Abstract
Background. Ischaemic kidney injury continues to play a dominant role in the pathogenesis of acute renal failure (ARF) in many surgical and medical settings. A major event in the induction of renal injury is related to the generation of oxygen-free radicals. Hyperbaric oxygen therapy (HBO) is indicated for treatment of many ischaemic events but not for ARF. Therefore, the present study examined the effects of HBO on kidney function and renal haemodynamics in rats with ischaemic ARF.
Methods. Renal ischaemia was induced by unilateral renal artery clamping (45 min) in rats. Within 24 h following ischaemia, rats were treated twice with HBO of 100% O2 at 2.5 absolute atmospheres for 90 min each (+HBO). Untreated rats (−HBO) served as a control. Forty-eight hours later, GFR, RBF and endothelial-dependent vasorelaxation were measured. In addition, the immunoreactive staining of 4-hydroxy-2-noneal (4-HNE), a major product of endogenous lipid peroxidation, and superoxide dismutase (SOD) were assessed.
Results. In the −HBO group, GFR was reduced by 94% compared with the untouched normal kidney (ischaemic: 0.06 ± 0.03 ml/min, normal: 1.02 ± 0.13 ml). In contrast, in the +HBO group, GFR of the ischaemic kidney (0.36 ± 0.07 ml/min) was reduced only by 68% compared with the contralateral normal kidney (1.12 ± 0.12 ml/min). In line with these findings, HBO improved the vasodilatory response to ACh as expressed in enhancement of both total and regional renal blood flow. In addition, HBO reduced the formation of 4-HNE by 33% and 76% and increased SOD by 30% and 70% in the cortex and outer stripe region of the medulla of the ischaemic kidney, respectively.
Conclusion. HBO attenuates the decline in GFR following renal ischaemia, and improves endothelial-dependent vasorelaxation, suggesting that treatment with HBO may be beneficial in the setting of ischaemic ARF.
Keywords: acute kidney injury, hyperbaric oxygen, ischaemia-reperfusion injury, rat
Introduction
Acute renal failure (ARF) continues to be a frequent threatening complication following trauma and complex surgical procedures, and in patients hospitalized in intensive care units [1,2]. Despite the increasing awareness of this syndrome, the advances in clinical care and extensive research of its pathophysiology, the mortality from ARF remains high and has not changed significantly during the last several decades [2].
Ischaemic kidney injury continues to play a dominant role in the pathogenesis of ARF in many surgical and medical settings. Recent investigations into the mechanisms of renal damage following ischaemia in animal models suggest an early contribution of endothelial dysfunction and a local inflammatory response as major components of the evolving ischaemic injury to the kidney [3–5]. As a consequence of these events, the recovery of blood supply to the ischaemic kidney may be significantly delayed and reduced leading to further impairment of tissue oxygenation.
A major event in the induction of tissue injury is related to the generation of oxygen-free radicals and the associated oxidative and nitrosative stress [4–6]. In particular, it has been demonstrated that the highly reactive hydroxyl radical and the nitric oxide (NO) metabolic product, peroxynitrite (ONOO−), are generated during the renal reperfusion phase and have diverse cytotoxic properties. These include damage to DNA, protein oxidation and nitrosylation, lipid peroxidation and induction of apoptosis [3,6,7].
Hyperbaric oxygen therapy (HBO) is defined as a treatment in which a patient is intermittently exposed to 100% oxygen while the treatment chamber is pressurized to a pressure above sea level (>1 ATA, 760 mmHg) [8]. HBO therapy has been used in a number of medical conditions with a proven efficacy in a limited number of disorders [8–10]. In ischaemic rat tissues, HBO was shown to inhibit neutrophil adherence to the wall of ischaemic vessels and to decrease post-ischaemic vasoconstriction in skin grafts [11]. Furthermore, we showed in our laboratory that HBO has striking beneficial anti-inflammatory effects on experimental colitis in rats [12]. In several experimental conditions, HBO has been claimed to exert its beneficial effects in part by increasing the activity of Cu/Zn superoxide dismutase (Cu/Zn-SOD) and other antioxidant cellular defence mechanisms, thereby altering the balance between generation and removal of oxygen-free radicals [13–15].
Several investigators reported that HBO treatment may modulate kidney function in rats with sepsis [16], rats with adriamycin-induced nephrotic syndrome [17] and experimental cyclosporine nephrotoxicity [18]. Recently, Solmazgul et al. [19] demonstrated that HBO attenuated the elevation in plasma creatinine and histological damage in Sprague-Dawley rats subjected to renal ischaemia/reperfusion injury. Moreover, HBO treatment has been reported to be beneficial in the management of the muscle compartment syndrome, a devastating complication of crush syndrome, where kidney function is often impaired [20] (reviewed in Reis ND and Better OS [21]).
