement of dual-chamber pacemakers (PM) using a telecardiology-based ambulatory surveillance programme.
Methods and results
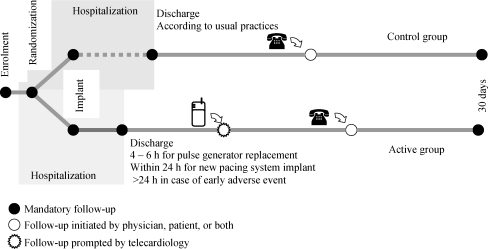
Patients were randomly assigned to (i) an active group, discharged from the hospital 24 h after a first PM implant or 4–6 h after replacement, and followed for 4 weeks with Home-Monitoring (HM), or (ii) a control group followed for 4 weeks according to usual medical practices. The primary objective was to confirm that the proportion of patients who experienced one or more major adverse events (MAE) was not higher in the active than in the control group. The study included 379 patients. At least one treatment-related MAE was observed in 9.2% of patients (n = 17) assigned to the active group vs. 13.3% of patients (n = 26) in the control group (P = 0.21), a 4.1% absolute risk reduction (95% CI −2.2 to 10.4; P = 0.98). By study design, the mean hospitalization duration was 34% shorter in the active than in the control group (P < 0.001), and HM facilitated the early detection of technical issues and detectable clinical anomalies.
Conclusion
Early discharge with HM after PM implantation or replacement was safe and facilitated the monitoring of patients in the month following the procedure.
Keywords: Telecardiology, Telemedicine, Ambulatory monitoring, Pacemaker implantation
Introduction
Telecardiology is offering new choices for the long-term surveillance of cardiac pacemakers (PM) and automatic defibrillators. Home-Monitoring® (HM) (Biotronik GmbH & Co. KG, Berlin, Germany) is a proven telecardiology system, which enables the remote follow-up of these devices, either continuously, for the early detection of adverse clinical or technical events, or intermittently, as a substitute for scheduled follow-up office visits.1–7 The OnE Day pacemaker Implantation Program with homE-monitoring (OEDIPE) trial was conducted to examine the safety and effectiveness of HM when implemented during the relatively high-risk first month that follows the primary implantation or replacement of the dual-chamber PM.
Methods
Primary study objective
The primary objective of the OEDIPE trial was to determine whether, by continuously monitoring all parameters of device function, this telecardiology system enables a significant shortening of post-operative hospitalization, while preserving a safety level equivalent to that associated with conventional management, usually associated with a longer hospital stay. The primary study objective was to confirm that the proportion of patients who experienced one or more major adverse events (MAE) was not higher in the group of patients assigned to early discharge from the hospital with HM (active group) than in a control group. Early discharge from the hospital was defined as (i) within 24 h after first implant, or (ii) within 4–6 h after pulse generator replacement.
Secondary objectives
The secondary objectives of the trial included (i) an evaluation of the performance of telecardiology in the detection of pacing system dysfunction, (ii) a comparison of the duration of hospitalization between the two study groups, (iii) an estimation of the putative cost saving associated with device implantation in the group assigned to shorter hospitalization, and (iv) a measurement of the possible impact of telecardiology on quality of life.8
Telecardiology system
All patients included in this trial underwent implantation of a Philos II DR-T PM (Biotronik), equipped with telecardiology, a system capable of automatically transmitting the data stored in implantable devices. Enrolment was limited to recipients of the dual-chamber PM, as, when the study was conducted, HM had not been installed in single chamber devices. The pulse generator includes a radiofrequency circuitry and an antenna, which emit the data daily to the Cardio-Messenger®. This base station automatically re-routs the data over a wireless global system for mobile communications (GSM) network to the Biotronik service centre. After an automatic analysis, the data (daily messages) are made available on a secure Internet site to the physician responsible for the patient. In case of clinical or technical anomaly (or both), the device emits additional warning messages, which are immediately delivered via the service centre to the physician, by e-mail, fac simile, or text messages, or by all three means (Table 1).
Table 1.
