Abstract
Background. Antagonists of relevant Gs protein-coupled and agonists of relevant Gi protein-coupled receptors lower renal cAMP and inhibit growth of renal cysts in animal models of human ARPKD (PCK rat) and/or ADPKD (Pkd2−/WS25 mouse). A calcium-sensing receptor (CaR) is expressed in various tubular segments and couples to Gq, thereby activating phospholipase Cγ, InsP3 generation and calcium mobilization from intracellular stores, and Gi proteins. By both mechanisms, CaR activation could lower intracellular cAMP and inhibit renal cyst growth.
Methods. PCK rat and Pkd2−/WS25 mouse littermates were fed rodent chow without or with R-568, a type 2 calcimimetic, at a concentration of 0.05% or 0.1% between 3 and 10 or 16 weeks of age. Histomorphometric analysis was performed with Meta-Morph software. Western analysis and immunohistochemical staining were performed using antibodies for aquaporin-2, urea transporter UT-A1 and CaR. Northern blot hybridization was used to quantify the expression of vasopressin V2 receptor and aquaporin 2 mRNAs. Cyclic AMP was measured using an enzyme immunoassay kit.
Results. R-568 had no effect on kidney weight, cyst volume, plasma BUN concentration or severity of the polycystic liver disease. A significant reduction in renal interstitial fibrosis was detected in PCK rats, but not in Pkd2−/WS25 mice. R-568 administration, as anticipated, resulted in hypocalcemia and hyperphosphatemia, and significant increases in urine output, osmolar clearance, and urinary excretions of sodium, potassium and calcium.
Conclusions. CaR activation had no detectable effect on cystogenesis in models of autosomal recessive or dominant polycystic kidney disease. The lack of protective effect could be due to the absence of CaR in the outer medullary and cortical collecting ducts, the reduction in extracellular calcium and the unaffected levels of renal cAMP and renal expression of cAMP-dependent genes. A possible beneficial effect on interstitial fibrosis deserves further study at more advanced stages of the disease.
Keywords: calcimimetic, cyclic AMP, PCK rat, Pkd2−/WS25 mouse, polycystic kidney disease
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD) are important causes of ESRD, morbidity and mortality in children and adults [1,2]. ADPKD is genetically heterogeneous with two disease loci, PKD1 and PKD2. ARPKD is caused by mutations at a single locus, PKHD1 [3]. The cysts in ARPKD derive mostly from the collecting ducts. The cysts in ADPKD may derive from any tubular segment, but the distal nephron and collecting ducts are predominantly involved.
The PKD1- and PKD2-encoded proteins, polycystin-1 and -2, and the PKHD1-encoded protein, fibrocystin, are membrane-associated glycoproteins. Polycystin-1 and fibrocystin are receptors for unknown ligands and may undergo regulated intramembrane proteolysis. Polycystin-2 is a calcium permeable cation channel. Polycystin-1 directly and fibrocystin indirectly interact with polycystin-2 and regulate its channel function. Polycystin-1, polycystin-2 and fibrocystin are localized to primary cilia [4–7] and are required for the rise in intracellular calcium triggered by mechanical stimulation of these organelles [6,8,9]. Polycystin-2 is also found in the endoplasmic reticulum, functionally interacts with inositol 1,4,5-triphosphate (InsP3) and ryanodine receptors, and modulates intracellular calcium signaling [10,11].
Alterations in two major interacting second messengers, intracellular calcium and adenosine 3′,5′-cyclic monophosphate (cAMP) play a role in the pathogenesis of polycystic kidney disease. Cyclic AMP levels reflect a balance between synthesis by adenylyl cyclases (activated by Gs protein-coupled, GsPCR, and inhibited by Gi protein-coupled, GiPCR, receptors) and degradation by phosphodiesterases [12]. Stimulation of calcium inhibitable adenylyl cyclases and inhibition of calcium-dependent cAMP phosphodiesterases may account for increased renal levels of cAMP and increased expression of cAMP-dependent genes in various animal models of polycystic kidney disease [13–15]. Cyclic AMP stimulates tubular cells beyond the loop of Henle to secrete chloride and fluid [16]. In addition, cAMP stimulates mitogen-activated protein kinase and extracellularly regulated kinase signaling and cell proliferation in renal epithelial cells of patients with polycystic kidney disease, whereas it has an inhibitory effect in wild-type cells [17,18]. This abnormal proliferative response is linked to alterations in intracellular calcium since it can be reproduced in wild-type cells by lowering intracellular calcium [19]. Conversely, calcium ionophores or channel activators rescue the abnormal response of cyst-derived cells [20].
Recently, antagonists of relevant GsPCR (vasopressin V2 receptor antagonists OPC31260 and OPC41061) or agonists of relevant GiPCR (octreotide) have been shown to lower renal cAMP [14,15,21,22] and inhibit the development and progression of the renal cystic disease in animal models orthologous to human ADPKD and/or ARPKD. Type 2 calcimimetic drugs act as selective allosteric modulators of calcium action at the level of the calcium-sensing receptor (CaR) [23,24]. CaR, a member of subfamily C of the GPCR superfamily, is capable of coupling to Gq (activating phospholipase Cγ, InsP3 generation and mobilization of calcium from intracellular stores) and Gi proteins, lowering intracellular cAMP and inhibiting cAMP signaling [25]. Since CaR is expressed in many tubular segments (apical membrane of the proximal tubule; basolateral membrane of the medullary thick ascending limb, cortical thick ascending limb and distal convoluted tubules; and apical surface of the inner medullary collecting duct) [26], we decided to ascertain whether activation of CaR by a type 2 calcimimetic drug would attenuate or prevent the development of polycystic kidney disease.