Here we report that HBO treatment following renal ischaemia/reperfusion attenuated the deterioration in glomerular filtration rate (GFR) in the ischaemic kidney, in parallel with an improvement in the antioxidant/oxidant balance.
Materials and methods
Studies were conducted on male Sprague-Dawley rats (Harlan Laboratories, Jerusalem, Israel) weighing 280– 320 g. Animals were maintained on regular rodent chow (0.45% NaCl) and water ad libitum prior to experiments. The experiments were conducted according to the guidelines of the institutional Animal Use and Care Committee, Technion, Israel.
In vivo protocols
Induction of ischaemic ARF.
Forty-eight hours prior to clearance and renal haemodynamic studies, rats were lightly anaesthetized by intraperitoneal injection of Nembutal (pentobarbitone sodium, 30 mg/kg BW) and placed on a heated surgical table to maintain body temperature. The left kidney was then exposed through a flank incision, and renal ischaemia was induced by complete occlusion of the left renal artery for 45 min by a surgical clamp. The contralateral, right kidney was left untouched and served as an internal control. Following ischaemia, the clamp was released, the incision was sutured and the rat was allowed to recover.
Hyperbaric oxygen treatment.
Rats with left kidney ischaemia were randomly divided into two experimental groups. The first group (+HBO, n = 9) was placed in a hyperbaric chamber for two HBO treatments of 90 min each, 2 h and 22 h after renal ischaemia. This protocol was chosen based on previous experiments from our laboratory in rats with crush syndrome where HBO exerted beneficial effects on muscle function (unpublished observations). The chamber was pressurized with 100% oxygen to 2.5 absolute atmospheres (ATA). The second group (−HBO, n = 6) was left at room atmosphere for 48 h, and served as a control for group 1.
Clearance studies.
On the second day, ∼48 h after renal ischaemia, animals from both groups were anaesthetized by injection of Inactin (100 mg/kg BW, i.p.), and prepared for clearance experiments as previously described from this laboratory [22,23]. In short, after tracheotomy, the rat was placed on a heated surgical table and the left carotid artery and right jugular vein were cannulated by polyethylene tubing (Portex, Hythe, Kent, UK) for continuous monitoring of mean arterial pressure (MAP) and infusion of various solutions, respectively. A solution of 2% inulin in 0.9% saline was infused at a rate of 1.0–1.5% BW/h, throughout the experiment. The abdominal cavity was then exposed through a mid-abdominal incision, and the left ureter was identified, separated from the surrounding fat and cannulated by a PE-10 tube for urine collection. The urinary bladder was exposed, and a funnel-shaped PE-50 catheter was inserted for urine collection from the contralateral right kidney. Urine was collected separately from each kidney into pre-weighed tubes containing mineral oil, and the urine volume was measured gravimetrically. Following surgery, a 60-min equilibration period was allowed before the first clearance period was obtained. Four clearance periods of 20–30 min each were obtained, and blood samples (0.3 ml) were withdrawn every second clearance period. Blood samples were separated by centrifugation, and plasma and urine aliquots were kept at −20°C for determination of inulin concentration. For each experimental group, GFR was determined for the left ischaemic kidney, the right control kidney and the ratio of GFR in ischaemic/control kidneys were calculated.
Renal haemodynamic measurements
Total renal blood flow: to evaluate the effects of HBO on renal haemodynamic parameters, studies were conducted in rats subjected to unilateral ischaemia 48 h earlier, treated (+HBO, n = 9) or untreated (−HBO, n = 6) with hyperbaric oxygen, as described in the previous protocol. An additional group of sham-operated control rats (n = 6) was used to test the effects of the operation itself on relevant haemodynamic parameters. In these rats, a flank incision was performed, but the left renal artery was not clamped. The rats were anaesthetized by Inactin as described in the previous protocol. The left renal artery was exposed through a mid-abdominal incision. Measurements of total renal blood flow (RBF) were performed by an ultrasonic flowmeter (model T206, Transonic Corp., Ithaca, NY, USA), using a flow-probe (1RB) placed around the left renal artery, as previously described [23,24]. MAP was continuously monitored by a pressure transducer, and RVR was calculated on-line according to the formula RVR = MAP/RBF using data acquisition software (Labtech Notebook®). After surgery and equilibration, baseline measurements were obtained for 20 min. The endothelial-dependent renal vasodilatation in response to incremental doses of acetylcholine (ACh) infusion (1, 10 and 100 μg/kg/min, for 20 min each dose, followed by a recovery period) was determined as previously described [22].