Technical and clinical information transmitted daily, along with corresponding warnings in case of detected anomaly
| Daily transmission | Associated warning messages | Device programming in both study groups |
|---|---|---|
| Technical information | DDD or DDDR mode | |
| Battery status | Elective replacement indicator | |
| Atrial lead impedance | Atrial lead impedance <200 or >3000 Ω | Automatic atrial lead test: ON |
| Ventricular lead impedance | Ventricular lead impedance <200 or >3000 Ω | Automatic ventricular lead test: ON |
| R wave safety margin | R wave safety margin <50% | |
| P wave safety margin | P wave safety margin <50% | |
| Ventricular capture threshold | Autothreshold deactivated | Autothreshold: ON |
| Variations in ventricular threshold >1 V | ||
| Ventricular threshold >2.4 V | ||
| No transmission | No transmission in last 36 h | Transmission at 3:00 a.m. |
| Clinical information | ||
| Mean ventricular rate/24 h | ||
| Number and duration of mode switch | Mode switch duration >18 h | Mode switch: DDIR/160 b.p.m. (X/Z 5/8) |
| Number and type of atrial arrhythmia | First mode switch since onset of follow-up | |
| Peak ventricular rate during mode switch | IEGM recording for mode switch: ON | |
| Daily peak ventricular rate | IEGM recording for ventricular rate: ON | |
| Duration of episode with fastest ventricular rate | Ventricular rate >160 b.p.m. | |
| Number of ventricular salvos | ||
| Number of ventricular episodes | Ventricular episode (>8 consecutive PVC) | |
| Peak hourly/daily rate of PVC |
PVC, premature ventricular complex; IEGM, intracardiac electrogram.
Patient selection
The criteria for inclusion in the OEDIPE trial were: (i) age >18 years; (ii) indication for first implant or replacement of a dual-chamber pulse generator; (iii) patient ability to comply with the study protocol and signature of an informed consent; (iv) stable medical and residential status; (v) ability to discharge the patient from the hospital 1 day after first device implant, or on the day of pulse generator replacement; (vi) absence of exclusion criterion. To optimize the safety of the trial and facilitate compliance with the protocol, patients were excluded if they (i) had a spontaneous ventricular rate <30 b.p.m.; (ii) were in overt heart failure; (iii) had a history of cardiac surgery or myocardial infarction within 1 month; (iv) were systemically anticoagulated; (v) were unable to understand telecardiology; (vi) lived in an area with insufficient GSM coverage.
Study protocol
OEDIPE was a randomized, open-label, parallel- and non-inferiority design trial in which 38 French and 1 Belgian medical centres participated, including 22 public and 17 private institutions (appendices). The trial protocol, which complied with the declaration of Helsinki, was reviewed and approved by the pertinent National Ethics Committees, and all patients granted their written informed consent to participate. Patients who fulfilled the inclusion criteria and indications for permanent pacing described in the guidelines issued by professional societies were randomly assigned to an active vs. a control group by means of sealed envelopes.9
In the absence of interim adverse event (AE), patients assigned to the active group were discharged 24 h after a first implant or 4–6 h after the replacement of the pulse generator. In patients who had undergone subclavian puncture, a chest radiograph was obtained before their discharge from the hospital. Patients in this group were monitored daily by telecardiology, with option of one or more visits by a home nurse.10 The data transmitted were analysed daily throughout the trial. In the event of a device dysfunction (technical issue) or clinical event (medical issue), the cardiologist investigator was notified by e-mail, fac simile, or text message, allowing the rescheduling of the next follow-up visit, if necessary. It is noteworthy, however, that HM was not a substitute for emergency medical services.
Patients in the control group were managed according to the usual practice of each participating medical centre and discharged on the basis of their medical status and institutional guidelines. Although transmitted daily, these telecardiology data were not made available to the investigators and were analysed retrospectively. The option of one or more visits by a home nurse was also available. The general design of the trial is shown in Figure 1.
Figure 1.
Overall study design.
At the end of the trial, 30 days after inclusion of the patient, or at the time of an additional follow-up visit, the investigator interrogated the pacing system and recorded the possible occurrence of an AE. In the event of MAE, a report was filed within 24 h to the Safety Monitoring Committee (SMC), which comprised the Principal Investigator and two medical experts (appendices). Quality of life was estimated at the end of the study by means of an SF-36 questionnaire.11 Cost of care was calculated in both study groups by review of the billing documents for private medical institutions and by compilation of customary reimbursement costs for the public medical centres.