Subjects and methods
Experimental animals and study design
Colonies of PCK rats and of Pkd2+/− and Pkd2WS25/WS25 mice have been maintained in the Animal Facilities of the Department of Veterinary Medicine at the Mayo Clinic, Rochester, MN, since 1999. The PCK rat is a model of human ARPKD caused by a splicing mutation (IVS35–2A→T) that skips exon 36 and leads to a frameshift in the ortholog, Pkhd1. The Pkd2-null mutation was generated by disrupting the wild-type exon 1 of the Pkd2 with a selectable neocassette that introduces an in-frame stop codon. The WS25 mutation was generated by the integration of an exon 1 disrupted by the introduction of a selectable neocassette into the first intron of Pkd2 without replacing the wild-type exon 1. This causes an increased rate of somatic mutations in the Pkd2 gene (intragenic homologous recombinations between tandemly repeated portions of the wild-type and mutant exon 1). We crossed Pkd2+/− and Pkd2WS25/WS25 mice to generate Pkd2−/WS25 mice. We used these double heterozygote mice because, unlike other Pkd1+/− or Pkd2+/− models, they reliably develop a slowly progressive renal cystic disease that greatly resembles human ADPKD. At the time of weaning or 3 weeks of age, PCK rat and Pkd2−/WS25 mouse littermates were divided into three groups of 10 male and 10 female animals each receiving a standard ground rodent chow (Teklad 7017, Madison WI, USA) or the same chow containing R-568 at a concentration of 0.1% and 0.05 %, estimated to provide a daily dose of 50 and 25 mg/kg body weight, respectively. Rats were euthanized at 10 weeks and mice at 16 weeks of age, times by which, in our experience, the disease has developed consistently in the absence of treatment. Tail-cuff blood pressures and 24-h urine collections in metabolic cages for determination of urine output, osmolality and urinary excretions of sodium, potassium, calcium and phosphorus were obtained weekly on three consecutive weeks before killing at 10 or 16 weeks of age. At killing, the animals were weighed and anesthetized with ketamine (50 mg/kg, rats; 60 mg/kg, mice) and xylazine (10 mg/kg), intraperitoneally. Blood was obtained by cardiac puncture for determination of plasma BUN, electrolytes, osmolality, calcium and phosphorus. The right kidney and part of the liver were placed into pre-weighed vials containing 10% formaldehyde in a phosphate buffer (pH 7.4). These tissues were embedded in paraffin for histological studies. The left kidney and part of the liver were immediately frozen in liquid nitrogen for determination of cyclic AMP and Western and Northern blot analysis.
Histomorphometric analysis
Whole 4 μm transverse tissue sections stained with hematoxylin-eosin and with picrosirius red were used to measure cyst volumes and fibrosis, respectively. Image analysis procedures were performed with Meta-Morph software (Universal Imaging, West Chester, PA, USA). The Meta-Morph software system includes a light microscope with a color digital camera (Nikon DXM 1200) and a Pentium IBM-compatible computer (Dell OptiPlex). Stained sections are visualized under a Nikon microscope and digital images are acquired using a high-resolution Nikon Digital camera and displayed on the monitor. The observer interactively applies techniques of enhancement for a better definition of interested structures and to exclude fields too damaged to be analyzed. A colored threshold is applied at a level that separates cysts from non-cystic tissue in order to calculate cyst volumes. Areas of interest are expressed as a percentage of total tissue. All the histomorphometric analyses were performed blindly, without knowledge of group assignment.
Western blot analysis and immunohistochemistry
Western analysis and immunohistochemical staining were performed using antibodies for aquaporin-2 (Santa Cruz Biotechnology, Santa Cruz, California), urea transporter UT-A1 (gift from Dr Jeff M. Sands, Emory University School of Medicine, Atlanta, GA, USA) and CaR (Abcam Inc., Cambridge, MA, USA).
Renal expression of vasopressin V2 receptor and aquaporin 2 mRNAs
Total RNA extracted from wild-type and cystic kidney pairs using TRIzol reagent (Invitrogen, Carlsbad, CA) was analyzed by northern blot hybridization using formaldehyde–agarose gel electrophoresis with transfer of RNA to nylon membranes. 32P-labeled probes to vasopressin V2 receptor and aquaporin 2 were used and the hybridized blots were exposed to X-ray film to obtain autoradiographs. Autoradiographs of hybridized blots were scanned and after background was subtracted and corrected for RNA loading, the pixel density of the band was divided by that of an internal control (L32) providing mRNA expression normalized to L32.