Intrarenal blood flow: to evaluate the effects of HBO on intrarenal haemodynamic parameters, studies were conducted in rats subjected to unilateral ischaemia 48 h earlier, treated (+HBO, n = 9) or untreated (−HBO, n = 6) with hyperbaric oxygen. For this purpose, the left kidney was exposed through a mid-abdominal incision. Cortical and medullary blood flow (CBF and MBF, respectively) were measured simultaneously by a dual channel laser-Doppler flowmeter (Master Perimed AB, model 4001) using two needle probes as previously described [23]. ACh was administered at a dose of 10 μg/kg/min, and CBF was measured at 5-min intervals for 30 min after administration of the drug. The location of the laser needle probes was verified at the end of each experiment.
In vitro protocols
To test the hypothesis that HBO exerted its beneficial effects on renal function in part by improving the antioxidant/oxidant balance, we evaluated the immunohistochemical localization of the activities of the antioxidant enzyme Cu/Zn-SOD and that of 4-hydroxy-2-noneal (4-HNE), a marker of lipid peroxidation.
The unclamped, normal kidney and the ischaemic kidney were removed 48 h following ischaemia/reperfusion in the −HBO and +HBO groups under pentobarbitone sodium anaesthesia. Kidneys were fixed in 10% neutral buffered formalin, dehydrated in ascending ethanols and embedded in paraffin wax. Five-micrometre thick microtome sections were mounted on SuperFrost® Plus slides (Menzel-Glaser, Braunschweig, Germany).
Immunostaining was performed with an antibody against 4-HNE (1:600, Alpha Diagnostic Inc., San Antonio, TX, USA) and anti-superoxide dismutase–CuZn enzyme (1:600, Oxis International Inc., Portland, OR, USA), using a histostain-SP kit (Zymed Laboratories, San Francisco, CA, USA).
The immunoreactive stained areas of the end-products were observed using an Olympus CH-40 brightfield microscope (Olympus, Tokyo, Japan) with an attached CCD camera. The areas observed and analysed in the sections were taken from the medulla and from throughout the cortex apart from that immediately under the capsule. Quantitative histomorphometric analysis was determined in randomly standardized fields of view by means of commercial image analysis software (analySIS® 3.0, Soft Imaging System, Münster, Germany).
Chemical analysis
Inulin concentration in plasma and urine samples was determined by the anthrone method [25]. GFR was equated with the clearance of inulin. Sodium concentrations were determined by flame photometry (model IL 943, Instrumentation Laboratories, Milano, Italy).
Statistical analysis
The paired student t-test (for comparison between the ischaemic and untouched, normal, kidney in the same animal) and either the unpaired t-test or analysis of variance (ANOVA), as appropriate, were used for statistical analysis of the data. A P-value of 0.05 or less was considered statistically significant. Data are expressed as means ± standard error of mean (S.E.M).
Results
Clearance protocols
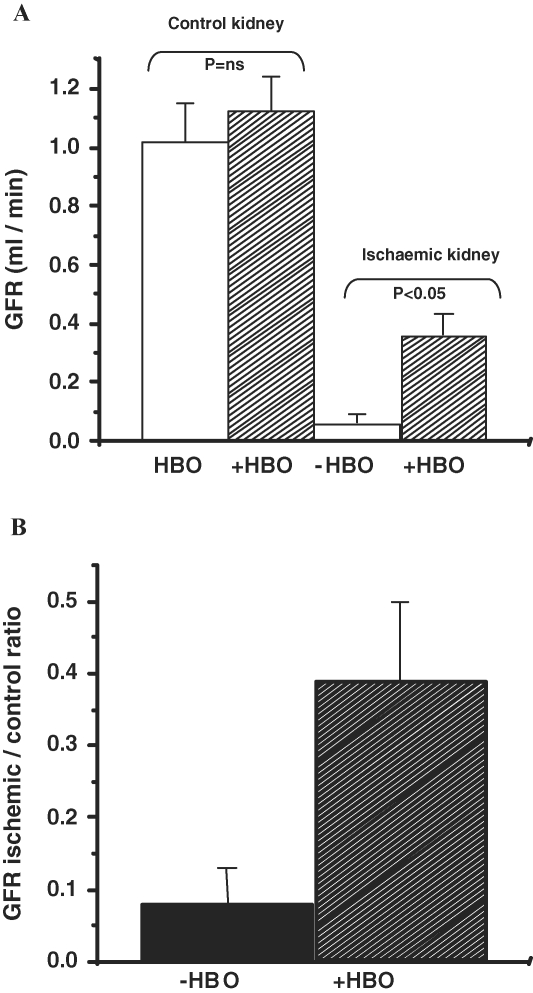
Figure 1 summarizes the results of the clearance experiments in the −HBO and +HBO groups. In the −HBO group, 48 h after renal ischaemia, GFR was reduced by 94% compared with the untouched, normal kidney (ischaemic: 0.06 ± 0.03 ml/min, normal: 1.02 ± 0.13 ml/min). In contrast, in the +HBO group, GFR in the ischaemic kidney (0.36 ± 0.07 ml/min) was reduced only by 68% compared with the contralateral normal kidney (1.12 ± 0.12 ml/min). The difference in GFR of the ischaemic kidneys in the groups treated or untreated with HBO was statistically significant (P < 0.05, Figure 1). Also, when expressed as GFR of ischaemic/normal kidney, this ratio was significantly higher in the +HBO group compared with the −HBO group (0.39 ± 0.1 versus 0.08 ± 0.05, respectively, P < 0.05, Figure 1B). In spite of the improvement in GFR following HBO treatment, there was no detectable change in either fractional sodium excretion (−HBO: 0.34 ± 0.09, +HBO: 0.33 ± 0.07, P = N.S.) or in the urine flow rate (−HBO: 3.86 ± 0.86 μl/min, +HBO: 2.25 ± 0.31 μl/min, P = N.S.) in the ischaemic kidney.