Definitions of adverse events
Major adverse event was defined as an event experienced by a patient, related to the PM implant procedure or the underlying medical condition, prompting an intervention, and with serious or potentially serious consequences, including death, change in prognosis, prolongation of hospitalization for peri- or post-operative complications, and re-admission to the hospital. Non-major AE (NMAE) was defined as an event related to the PM implant procedure or the underlying medical condition without serious or potentially serious consequences, such as prolongation of hospitalization or the need to re-admit the patient to the hospital. For example, an episode of atrial fibrillation might have been classified as MAE or NMAE depending on its tolerability and need for continued or repetitive in-hospital care. Adverse events unrelated to the PM implant procedure or to the underlying medical condition were not included in this analysis. All AE that occurred in the active group were reported to the SMC.
Statistical analyses
On the basis of a primary non-inferiority hypothesis and an 80% power (1-β), we estimated that a sample of 400 patients needed to be enrolled to reach a 5% significance level (α error), assuming a 10% rate of non-compliance and 5% equivalence margin. The patients were randomly assigned to an active vs. a control group by means of sealed envelopes in even blocks among study centres. Comparisons between the two study groups were made by Fisher's exact test and χ2 test for nominal, qualitative variables and by Student's parametric test for normal distributions of quantitative, continuous, and discrete variables. Absolute risk reduction (RR) and 95% confidence intervals (CI) were calculated. A P-value <0.05 was considered significant.
Results
Patient population
Between April 2005 and December 2006, 406 patients were initially included in the trial. After the exclusion of (i) two enrolling centres because of randomization and protocol violations, and (ii) seven patients because of exclusion criteria, the final analysis included 379 patients (mean age = 75 ± 9.8 years, 61% men), of whom 184 were assigned to the active and 195 to the control group. The baseline clinical characteristics, similar in both study groups, and the electrocardiographic observations prompting the implantation of permanent PM, are shown in Table 2. The percentages of first pacing system implants (86 vs. 87%), overall procedure duration (45 ± 20 vs. 47 ± 21 min), and duration of fluoroscopic exposure (4.3 ± 4.1 vs. 5.0 ± 4.6 min) were also similar in both groups.
Table 2.
Underlying heart disease, disease manifestations and electrocardiographic indications for pacing in each study group
| Study groups |
P | ||
|---|---|---|---|
| Active (n = 184) | Control (n = 195) | ||
| Underlying heart disease | |||
| Primary conduction system disease | 31.1 | 25.9 | 0.21 |
| None known | 39.9 | 46.3 | 0.2 |
| Hypertensive | 7.3 | 9 | 0.54 |
| Ischaemic | 6.7 | 4.5 | 0.33 |
| Cardiomyopathy | 3.6 | 5 | 0.51 |
| Congenital | 1.6 | 0 | 0.07 |
| Disease manifestations | |||
| None | 14.5 | 9.4 | 0.1 |
| Syncope | 44.5 | 38.2 | 0.18 |
| Light-headedness | 2.5 | 6.4 | 0.06 |
| Dyspnoea | 15 | 18 | 0.4 |
| Fatigue | 18 | 16.3 | 0.64 |
| Palpitation | 2.5 | 6 | 0.07 |
| Others | 3 | 5.6 | 0.19 |
| Electrocardiographic indications | |||
| Bradycardia–tachycardia or sick sinus syndrome | 28.3 | 32.5 | 0.37 |
| First- or second-degree atrioventricular block | 31.6 | 24.5 | 0.12 |
| Third-degree atrioventricular block | 25.1 | 26 | 0.84 |
| Missing information | 2.7 | 4 | 0.47 |
| Others | 12.3 | 13 | 0.83 |
Values indicate percentage of patients in the corresponding group.
The mean duration of the trial was 31 ± 6.3 days (range 7–89) following implantation of the pacing systems.
Adverse events
By the end of the trial, the proportion of patients with at least one treatment-related AE was 19.5% (n = 74) in the overall population (95% CI 15.5–23.5), 20.1% (n = 37) in the active group, and 19.0% (n = 37) in the control group (absolute RR 1.1%; 95% CI −6.9 to 9.1; P=0.78). At least one treatment-related MAE (primary study endpoint) was observed in 11.3% of patients (n = 43) in the overall population (95% CI 8.2–14.5), and in 9.2% of patients (n = 17) assigned to the active group, vs. 13.3% of patients (n = 26) in the control group. This 4.1% absolute RR (95% CI −2.2 to 10.4; P=0.98) confirmed the non-inferiority of the abbreviated hospitalization when compared with the standard management programme. At least one treatment-related NMAE was observed in 8.7% (n = 33) of the overall population (95% CI 5.9–11.5) and in 11.4% of patients (n = 21) assigned to the active group vs. 6.2% of patients (n = 12) in the control group (P = 0.07).