Cyclic AMP content of whole kidneys
The kidneys were ground to fine powder under liquid nitrogen in a stainless steel mortar and homogenized in 10 volumes of cold 5% TCA in a glass-Teflon tissue grinder. After centrifugation at 600 g for 10 min, the supernatants were extracted with three volumes of water-saturated ether. After drying the aqueous extracts, the reconstituted samples were processed without acetylation using an enzyme immunoassay kit (Sigma-Aldrich, Inc., St. Louis, MO, USA). The results were expressed in pmol/mg of protein.
Chemical determinations
Plasma blood urea nitrogen (BUN), phosphorus, calcium and electrolytes were measured with a Hitachi Modular System. Urine phosphorus, calcium and electrolytes were measured using a Hitachi 912 analyzer and Perkin-Elmer Optima 2100 DV instrument, respectively. Plasma and urine osmolalities were measured using a computerized micro-osmometer.
Statistical methods
Comparisons between groups were made using two-way analysis of variance (ANOVA) with least significant difference comparisons of the means. Data are expressed as means ± SD.
Results
The administration of R-568 to PCK rats (Table 1) and Pkd2−/WS25 mice (Table 2) was accompanied by a small but significant reduction in body weight, but it was otherwise well tolerated without apparent toxicity. In both animal models, it induced a reduction in serum calcium and an increase in serum phosphorus, which were accompanied in PCK rats by an increase in the urinary excretion of calcium and a reduction in the urinary excretion of phosphorus.
Table 1.
Effect of R-568 on the development of polycystic kidney disease in PCK rats
| Male | Female | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.05% | 0.1% | Control | 0.05% | 0.1% | Gender | R-568 | |
| (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | (n = 10) | |||
| Body weight (g) | 340 ± 19 | 330 ± 20 | 299 ± 27* | 234 ± 10 | 221 ± 15* | 218 ± 10* | < 0.001 | < 0.001 |
| Kidney weight (% BW) | 1.97 ± 0.30 | 1.95 ± 0.20 | 1.85 ± 0.21 | 1.57 ± 0.26 | 1.61 ± 0.20 | 1.63 ± 0.20 | < 0.001 | NS |
| Kidney cyst volume (%) | 22.7 ± 5.4 | 20.2 ± 5.5 | 20.9 ± 2.3 | 21.1 ± 5.6 | 19.3 ± 6.3 | 18.9 ± 4.0 | NS | NS |
| Kidney fibrosis volume (%) | 4.53 ± 1.71 | 2.17 ± 0.92* | 1.58 ± 0.41* | 2.94 ± 1.17 | 1.28 ± 0.57* | 1.03 ± 0.41* | < 0.001 | < 0.001 |
| Kidney cAMP (pmol/mg protein) | 25.2 ± 8.9 | 36.6 ± 12.1 | 33.9 ± 16.7 | 20.8 ± 10.0 | 19.3 ± 5.6 | 17.0 ± 2.7 | < 0.001 | NS |
| Plasma BUN (mg/dL) | 16.1 ± 1.7 | 16.7 ± 2.0 | 20.3 ± 4.6* | 16.4 ± 0.8 | 16.9 ± 2.2 | 16.3 ± 1.6 | NS | 0.031 |
| Plasma osmolality (mosm/L) | 310 ± 10 | 310 ± 14 | 306 ± 16 | 318 ± 13 | 309 ± 10 | 310 ± 8 | NS | NS |
| Plasma phosphorus (mg/dL) | 8.4 ± 1.0 | 10.4 ± 1.5* | 12.5 ± 1.4* | 7.4 ± 0.7 | 9.9 ± 1.3* | 11.1 ± 0.9* | 0.003 | < 0.001 |
| Plasma calcium (mg/dL) | 10.3 ± 0.4 | 8.4 ± 0.4* | 8.1 ± 0.5* | 10.6 ± 0.2 | 8.6 ± 0.4* | 8.1 ± 0.2* | 0.025 | < 0.001 |
| Urine volume (μL/min) | 8.3 ± 2.4 | 14.2 ± 2.0* | 13.5 ± 1.6* | 4.0 ± 0.5 | 10.0 ± 2.0* | 10.1 ± 1.5* | < 0.001 | < 0.001 |
| Urine osmolality (mosm/L) | 869 ± 391 | 863 ± 413 | 1064 ± 420 | 1785 ± 577 | 1795 ± 523 | 1840 ± 352 | < 0.001 | NS |
| Osmolar clearance (μL/min) | 21.3 ± 7.9 | 38.7 ± 17.3* | 48.1 ± 21.6* | 22.3 ± 7.9 | 55.6 ± 11.4* | 60.0 ± 14.5* | 0.009 | < 0.001 |
| Free water clearance (μL/min) | −13.0 ± 9.4 | −24.4 ± 17.9 | −34.6 ± 20.5* | −18.3 ± 7.7 | −45.6 ± 12.2* | −49.9 ± 13.8* | < 0.001 | < 0.001 |
| Urine sodium (mEq/24 h) | 0.56 ± 0.22 | 3.03 ± 0.37* | 2.54 ± 0.88* | 0.42 ± 0.15 | 2.02 ± 0.48* | 2.22 ± 0.54* | < 0.001 | < 0.001 |
| Urine potassium (mEq/24 h) | 2.02 ± 0.71 | 5.44 ± 0.86* | 5.01 ± 0.85* | 1.43 ± 0.29 | 3.58 ± 0.93* | 4.28 ± 0.99* | < 0.001 | < 0.001 |
| Urine phosphorus (mg/24 h) | 18.2 ± 8.4 | 13.5 ± 4.2 | 8.6 ± 7.7* | 13.8 ± 4.2 | 8.5 ± 4.8* | 9.0 ± 1.7* | NS | 0.002 |
| Urine calcium (mg/24 h) | 1.04 ± 0.63 | 2.27 ± 0.87* | 5.48 ± 2.00* | 0.79 ± 0.41 | 3.02 ± 0.95* | 4.30 ± 0.92* | NS | < 0.001 |
*P < 0.01 versus control.