Fig. 1.
(A) Effect of HBO treatment on glomerular filtration rate (GFR) of control and ischaemic kidneys. (B) GFR ratio of ischaemic/normal kidney in the +HBO group and the −HBO group. n = 6–9 in each group. (A) shows that in the −HBO group, GFR was reduced by 94% compared with the untouched normal kidney. In contrast, in the +HBO group GFR of the ischaemic kidney was reduced only by 68% compared with the contralateral normal kidney. (B) demonstrates that the ratio of GFR of ischaemic/normal kidney is significantly higher in the +HBO group compared with the −HBO group.
Renal and intrarenal haemodynamics measurements
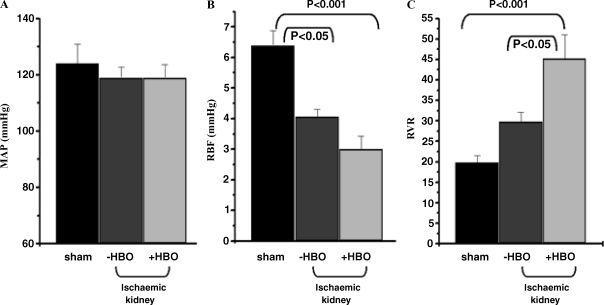
Baseline values of MAP, RBF and calculated RVR in the sham-operated control group and in the ischaemic kidney of −HBO and +HBO groups are summarized in Figure 2. Baseline MAP did not differ significantly in the three experimental groups. Baseline RBF decreased by 26% in +HBO rats versus −HBO rats. However, both were significantly lower compared with normal control kidney. In line with these measurements, calculated baseline RVR was higher (by 52%) in the ischaemic kidney of the +HBO group compared with the −HBO group.
Fig. 2.
Baseline values of (A) mean arterial pressure (MAP), (B) renal blood flow (RBF) and renal vascular resistance (RVR), in the sham-operated control group and in the ischaemic kidney of −HBO and +HBO treated groups, 48 h after the induction of ischaemia. n = 6–9 in each group. Baseline values of MAP, RBF and calculated RVR in the sham-operated control group and in the ischaemic kidney of −HBO and +HBO groups are summarized in Figure 3. It is clear that baseline RBF decreased by 26% in +HBO rats versus −HBO rats. However, both were significantly lower compared with normal control kidney. Since MAP does not differ significantly in the three experimental groups, the calculated baseline RVR is higher (by 52%) in the ischaemic kidney of the +HBO group compared with the −HBO group.