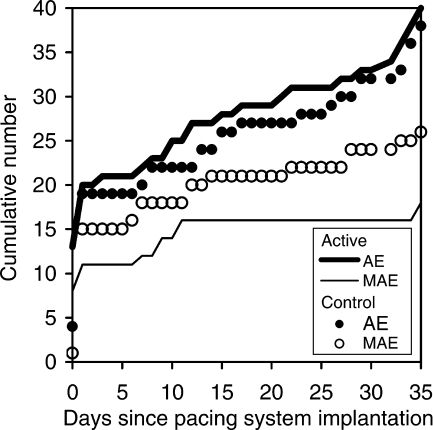
There were 40 (17 technical and 23 medical) AE in the active group vs. 38 (11 technical and 27 medical) in the control group. The types of technical and medical AE that occurred in the overall population and in each study group, before and after discharge from the index hospitalization, are detailed in Table 3. In the active group, 45% (18/40) of all AE were MAE, vs. 68% (26/38) in the control group (P = 0.04). Conversely, the proportions of NMAE relative to all AE were 55% (22/40) in the active group, vs. 32% (12/38) in the control group (P = 0.04). The cumulative incidence of MAE and NMAE in each study group is shown in Figure 2.
Table 3.
Technical and medical adverse events before/after discharge from the hospital in each study group
| Adverse event | All patients | Active group | Control group |
|---|---|---|---|
| Major | |||
| Technical | |||
| Lead dislodgement and twiddler syndrome | 6/8 | 1/4 | 5/4 |
| Intermittent pacing; undersensing | 1/1 | 1/0 | 0/1 |
| Medical | |||
| Death from lung cancera | 0/1 | 0/0 | 0/1 |
| Arrhythmia | 12/2 | 5/2 | 7/0 |
| Pneumothorax | 1/0 | 1/0 | 0/0 |
| Myocardial perforation and tamponade | 4/0 | 3/0 | 1/0 |
| Pulse generator pocket complications | 1/3 | 0/1 | 1/2 |
| Hypotension, hypertension, near-syncope, syncope | 1/2 | 0/0 | 1/2 |
| Acute pulmonary oedema | 0/1 | 0/0 | 0/1 |
| All major adverse events, before/after (total) | 26/18 (44) | 11/7 (18) | 15/11 (26) |
| Non-major | |||
| Technical | |||
| Rise in ventricular capture threshold | 0/2 | 0/2 | 0/0 |
| Permanent atrial undersensing | 0/4 | 0/4 | 0/0 |
| Deactivation of autothreshold | 2/2 | 2/2 | 0/0 |
| Telemetry-related stressa | 0/2 | 0/1 | 0/1 |
| Medical | |||
| Pulse generator pocket complication | 0/1 | 0/1 | 0/0 |
| Arrhythmia | 1/7 | 0/3 | 1/4 |
| Fever | 1/0 | 1/0 | 0/0 |
| Hypotension, light-headedness | 2/1 | 2/0 | 0/1 |
| Haematoma | 6/2 | 3/0 | 3/2 |
| Cutaneous allergic reaction | 1/0 | 1/0 | 0/0 |
| All non-major adverse events, before/after (total) | 13/21 (34) | 9/13 (22) | 4/8 (12) |
| All adverse events | 39/39 (78) | 20/20 (40) | 19/19 (38) |
Values indicate numbers of observations before/after discharge from the hospital.
aNo associated telemetry transmission.
Figure 2.
Cumulative number of adverse events (AE) and major adverse events (MAE), up to 35 days of follow-up, in each study group.
Adverse events not included in the analysis
Twelve AE unrelated to the implantation procedure or to the patient clinical presentation or management (one programmer dysfunction, eight miscellaneous organizational issues, one surgical intervention for inguinal hernia, one convulsive episode, and one acute urinary tract infection) were not included in the analysis.
Implementation of telecardiology
Telecardiology was successfully implemented and operational in 346 of the 379 patients (91% of the overall population). The transmission functions were implemented for 85% of the time elapsed between the activation of telecardiology and the end of the study, representing 24.0 ± 9.3 days of follow-up and a total of 8144 transmissions.