Table 2.
Effect of R-568 on the development of polycystic kidney disease in Pkd2−/WS25 mice
| Male | Female | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.05% | 0.1% | Control | 0.05% | 0.1% | Gender | R-568 | |
| (n = 9) | (n = 10) | (n = 10) | (n = 7) | (n = 8) | (n = 8) | |||
| Body weight (g) | 28.4 ± 2.8 | 27.1 ± 1.1 | 26.1 ± 2.0 | 22.4 ± 1.4 | 22.0 ± 1.7 | 21.2 ± 1.5 | < 0.001 | 0.027 |
| Kidney weight (%BW) | 1.94 ± 0.76 | 1.60 ± 0.46 | 1.77 ± 0.62 | 1.65 ± 0.32 | 1.45 ± 0.25 | 1.82 ± 0.85 | NS | NS |
| Kidney cyst volume (%) | 21.3 ± 17.8 | 11.0 ± 18.6 | 16.0 ± 17.1 | 26.0 ± 11.8 | 17.8 ± 16.8 | 22.3 ± 16.4 | NS | NS |
| Kidney fibrosis volume (%) | 2.44 ± 1.61 | 1.74 ± 1.68 | 3.07 ± 3.05 | 3.13 ± 2.16 | 1.98 ± 1.91 | 3.40 ± 3.26 | NS | NS |
| Kidney cAMP (pmol/mg protein) | 5.90 ± 2.21 | 5.75 ± 1.82 | 5.85 ± 2.20 | 7.04 ± 1.67 | 7.24 ± 2.17 | 6.50 ± 3.71 | NS | NS |
| Plasma.BUN (mg/dL) | 31.1 ± 3.4 | 29.0 ± 4.2 | 29.2 ± 6.6 | 30.2 ± 7.7 | 28.9 ± 5.0 | 28.0 ± 4.0 | NS | NS |
| Plasma osmolality (mosm/L) | 317 ± 11 | 320 ± 10 | 319 ± 9 | 319 ± 10 | 319 ± 10 | 319 ± 11 | NS | NS |
| Plasma phosphorus (mg/dL) | 7.55 ± 0.72 | 8.25 ± 0.77 | 8.58 ± 0.95 | 7.25 ± 1.42 | 7.63 ± 0.71 | 7.87 ± 1.02 | NS | NS |
| Plasma calcium (mg/dL) | 8.53 ± 0.56 | 7.95 ± 0.67 | 7.30 ± 0.89* | 7.63 ± 0.74 | 7.37 ± 0.82 | 6.68 ± 1.21* | 0.009 | 0.004 |
| Urine volume (μL/min) | 0.56 ± 0.48 | 1.01 ± 0.53 | 0.72 ± 0.22 | 0.32 ± 0.22 | 0.80 ± 0.37* | 0.52 ± 0.54 | NS | 0.013 |
| Urine osmolality (mosm/L) | 898 ± 265 | 1076 ± 339 | 936 ± 288 | 1514 ± 752 | 835 ± 333 | 885 ± 418 | NS | NS |
| Osmolar clearance (μl/min) | 1.60 ± 1.37 | 3.28 ± 1.84* | 2.22 ± 1.26 | 1.35 ± 0.67 | 2.20 ± 1.24 | 1.47 ± 1.46 | NS | 0.044 |
| Free water clearance (μl/min) | −1.04 ± 0.92 | −2.26 ± 1.45* | −1.51 ± 1.08 | −1.03 ± 0.59 | −1.40 ± 0.97 | −0.95 ± 0.96 | NS | NS |
| Urine sodium (mEq/24 h) | 0.13 ± 0.07 | 0.23 ± 0.11* | 0.20 ± 0.06* | 0.10 ± 0.06 | 0.25 ± 0.07* | 0.22 ± 0.17 | NS | 0.004 |
| Urine potassium (mEq/24 h) | 0.15 ± 0.07 | 0.32 ± 0.18* | 0.23 ± 0.08* | 0.11 ± 0.04 | 0.30 ± 0.08* | 0.28 ± 0.22 | NS | 0.003 |
| Urine phosphorus (mg/24 h) | 0.85 ± 0.98 | 2.32 ± 1.32* | 1.17 ± 0.88 | 0.84 ± 0.49 | 1.58 ± 0.91 | 0.96 ± 1.11 | NS | 0.009 |
| Urine calcium (mg/24 h) | 0.07 ± 0.04 | 0.08 ± 0.03 | 0.06 ± 0.03 | 0.03 ± 0.02 | 0.07 ± 0.04* | 0.07 ± 0.05 | NS | NS |
*P < 0.01 versus control.