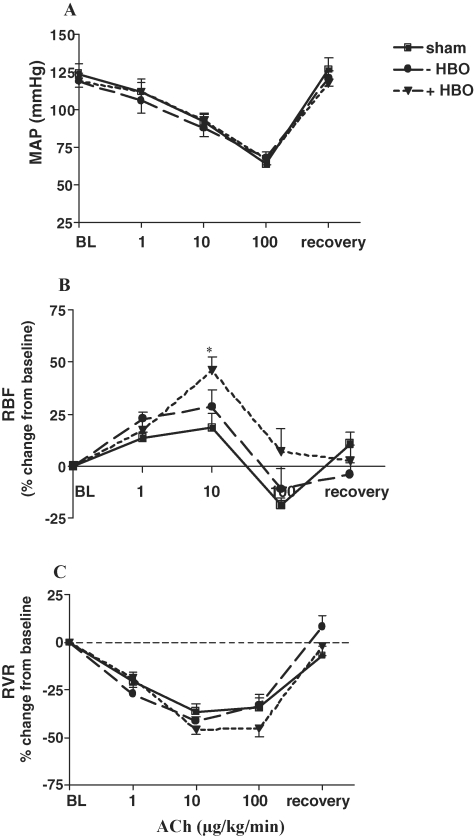
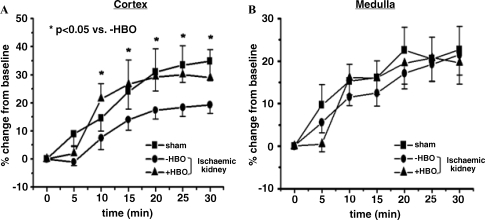
The results of the endothelial-dependent renal vasodilatation in response to ACh administration are depicted in Figure 3. As shown, infusion of incremental doses of ACh produced a similar decrease in MAP in the three experimental groups (sham, ischaemic −HBO and ischaemic +HBO), followed by a similar recovery response upon cessation of ACh infusion. In spite of the decrease in MAP, there was a dose-related rise in RBF in response to ACh in all groups, apparently as a result of an endothelial-dependent renal vasodilatation. Of interest, treatment with HBO (ischaemic kidney of +HBO) significantly improved the renal vasodilatory response to ACh as compared to ischaemic kidney of −HBO. Indeed, as shown in the lower panel of Figure 3, there was a dose-dependent decrease in RVR in all three experimental groups, especially in ischaemic kidney of +HBO rats. This notion is further supported by the measurements of intrarenal blood flow in response to the ACh as depicted in Figure 4. As shown, administration of ACh to control rats caused a gradual increase in CBF, which reached maximal magnitude of 35% ± 8% above baseline value at 30 min after the drug injection (Figure 4A), whereas MBF did not change significantly (Figure 4B). In contrast, in the ischaemic kidney of the −HBO group, ACh elicited an attenuated increase in CBF (18 ± 3% peak increase at 30 min after injection of the drug). The cortical vasodilatory response to ACh significantly improved following HBO treatment (CBF reached a peak increase of 28 ± 11% at 30 min after injection of the drug). These findings may explain the observed stimulatory effects of HBO on total RBF, and suggest that treatment with HBO may improve endothelial-dependent vasorelaxation mediated by eNOS. It should be emphasized that basal CBF of normal kidney, ischaemic kidney and ischaemic kidney following HBO treatment was not significantly different: 309.9 ± 11.95; 323.9 ± 25.4 and 330.6 ± 26.0 perfusion units, respectively. Likewise, baseline MBF values of these experimental groups were comparable: 82.05 ± 2.17; 94.08 ± 5.68 and 95.30 ± 4.41 perfusion units, respectively.
Fig. 3.
Effects of acetylcholine (ACh) administration (1, 10 and 100 μg/kg/min) on (A) mean arterial pressure (MAP), (B) renal blood flow (RBF) and (C) renal vascular resistance (RVR), in the sham-operated control group and in the ischaemic kidney of −HBO and +HBO groups 48 h after the induction of ischaemia. n = 6–9 in each group. *P < 0.05 versus sham. As shown, infusion of incremental doses of ACh produced a similar decrease in MAP in association with rise in RBF in response to ACh in all the groups, apparently as a result of an endothelial-dependent renal vasodilatation.
Fig. 4.
Effects of acetylcholine (ACh) administration (10 μg/kg/min) on (A) cortical blood flow (CBF) and (B) medullary blood flow (MBF) in the sham-operated control group and in the ischaemic kidney of −HBO and +HBO groups 48 h after the induction of ischaemia. n = 6–9 in each group. It is evident that administration of ACh to control rats caused a gradual increase in CBF, whereas MBF did not change significantly. In contrast, in the ischaemic kidney of the −HBO group, ACh elicited an attenuated increase in CBF. The cortical vasodilatory response to ACh significantly improved following HBO treatment. These findings may explain the observed stimulatory effects of HBO on total RBF, and suggest that treatment with HBO may improve endothelial-dependent vasorelaxation mediated by eNOS. P < 0.05 vs. −HBO.