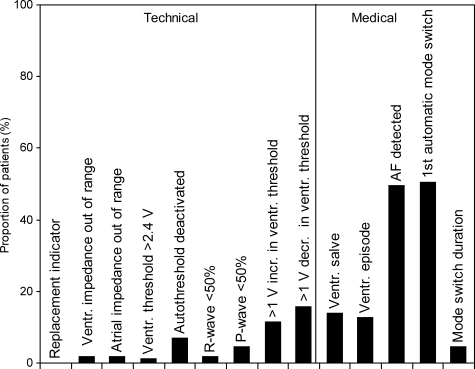
In the active group, 12 patients (6.5%) from seven centres sent no transmission because of the improper use of the transmitter, including 10 devices that were turned off or out of reach of the PM and two instances of unmanageable stress caused by the telecardiology system. Excluding duplicate messages, 108 of the 172 patients whose telecardiology was implemented transmitted a total of 167 warning messages during the trial, with a higher percentage of warnings prompted by medical than by technical AE (Figure 3).
Figure 3.
Types of messages transmitted by 108 patients assigned to the active group.
Management of patients by telecardiology
In the active group, after discharge of the patients from the hospital, 12 warning messages (of which 2 were accompanied by telephone communications) prompted patient visits and the detection of 4 MAE and 8 NMAE, representing 60% of the 20 AE observed in this group. Technical issues prompted 9 of these 12 messages (75%).
A retrospective analysis including the overall population revealed that 39 of the 78 AE occurred after discharge of the patient from the hospital (Table 3). Among these 39 AE, 3 were not detectable because of absence of transmission and 21 of the remaining 36 (58%) were detectable.
The additional workload imposed by the reception of an estimated 1 ± 1.5 messages/patient/month decreased to 0.5 message/patient/month past the fifth day after the implantation procedure.
After a warning issued by the device, the mean medical reaction time, defined as the time elapsed between the reception of a first warning message and the patient contact, was 3.0 ± 3.5 days for four MAE and 8.2 ± 8.7 days for eight NMAE in the active group (prospective analysis), vs. 6.6 ± 10.0 days (n = 5) and 17.5 ± 11.6 days (n = 4), respectively, in the control group (retrospective analysis). The mean medical reaction time for all 12 AE observed in the active group was 6.5 ± 7.6 days, vs. 11.4 ± 11.6 days for the 9 AE observed in the control group. Furthermore, in the active group, the mean advancement of follow-up, defined as the interval between the date of supplemental patient visit and the date of routinely scheduled 1-month follow-up, was 20.0 ± 1.6 days for MAE and 12.0 ± 9.3 days for NMAE.
Patients without warning messages
In the overall population of 346 patients with operational HM systems, 139 (40.2%) transmitted no warning message. Among these 139 patients, 6 experienced an MAE and 1 experienced an NMAE. The six MAE included two infected pulse generator pockets, two hypotensive episodes, one episode of acute pulmonary oedema, one atypical, non-cardiovascular episode of malaise, and one haematoma. Physicians were contacted by five patients for MAE. The negative predictive value of the absence of warning by HM was 94% (95% CI 91–98).
Duration of hospitalization
By study design, the mean duration of hospitalization was 34% shorter in the active than in the control group (95% CI 19–49%), corresponding to 1.6 ± 4.9 fewer days (95% CI 0.9–2.3; P < 0.001) spent in the hospital (3.2 ± 3.2 vs. 4.8 ± 3.7 days). This shorter mean hospital stay was attributable to a 49% shorter post-operative period in the active group (95% CI 32–66%), corresponding to a gain of 1.4 ± 3.3 days (95% CI 0.89–1.85; P < 0.001) compared with the control group (1.4 ± 2.5 vs. 2.8 ± 2.2 days). Thus, 87% of patients left the hospital on the day of (for pulse generator replacements) or the day after (for new systems) the implantation procedure, in contrast with 29% of patients managed by standard methods.
Cost analysis
The cost calculations were based on (i) the institutional and patient care charges listed in the 2005 and 2006 ‘Groupe Homogène de Séjour dans le secteur public et privé’ publication, (ii) the billable items included on the ‘list of products and medical acts’ (costs of telecardiology excluded), (iii) the medical and para-medical fees listed in the ‘Classification Commune des Actes Médicaux’, (iv) the laboratory costs (from the private sector billing contract), and (v) the transportation costs (http://www.ameli.fr). Expenses related to the Biotronik service centre were provided by the manufacturer. The mean costs for the duration of the trial were €7125 ± 1543/patient for the 178 patients assigned to the active group vs. €7414 ± 1659 for the 187 patients assigned to the control group (P = 0.08).