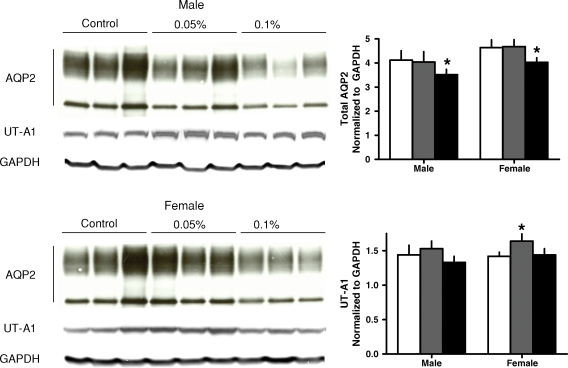
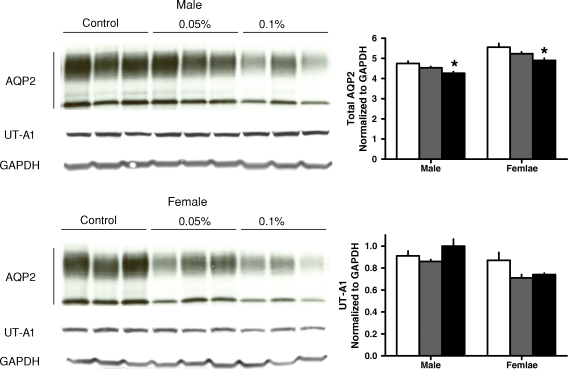
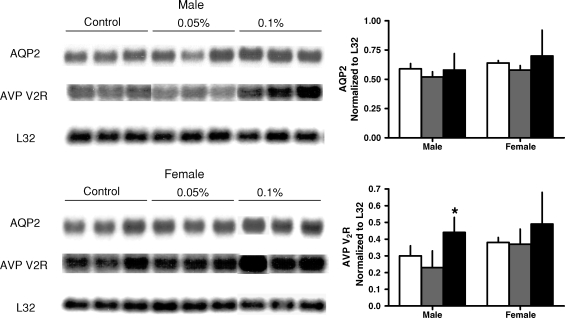
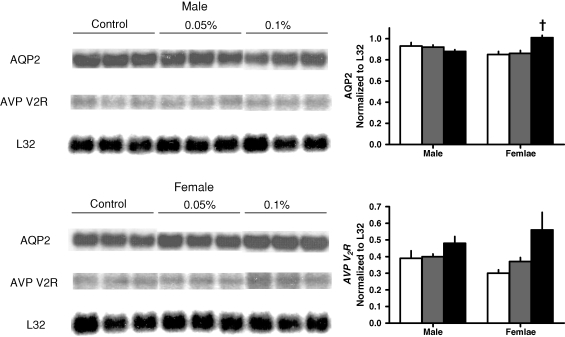
The administration of R-568 also resulted in a significant increase in urine output, osmolar clearance and urinary excretions of sodium and potassium in both rats and mice, without significant changes in plasma or urine osmolality (Tables 1 and 2). The increase in urine output was accompanied by a significant reduction in the renal aquaporin-2 protein in both animal models (Figures 1 and 2). The levels of cAMP (Tables 1 and 2) and mRNA expression of aquaporin-2 were not affected significantly by R-568 administration (Figures 3 and 4). Female PCK rats treated with the lower R-568 dose had a higher expression of the UT-A1 protein than control rats or those treated with the higher dose and male rats treated with the higher R-568 dose had an increased expression of V2R mRNA compared to controls (Figures 1–4).
Fig. 1.
Renal expression (Western blots) of aquaporin 2 and urea transporter UT-A1 in male and female PCK rats on no treatment (white bars) or treated with 0.05% (gray bars) or 0.1% (black bars) R-568 added to their diet. *P < 0.05 versus no treatment.
Fig. 2.
Renal expression (Western blots) of aquaporin 2 and urea transporter UT-A1 in male and female Pkd2−/WS25 mice on no treatment (white bars) or treated with 0.05% (gray bars) or 0.1% (black bars) R-568 added to their diet. *P < 0.05 versus no treatment.
Fig. 3.
Renal expression (Northern blots) of aquaporin 2 and vasopressin V2 receptor mRNA in male and female PCK rats on no treatment (white bars) or treated with 0.05% (gray bars) or 0.1% (black bars) R-568 added to their diet. *P < 0.05 versus no treatment.
Fig. 4.
Renal expression (Northern blots) of aquaporin 2 and vasopressin V2 receptor mRNA in male and female Pkd2−/WS25 mice on no treatment (white bars) or treated with 0.05% (gray bars) or 0.1% (black bars) R-568 added to their diet. *†P < 0.01 versus no treatment.