Immunohistological staining
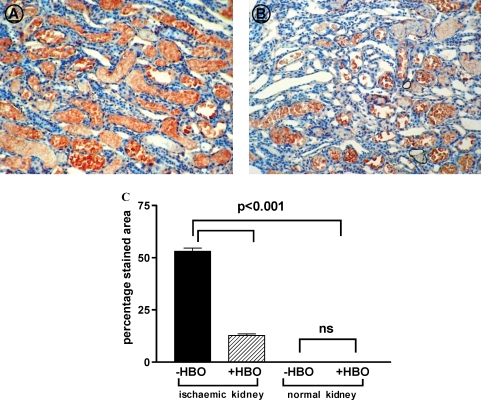
Figure 5 summarizes the results of immunostaining for 4-HNE, a marker for lipid peroxidation. Immunostaining of 4-HNE was localized primarily in the renal tubular cells of the outer stripe region of the medulla of the ischaemic kidneys, and was almost undetectable in the cortex of normal or ischaemic kidneys of both +HBO and −HBO groups. The vascular elements and the glomeruli were unstained in all cases, treated and controls. In contrast to the findings in the cortex, the area in the outer stripe of the medulla stained for 4-HNE was significantly lower in the ischaemic kidneys of rats treated with hyperbaric oxygen than in untreated rats (+HBO: 12.7 ± 0.84%, −HBO: 53.1 ± 1.55, P < 0.001, Figure 5).
Fig. 5.
Representative pictures of 4-HNE staining in the medulla of −HBO (A) and +HBO (B) ischaemic kidneys (objective ×20). (C) Percentage staining area of 4HNE in the renal outer strip of the outer of −HBO and +HBO of control and ischaemic kidneys. n = 6–9 in each group. This figure indicates that 4-HNE is localized primarily in the renal tubular cells of the outer stripe of the medulla of the ischaemic kidneys, and is almost undetectable in the cortex. The area of the outer stripe of the medulla stained for 4-HNE is significantly less than in the ischaemic kidneys of rats treated with hyperbaric oxygen than in untreated rats.
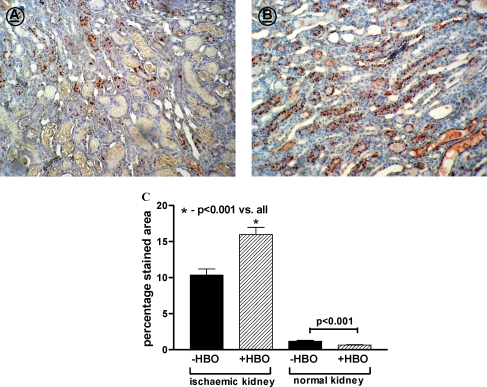
Figure 6 summarizes the immunohistological data on Cu/Zn-SOD in the cortex and outer stripe region of the medulla of control and ischaemic kidneys treated with or without HBO. In the control, untouched kidneys, immunostaining for Cu/Zn-SOD in the cortex and the medulla was negligible. There was a marked increase in Cu/Zn-SOD immunoreactive staining in the outer stripe of the medulla of the ischaemic kidneys in both −HBO and +HBO groups, compared with the control non-ischaemic kidneys. However, in the +HBO group, the percentage of staining for Cu/Zn-SOD was significantly higher than that in the −HBO group (+HBO: 17.3 ± 1.11%, −HBO: 10.2 ± 0.83%, P < 0.001, Figure 6).
Fig. 6.
Representative pictures of CuZn-SOD staining in the medulla of −HBO (A) and +HBO (B) ischaemic kidneys (objective × 20). (C) Percentage staining area of CuZn-SOD in the renal medulla of −HBO and +HBO control and ischaemic kidney. n = 6–9 in each group. It is evident that immunostaining for Cu/Zn-SOD in the cortex and the outer stripe of the medulla of untouched kidneys is negligible. A marked increase in Cu/Zn-SOD immunoreactive staining in the outer stripe of the medulla of the ischaemic kidneys in both −HBO and +HBO groups, compared with the control non-ischaemic kidneys, is notable. However, in the +HBO group, the percentage of staining for Cu/Zn-SOD is significantly higher than that in the −HBO group. Thus, HBO treatment induces an increase in the immunohistochemical staining of the antioxidant Cu/Zn-SOD in the outer stripe of the medulla of the ischaemic kidneys.
Thus, HBO treatment was associated with an increase in the immunohistochemical staining of the antioxidant Cu/Zn-SOD and a parallel decrease in the immunohistochemical staining for the lipid peroxidation marker, 4-HNE, in the outer stripe of the medulla of the ischaemic kidneys.
Discussion
The findings of the present study provide novel information on the mechanisms underlying ARF and support the use of HBO for treatment of ischaemic renal injury. Our data clearly demonstrate that treatment with HBO substantially attenuated the reduction in GFR characterizing ARF. The HBO-induced renal vasodilatation was associated with a significant increase in total RBF in response to ACh, due to enhanced perfusion of the renal cortex in the ischaemic kidney. Despite the improvement in renal haemodynamics, HBO did not cause a concomitant increase in urine flow or sodium excretion. Moreover, our findings demonstrate that the improvement in renal function following HBO occurs in parallel with a similar increase in the antioxidant/oxidant ratio in the ischaemic kidney. This suggests that the latter mechanism may partially contribute to the improvement in renal function in kidneys recovering from ischaemic injury, and support the use of HBO for treatment of ARF.