Quality-of-life estimates
The SF-36 quality-of-life questionnaire was completed at 1 month after the index implant procedure by 107 study participants assigned to the active group and 110 patients assigned to the control group. The mean psychological, physical, and overall scores were 66 ± 20, 60 ± 20, and 64 ± 19, respectively, in the active group vs. 68 ± 18, 62 ± 19, and 67 ± 19, respectively, in the control group. These differences were all statistically non-significant.
Discussion
Telecardiology has unequivocally made positive contributions to the follow-up of patients, particularly for recipients of permanent PM, cardioverter defibrillators, and cardiac resynchronization devices. All manufacturers of implantable devices are currently involved in developing and perfecting these systems, which are all capable of remote consultations. However, their implementation remains heterogeneous, because of variable organizational or administrative factors among countries, including reimbursement for telemedicine. The HM system used in this trial allows a time-limited follow-up of the functions of implanted devices, as well as the continuous, daily surveillance of transmitted data, which can be reviewed on a dedicated and secure Internet site. A particularly notable characteristic of HM, besides its scheduled transmissions, is the warning messages that are issued in case of technical dysfunction or detectable clinical abnormality and that are addressed to the physician in charge of the patient via e-mail, fac simile, or text message or via all these means of communication. These warnings allow the anticipation and, when appropriate, the management of potentially harmful medical events. It is, however, noteworthy that HM is not a means of real-time management of emergencies, rather than a close surveillance system.
OEDIPE was the first trial designed to examine prospectively the role that HM might play in the first month after implantation of pacing systems or replacement of pulse generators. The increased risk of complications incurred in the weeks following implants of PM, as well as the medical and economic importance of shortening the hospital stay after surgical procedures, is well known.12,13 By study design, nearly 90% of the patients in the active group were discharged from the hospital within 24 h, compared with <30% in the control group. Although overnight hospitalizations for first implants and ambulatory replacements of pulse generators have been implemented in experienced medical centres, the mean post-operative hospital stay of 2.8 days in our control group indicates that these practices did not represent standard care among the 39 centres included in this study. The ability to remotely follow recipients of implanted devices post-operatively might facilitate the shortening of hospital stays.
The proportion of patients with at least one treatment- related MAE was similar in both study groups. This even distribution between the two groups satisfied the primary objective of the study, which was to confirm that HM allowed a significant shortening of the post-procedural hospitalization, while preserving a safety level similar to that associated with standard patient care. It is noteworthy that whereas the cumulative number of MAE was significantly lower in the active than in the control group, that of NMAE was significantly higher, such that the overall number of AE was similar in both groups. Instead of reflecting a higher morbidity, the higher number of NMAE in the active group might be attributable to the early detection of technical or clinical anomalies, which, otherwise, might have remained unnoticed without telecardiology surveillance. It is also noteworthy that, in both groups, 50% of the AE occurred in the hospital and could have been detected without the assistance of HM (Table 3). However, in the active group, after discharge from the hospital and up to 30 days of follow-up, 12 warning messages prompted the early detection of 4 MAE and 8 NMAE out of a total of 20 AE. The relatively high number of post-operative AE observed in this study was probably due to the inclusion of events that were not procedure-related, such as atrial or ventricular arrhythmias. However, these rhythm disturbances developing in the immediate post-operative period might be, in some cases, the first indication of an early complication. The single non-cardiac death observed in this trial was not related to the procedure, though was classified as MAE according to the pre-specified definitions of our protocol. This single death had no effect on the overall statistical outcome.
The retrospective analysis of the pooled data from the two groups, including the data from the control group available a posteriori to the expert committee, showed that over 80% of the technical AE could have been detected early. Although HM is sensitive in the detection of technical complications and arrhythmic episodes, its sensitivity is lower with respect to medical AE related to the surgical procedure, such as pulse generator pocket complications or development of a pneumothorax. Furthermore, warnings may be non-specific, as a single anomaly may trigger several different kinds of messages. From this perspective, HM is not an automatic surveillance system, and its data must be critically reviewed by the physician.