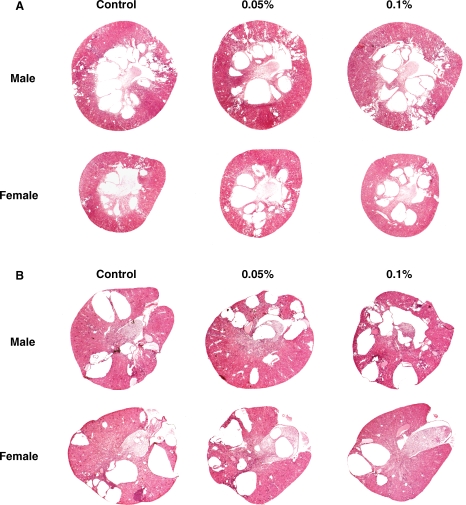
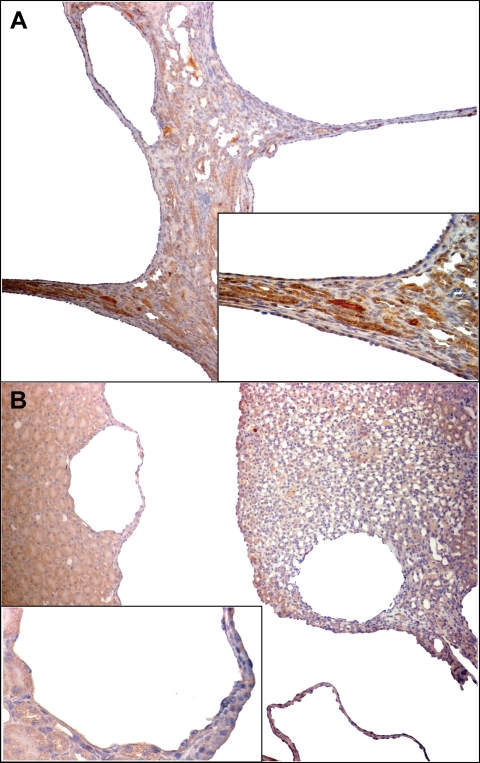
Despite the expected pharmacologic effects (hypocalcemia and hyperphosphatemia, increased urine output and osmolar clearance), R-568 had no significant effect on the development of polycystic kidney disease in PCK rats and Pkd2−/WS25 mice as reflected by kidney weights, cyst volumes and plasma BUN concentration (Tables 1 and 2 Figure 5). The majority of cysts in both animal models did not stain with the CaR antibodies (Figure 6). A significant reduction in interstitial fibrosis was detected in PCK rats that appeared to be dose dependent (Table 1); in Pkd2−/WS25 mice, however, a significant effect on fibrosis was not observed. Liver weights, cyst and fibrosis volumes were also similar in control and treated PCK rats and Pkd2−/WS25 mice (results not shown).
Fig. 5.
Representative kidney sections from male and female PCK rats (panel A) and Pkd2−/WS25 mice (panel B) on no treatment or treated with 0.05% or 0.1% R-568 added to their diet.
Fig. 6.
Immunohistochemical staining of kidney sections from PCK rats (panel A) and Pkd2−/WS25 mice (panel B) with a CaR antibody. Most cells lining the cysts show no staining. ×100, insets ×400.
Discussion
The administration of R-568 did not have a significant effect on cyst growth in two different models of PKD although it had pharmacologic effects on renal function and mineral metabolism [27]. The hypercalciuric effect of R-568 (Tables 1 and 2) is due to the activation of the CaR in the thick ascending limb of Henle and distal convoluted tubule. In the thick ascending limb of Henle, activation of the CaR inhibits calcium reabsorption by two mechanisms. The first is by eliciting phospholipase A2 (PLA2)-mediated arachidonic acid release and production of 20-hydroxyeicosatetraenoic acid that inhibits the apical potassium channel Kir1.1 (ROMK) [28,29]. ROMK is necessary for the recycling of potassium, sustained reabsorption of sodium chloride via the Na–K–2Cl cotransporter and passive paracellular reabsorption of calcium in the thick ascending limb of Henle. The second mechanism is by inhibiting the vasopressin-dependent stimulation of adenylyl cyclase and downregulation of the Na–K–2Cl cotransporter expression [30]. It should be noted that a marked hypercalciuric effect of R-568 was observed only in the PCK rat (see below). The hypophosphaturic effect in the PCK rat (Table 1) is consistent with the inhibition of parathyroid hormone secretion and with the calcimimetic-induced blunting of the phosphaturic action of parathyroid hormone in the proximal tubule. Most likely, the hyperphosphatemic and hypocalcemic effects of R-568 are directly due to the inhibition of parathyroid hormone secretion and to a large extent independent of the observed hypercalciuric and hypophosphaturic effects (Tables 1 and 2) since they can also be observed in totally nephrectomized animals [31].