Previously, Solmazgul et al. [19] demonstrated that HBO treatment produced marked protection against ischaemia of 30 min followed by 24-h reperfusion after the right nephrectomy. These authors applied HBO therapy for 60 min, starting at the initial 15th min of reperfusion. Animals treated with HBO showed regression of the elevated plasma creatinine and blood urea nitrogen levels to normal concentrations in association with reversal of tubular damage and neutrophil infiltration. However, the mechanisms underlying the beneficial effects of HBO therapy were not studied in this study. Our data clearly show that HBO treatment partially corrected the decline in GFR complicating ARF and provide new insights into the mechanisms underlying the therapeutic actions of HBO in the I/R model of ARF. For instance, the beneficial effects of HBO on GFR in the +HBO group could be attributed to the observed concurrent improvement in endothelial function of the injured kidney following HBO. In that respect, rats with ARF that were treated with HBO displayed a more pronounced endothelial-dependent vasorelaxation in response to ACh compared with control animals, and the −HBO group. This improvement was accompanied by a concomitant decrease in the 4-HNE level in renal tissue. 4-HNE, a major product of endogenous lipid peroxidation, is considered as a reliable indicator of oxidative damage [26,27]. It is well established that ROS directly cause vasoconstriction in kidney microcirculation [28]. Furthermore, they indirectly increase vascular tone by affecting the action or production of vasodilatory substances of endothelial origin such as NO [29]. Therefore, the observed increased oxidative stress in the ischaemic kidney probably composes additional mechanism that could be responsible for the renal dysfunction occurring during ARF. The latter is compatible with our findings that the beneficial effect of HBO treatment on GFR was accompanied by enhancement of SOD abundance and decline in 4-HNE in the renal tissue. Similar results were obtained in sepsis-induced ARF, where renal dysfunction was associated with the reduction of renal SOD and catalase (CAT) activities and the increase in malondialdehyde (MDA) levels [16,30]. Interestingly, GFR recovered to normal values along with the reduction in oxidative damage by either HBO treatment [16] or administration of an SOD mimetic [29]. Beneficial renal effects of antioxidant agents such as vitamin E, edaravone (radical scavenger) and melatonin were also reported in experimental models of cyclosporine nephrotoxicity [31], ischaemic ARF [32] and glycerol-induced ARF [33], respectively. Taken together, these studies indicate that oxygen-free radicals play a major role in renal injury of different aetiologies and that HBO treatment has a beneficial effect on renal dysfunction. The beneficial renal effect of HBO could be attributed to its anti-oxidative capacity. This notion is supported by our findings that HBO augmented the SOD immunoreativity in the renal tissue to normal levels and abolished the increase in 4-HNE. Similarly, several studies have demonstrated that HBO treatment increased the SOD and/or CAT activities in rat extensor digitorum longus muscle [13], in pancreas and erythrocytes of rats with induced acute necrotizing pancreatitis [34], in the lungs of rats and guinea pigs [35] and in the erythrocytes of human patients with multiple sclerosis [36].
The stimulatory effects of HBO treatment on the endothelial-dependent vasorelaxation induced by ACh in the ischaemic kidney hint that HBO treatment improves the function of the NO system, which plays an important role in the regulation of renal haemodynamic and kidney function. In line with these findings, several studies have shown that HBO increases the NO levels in the perivascular part of the aorta [35], and the brain [37,38]. Similarly, Cabigas and coworkers [39] have recently demonstrated a cardioprotective effect of HBO in rats subjected to myocardial ischaemia/reperfusion. The authors suggested that the conferred cardioprotection was due to HBO-mediated increased association between heat-shock protein 90 (hsp90) and eNOS, which further augmented NO production [39]. Additional studies are required to elucidate if similar mechanisms contributed to the enhanced recovery of GFR in our rats with ischaemic renal injury.
Nevertheless, despite the restoration and even enhanced endothelial-dependent response to ACh 48 h after ischaemic injury, baseline RBF was still lower in the −HBO and +HBO ischaemic kidneys compared with the non-ischaemic control kidney. This could reflect the predominant effects of various vasoconstrictor, NO-independent, systems that are still present at this post-ischaemic stage and may control renal haemodynamics.