In the control group, the medical reaction time, based strictly on overtly abnormal clinical manifestations, was considerably longer than in the active group. The analysis of AWARE, a retrospective study of >11 000 patients in 23 countries, revealed that AE occurred, on average, 26 days after the last visit, advancing the diagnosis by a mean of 154 days.14 In the OEDIPE trial, besides the surveillance data routinely transmitted to the Internet site, an estimated 1 ± 1.5 additional warning messages/patient/month were issued during the study, decreasing to 0.5 warning message/patient/month past the fifth day of enrolment in the trial, an estimate concordant with the 0.6 message/patient/month reported during the long-term follow-up of AWARE. The gradual decrease in the rate of warning messages following device implantation appears related to the optimization of the alert settings and to the elimination of warnings after a first event, for example, a first automatic mode switch. It is noteworthy that 40% of the overall patient population transmitted no warning message, though seven patients did experience a medical AE that could not have been detected by telecardiology. This strongly suggests that the absence of warning message excludes reliably the occurrence of undetected technical AE. A similar 47% of patients regularly monitored by telecardiology transmitted no warning message in the AWARE study.
Although HM decreased the duration of post-operative hospitalization by nearly 50% compared with the control group, the decrease in overall costs associated with this shortening of the hospital stay was not statistically significant, because of the reimbursement scheme based on diagnosis-related groups. Finally, the SF-36 questionnaire showed that telecardiology had no negative effect on the patients' quality of life.
The results of the OEDIPE trial, which combines abbreviated hospitalization with telecardiology follow-up after the first implant of pacing systems or replacement of pulse generators, demonstrate that this programme was safe and facilitated the monitoring of patients in the month following the procedure.
Funding
This study was funded by Biotronik Inc. Funding to pay the Open Access publication charges for this article was provided by Biotronik Inc.
Acknowledgements
The authors thank Mr Nicolas Canot, Clinical Study Manager, Mr Xavier Laroche, Home-Monitoring Manager, and Ms Sophie Bader, Clinical Research Associate for their assistance in the conduct of the OEDIPE trial.
Conflict of interest: none declared.
Appendix
The following investigators participated in the ŒDIPE trial.
Xavier Dessenne, MD, Clinique du Mousseau, Evry; Walid Amara, MD, Centre Hospitalier Intercommunal, Montfermeil; Franck Halimi, MD, Centre Médico-Chirurgical Parly 2, Le Chesnay; Patrick Attuel, MD, Centre Médico-Chirurgical Parly 2, Le Chesnay; Michel Sportiche, MD, Centre Médico-Chirurgical Parly 2, Le Chesnay; Isabelle Robin, MD, Centre Hospitalier Régional Universitaire, Angers; Alain Thia, MD, Centre Hospitalier Régional Universitaire, Angers; Olivier Bizeau, MD, Centre Hospitalier Régional La Source, Orléans; Luc Kubler, MD, Polyclinique Gentilly, Nancy; Nourreddine El Hajjaji, MD, Centre Hospitalier Abbeville, Abbeville; Georges Nadji, MD, Centre Hospitalier Abbeville, Abbeville; Bouchaib Deriouich, MD, Centre Hospitalier, Evreux; Pierrick Boyer, MD, Clinique Draguignan, Draguignan; Armel Bonneau, MD, Centre Hospitalier, Châteauroux; Mourad Hadid, MD, Centre Hospitalier, Béziers; Dominique Bleinc, MD, Centre Hospitalier Claude Galien, Quincy S/ Sénart; Robert Frank, MD, Centre Hospitalier La Pitié Salpétrière, Paris; André Tardieu, MD, Clinique Pont de Chaume, Montauban; Olivier Thourot, MD, Centre Hospitalier, Bar le Duc; Sandrine Bellmont, MD, Centre Hospitalier, Colmar; Fouad El Ghelbazouri, MD, Clinique Montargis, Montargis; Jean-Marie Lefebvre, MD, Polyclinique La Louvière, Lille; Sébastien Caudmont, MD, Polyclinique Vauban, Valenciennes; Olivier Brimont, MD, Polyclinique Vauban, Valenciennes; Ali Benkaci, MD, Centre Hospitalier, Meaux; Joël Rivagorda, MD, Centre Hospitalier Paul d'Egine, Champigny Sur Marne; Christophe Boesch, MD, Centre Hospitalier Paul d'Egine, Champigny Sur Marne; Serge Boveda, MD, Clinique Pasteur, Toulouse; Pascal Laurentjoye, MD, Polyclinique Cauderan, Bordeaux; Jacques Clémenty, MD, Centre Hospitalier Universitaire Haut Lévèque, Pessac; Julien Laborderie, MD, Centre Hospitalier Universitaire Haut Lévèque, Pessac; Gilles Lascault, MD, Centre Cardiologique du Nord, Saint Denis; Olivier Paziaud, MD, CCN, Saint Denis; Gilles Boccara, MD, Polyclinique, Aix en Provence; Jean-Marc Chabernaud, MD, CliniqueI du Colombier, Limoges; Mohamed Belhameche, MD, Centre Hospitalier, Lagny; Jean-Luc Massing, MD, Hôpital Sainte Blandine, Metz; Patrice Scanu, MD, Centre Hospitalier Régional Universitaire, Caen; Frédéric Dumont, MD, Clinique Universitaire de Mont-Godinne, Louvain; Claude Gully, MD, Centre Hospitalier Départemental, La Roche sur Yon; Patrick Bechetoille, MD, Centre Hospitalier, Vesoul; Pierre Graux, MD, Groupe Hospitalier de l'Institut Catholique de Lille, Lomme; Patrick Messner, MD, Centre Hospitalier Universitaire, Nîmes; Marc Sagnol, MD, Centre Hospitalier, Châlon Sur Saône; Philippe Jarnier, MD, Centre Hospitalier, Perigueux; Denis Raguin, MD, Hôpital, Amiens.