Polyuria, as it occurred in this study, is also a known calcimimetic effect. It has been attributed to the disruption of urinary concentration by its action on the basolateral CaR in the thick ascending limb of Henle (inhibition of sodium chloride reabsorption and disruption of countercurrent multiplication mechanism) and on the apical CaR in the inner medullary collecting duct (inhibition of the vasopressin-induced effect on water permeability) [32,33]. Indeed, the administration of R-568 resulted in a significant reduction in the aquaporin-2 protein (Figures 1 and 2). As previously described in chronic hypercalcemic/hypercalciuric rats [32,33], the reduction in the aquaporin-2 protein was likely due to increased degradation rather than reduced synthesis since it occurred without a reduction in aquaporin-2 mRNA (Figures 3, 4). Moreover, renal cAMP levels were not affected by R-568. Female PCK rats treated with the lower R-568 dose had a higher expression of the UT-A1 protein than control rats or those treated with the higher dose, and male rats treated with the higher dose had an increased expression of V2R mRNA. These observations may point to dose-dependent effects that we have not explored fully. The calcimimetic-induced polyuria in our study was accompanied by a significant increase in osmolar clearance and urinary excretion of sodium, potassium and calcium. Therefore reduced expression or apical targeting of aquaporin-2 cannot entirely explain the increase in urine volume.
Despite the observation of the expected pharmacologic effects on renal function and mineral metabolism, R-568 had no significant effect on kidney cyst volume in PCK rats and Pkd2−/WS25 mice (Tables 1 and 2). There are at least three possible explanations for this lack of effect. First, CaR is not significantly expressed in the outer medullary and cortical collecting ducts [26], the main site of cyst development in the PCK rat and Pkd2−/WS25 mice [34]; this is consistent with the observation that most cysts did not stain with CaR antibodies. A recent study showing that CaR is expressed in mouse cortical collecting duct cell line (mpkCCDcl4) [35] makes this explanation less likely. Second, the administration of R-568 resulted in hypocalcemia and therefore it is possible that the effects of R-568 on intracellular calcium and cAMP are negated by the reduction in extracellular calcium. Lowering extracellular calcium has been shown to induce a phenotypic switch from wild type to polycystic [19]. It has also been shown to increase cAMP in rat collecting duct cells [36] and in vascular smooth muscle cells freshly dissected from PKD2WS25/− mice [37]. Third, the inhibition of the vasopressin elicited water permeability induced by the effect of high urinary calcium on the apical CaR in the inner medullary collecting duct may occur despite a fully functional adenylyl cyclase, as suggested by the fact that in models of chronic hypercalcemia vasopressin elicits increases in urea permeability [33] regulated through increases in intracellular cAMP [38]. Consistent with the last two explanations, the renal cAMP levels (Tables 1 and 2) and the expressions of the urea transporter UT-A1 protein and of vasopressin V2 receptor mRNA (Figures 1–4) in the treated PCK rats and Pkd2−/WS25 mice were similar to or higher than those in the control animals. The administration of R-568 had no significant effect on the development of polycystic liver disease in either animal model. This is consistent with the lack of expression of CaR in the biliary epithelium [39].
The extent of renal interstitial fibrosis in PCK rats and Pkd2−/WS25 mice at the ages evaluated in our study was modest. The administration of R-568 significantly reduced the extent of the renal fibrosis in the PCK rat, but it had no effect on renal fibrosis in Pkd2−/WS25 mice or on liver fibrosis in either model. This could reflect that in the rat the renal effects of R-568 are more robust as reflected by its greater effect on urinary calcium excretion. In addition, there may be a species difference since renal fibrosis may be easier to induce in rats than in mice. While the mechanism and significance of the favorable effect on interstitial fibrosis in PCK rats is uncertain, it must be noted that R-568 has been reported to ameliorate the tubulo-interstitial damage in subtotally nephrectomized rats, possibly by reducing the levels of parathyroid hormone [36]. This antifibrotic effect of calcimimetic therapy may have a therapeutic action that may deserve further study at more advanced stages of polycystic kidney disease.
In summary, despite a sound rationale for considering calcimimetics in the treatment of polycystic kidney disease, their administration failed to ameliorate the cystic growth in animal models orthologous to human ARPKD and ADPKD.
Acknowledgments
The authors thank Dr Jeff M. Sands for the gift of urea transporter UT-A1 antibody. The study was supported by NIDDK DK44863 and PKD Foundation. R-568 was kindly provided by Amgen, Inc., Thousand Oaks, CA.
Conflict of interest statement. None declared.