Members of the SMC:
Patrick Attuel, MD, Franck Halimi, MD, Centre Médico-Chirurgical Parly 2, Le Chesnay; Jacques Clémenty, MD, Centre Hospitalier Universitaire Haut Lévèque, Pessac.
References
- 1.Wallbrück K, Stellbrink C, Santini M, Gill J, Hartmann A, Wunderlich E. The value of permanent follow-up of implantable pacemakers—first results of an European trial. Biomed Tech. 2002;47:950–3. doi: 10.1515/bmte.2002.47.s1b.950. [DOI] [PubMed] [Google Scholar]
- 2.Stellbrink C, Hartmann A, Igidbashian D, Jaswinder G, Wunderlich E, Santini M. Home monitoring for pacemaker therapy: intermediate results of the first European multicenter study. Pacing Clin Electrophysiol. 2002;25:686. (abstract) [Google Scholar]
- 3.Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace. 2008;10:164–70. doi: 10.1093/europace/eum289. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network—Home Care Management System (TEN-HMS) Study. J Am Coll Cardiol. 2005;45:1654–64. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Brugada P. What evidence do we have to replace in-hospital implantable cardioverter defibrillator follow-up? Clin Res Cardiol. 2006;95:III/3–9. doi: 10.1007/s00392-006-1302-x. [DOI] [PubMed] [Google Scholar]
- 6.Ricci RP, Russo M, Santini M. Management of atrial fibrillation-what are the possibilities of early detection with home-monitoring? Clin Res Cardiol. 2006;95:III/10–6. doi: 10.1007/s00392-006-1303-9. [DOI] [PubMed] [Google Scholar]
- 7.Deharo JC, Djiane P. Home Monitoring: what can we expect in the future? Clin Res Cardiol. 2006;95:III/36–9. doi: 10.1007/s00392-006-1307-5. [DOI] [PubMed] [Google Scholar]
- 8.Fauchier L, Sadoul N, Kouakam C, Briand F, Chauvin M, Babuty D, et al. Potential cost savings by telemedicine-assisted long-term care of implantable cardioverter defibrillator recipients. Pacing Clin Electrophysiol. 2005;28:S255–9. doi: 10.1111/j.1540-8159.2005.00071.x. [DOI] [PubMed] [Google Scholar]
- 9.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, et al. European Society of Cardiology, European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959–98. doi: 10.1093/europace/eum189. [DOI] [PubMed] [Google Scholar]
- 10.Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162:705–12. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Jr, Sherbourne CD. The MOS 36-Item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 12.Aggarwal RK, Connelly DT, Ray SG, Ball J, Charles RG. Early complications of permanent pacemaker implantation: no difference between dual and single 1chamber systems. Br Heart J. 1995;73:571–5. doi: 10.1136/hrt.73.6.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irwin ME, Gulamhusein SS, Senaratne MP, St Clair WR. Outcomes of an ambulatory cardiac pacing program: indications, risks, benefits, and outcomes. Pacing Clin Electrophysiol. 1994;17:2027–31. doi: 10.1111/j.1540-8159.1994.tb03794.x. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30:S2–12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]