References
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Gunay-Aygun M, Avner ED, Bacallao RL, et al. Autosomal recessive polycystic kidney disease and congenital hepatic fibrosis: summary statement of a First National Institutes of Health/Office of Rare Diseases conference. J Pediatr. 2006;149:159–164. doi: 10.1016/j.jpeds.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2:40–54. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- 4.Pazour GJ, San Agustin JT, Follit JA, et al. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 5.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 6.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 7.Ward CJ, Yuan D, Masyuk TV, et al. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 8.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2002;232:1042–1044. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Zhang J, Nauli SM, et al. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koulen P, Cai Y, Geng L, et al. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Wright JM, Qian F, et al. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. [DOI] [PubMed] [Google Scholar]
- 12.Chabardes D, Imbert-Teboul M, Elalouf JM. Functional properties of Ca2+-inhibitable type 5 and type 6 adenylyl cyclases and role of Ca2+ increase in the inhibition of intracellular cAMP content. Cell Signal. 1999;11:651–663. doi: 10.1016/s0898-6568(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Nagao S, Kasahara M, et al. Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis. 1997;30:703–709. doi: 10.1016/s0272-6386(97)90496-0. [DOI] [PubMed] [Google Scholar]
- 14.Gattone VH, Wang X, Harris PC, et al. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nature Med. 2003;9:1323–1326. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 15.Torres VE, Wang X, Qian Q, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nature Med. 2004;10:363–364. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DP, Rome LA, Sullivan LP, et al. cAMP-dependent fluid secretion in rat inner medullary collecting ducts. Am J Physiol Renal Physiol. 2001;280:F1019–F1029. doi: 10.1152/ajprenal.2001.280.6.F1019. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi T, Nagao S, Wallace DP, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanaoka K, Guggino W. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J Am Soc Nephrol. 2000;11:1179–1187. doi: 10.1681/ASN.V1171179. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Wallace DP, Magenheimer BS, et al. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004;279:40419–40430. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Hempson SJ, Reif GA, et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Gattone VH, II, Harris PC, et al. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–851. doi: 10.1681/ASN.2004121090. [DOI] [PubMed] [Google Scholar]
- 22.Masyuk TV, Masyuk AI, Torres VE, et al. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Nagano N. Pharmacological and clinical properties of calcimimetics: calcium receptor activators that afford an innovative approach to controlling hyperparathyroidism. Pharmacol Ther. 2006;109:339–365. doi: 10.1016/j.pharmthera.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–133. doi: 10.1038/ncpendmet0388. [DOI] [PubMed] [Google Scholar]
- 25.Gerbino A, Ruder WC, Curci S, et al. Termination of cAMP signals by Ca2+ and Gαi via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations. J Cell Biol. 2005;171:303–312. doi: 10.1083/jcb.200507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riccardi D, Hall AE, Chattopadhyay N, et al. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol. 1998;274:F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 27.Ward DT, Riccardi D. Renal physiology of the extracellular calcium-sensing receptor. Pflugers Arch. 2002;445:169–176. doi: 10.1007/s00424-002-0914-x. [DOI] [PubMed] [Google Scholar]
- 28.Wang WH, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extracellular Ca(2+)-induced inhibition of apical K+ channels in the TAL. Am J Physiol. 1996;271:C103–C111. doi: 10.1152/ajpcell.1996.271.1.C103. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Lu M, Balazy M, et al. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am J Physiol. 1997;273:F421–F429. doi: 10.1152/ajprenal.1997.273.3.F421. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Li C, Kwon TH, et al. AQP3, p-AQP2, and AQP2 expression is reduced in polyuric rats with hypercalcemia: prevention by cAMP-PDE inhibitors. Am J Physiol Renal Physiol. 2002;283:F1313–F1325. doi: 10.1152/ajprenal.00040.2002. [DOI] [PubMed] [Google Scholar]
- 31.Fox J, Lowe SH, Petty BA, et al. NPS R-568: a type II calcimimetic compound that acts on parathyroid cell calcium receptor of rats to reduce plasma levels of parathyroid hormone and calcium. J Pharmacol Exp Ther. 1999;290:473–479. [PubMed] [Google Scholar]
- 32.Sands JM, Naruse M, Baum M, et al. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest. 1997;99:1399–1405. doi: 10.1172/JCI119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sands JM, Flores FX, Kato A, et al. Vasopressin-elicited water and urea permeabilities are altered in IMCD in hypercalcemic rats. Am J Physiol. 1998;274:F978–F985. doi: 10.1152/ajprenal.1998.274.5.F978. [DOI] [PubMed] [Google Scholar]
- 34.Lager DJ, Qian Q, Bengal RJ, et al. The PCK rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59:126–136. doi: 10.1046/j.1523-1755.2001.00473.x. [DOI] [PubMed] [Google Scholar]
- 35.Bustamante M, Hasler U, Leroy V, et al. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J Am Soc Nephrol. 2008;19:109–116. doi: 10.1681/ASN.2007010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaki M, McIntyre S, Rassier ME, et al. Cyclic 3′,5′-nucleotide diesterases in dynamics of cAMP and cGMP in rat collecting duct cells. Am J Physiol. 1992;262:F957–F964. doi: 10.1152/ajprenal.1992.262.6.F957. [DOI] [PubMed] [Google Scholar]
- 37.Kip SN, Hunter LW, Ren Q, et al. [Ca2+]i reduction increases cellular proliferation and apoptosis in vascular smooth muscle cells: relevance to the ADPKD phenotype. Circ Res. 2005;96:873–880. doi: 10.1161/01.RES.0000163278.68142.8a. [DOI] [PubMed] [Google Scholar]
- 38.Klein JD, Frohlich O, Blount MA, et al. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J Am Soc Nephrol. 2006;17:2680–2686. doi: 10.1681/ASN.2006030246. [DOI] [PubMed] [Google Scholar]
- 39.Canaff L, Petit JL, Kisiel M, et al. Extracellular calcium-sensing receptor is expressed in rat hepatocytes. Coupling to intracellular calcium mobilization and stimulation of bile flow. J Biol Chem. 2001;276:4070–4079. doi: 10.1074/jbc.M009317200. [DOI] [PubMed] [Google Scholar]