Since basal RBF was lower in the +HBO group as compared to the −HBO group, this further decline in basal RBF following HBO treatment is consistent with the vasoconstrictive action of hyperoxygenation [40]. The mechanism(s) by which HBO treatment caused the increase in GFR were not clarified in the present study. Thus, the finding that despite the reduction in basal RBF, HBO caused a concomitant increase in GFR of the ischaemic kidneys might suggest that HBO induced a preferential vasoconstriction of the efferent arteriole. Alternatively, it might be speculated that the observed enhancement in GFR was not related to haemodynamic changes, but rather to an improvement in the tubular integrity and function of the injured nephrons. Further studies will be required to elucidate the exact mechanisms of GFR improvement and the contribution of the alterations to oxidant/antioxidant balance in that regard.
In summary, this study demonstrates that acute ischaemic renal injury is associated with enhancement of renal oxidative stress and that HBO treatment has a potential in the recovery of GFR by improving the antioxidant/oxidant balance in the ischaemic kidney.
Acknowledgments
The authors thank Prof. Samuel Heyman for reading the manuscript and for his helpful suggestions. This study was supported by Lisa Kurzbauer Memorial Fund and by Michael and Helen Schaffer Military Medicine Research Center.
Conflict of interest statement. None declared.
References
- 1.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 2.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 4.Goligorsky MS, Brodsky SV, Noiri E. NO bioavailability, endothelial dysfunction, and acute renal failure: new insights into pathophysiology. Semin Nephrol. 2004;24:316–323. doi: 10.1016/j.semnephrol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 6.Noiri E, Nakao A, Uchida K, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 7.Devarajan P. Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr. 2005;17:193–199. doi: 10.1097/01.mop.0000152620.59425.eb. [DOI] [PubMed] [Google Scholar]
- 8.Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97:385–395. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- 9.Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 10.Hills BA. A role for oxygen-induced osmosis in hyperbaric oxygen therapy. Med Hypotheses. 1999;52:259–263. doi: 10.1054/mehy.1997.0640. [DOI] [PubMed] [Google Scholar]
- 11.Zamboni WA, Roth AC, Russell RC, et al. Morphologic analysis of the microcirculation during reperfusion of ischemic skeletal muscle and the effect of hyperbaric oxygen. Plast Reconstr Surg. 1993;91:1110–1123. doi: 10.1097/00006534-199305000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Rachmilewitz D, Karmeli F, Okon E, et al. Hyperbaric oxygen: a novel modality to ameliorate experimental colitis. Gut. 1998;43:512–518. doi: 10.1136/gut.43.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregorevic P, Lynch GS, Williams DA. Hyperbaric oxygen modulates antioxidant enzyme activity in rat skeletal muscles. Eur J Appl Physiol. 2001;86:24–27. doi: 10.1007/s004210100503. [DOI] [PubMed] [Google Scholar]
- 14.Mrsic-Pelcic J, Pelcic G, Vitezic D, et al. Hyperbaric oxygen treatment: the influence on the hippocampal superoxide dismutase and Na+,K+-ATPase activities in global cerebral ischemia-exposed rats. Neurochem Int. 2004;44:585–594. doi: 10.1016/j.neuint.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Wada K, Miyazawa T, Nomura N, et al. Mn-SOD and Bcl-2 expression after repeated hyperbaric oxygenation. Acta Neurochir Suppl. 2000;76:285–290. doi: 10.1007/978-3-7091-6346-7_59. [DOI] [PubMed] [Google Scholar]
- 16.Edremitlioglu M, Kilic D, Oter S, et al. The effect of hyperbaric oxygen treatment on the renal functions in septic rats: relation to oxidative damage. Surg Today. 2005;35:653–661. doi: 10.1007/s00595-004-3000-5. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz MI, Korkmaz A, Kaya A, et al. Hyperbaric oxygen treatment augments the efficacy of a losartan regime in an experimental nephrotic syndrome model. Nephron Exp Nephrol. 2006;104:e15–e22. doi: 10.1159/000093260. [DOI] [PubMed] [Google Scholar]
- 18.Atasoyu EM, Yildiz S, Cimsit M, et al. Investigation of the effect of hyperbaric oxygen on experimental cyclosporine nephrotoxicity. Basic Clin Pharmacol Toxicol. 2006;98:150–154. doi: 10.1111/j.1742-7843.2006.pto_307.x. [DOI] [PubMed] [Google Scholar]
- 19.Solmazgul E, Uzun G, Cermik H, et al. Hyperbaric oxygen therapy attenuates renal ischemia/reperfusion injury in rats. Urol Int. 2007;78:82–85. doi: 10.1159/000096941. [DOI] [PubMed] [Google Scholar]
- 20.Skyhar MJ, Hargens AR, Strauss MB, et al. Hyperbaric oxygen reduces edema and necrosis of skeletal muscle in compartment syndromes associated with hemorrhagic hypotension. J Bone Joint Surg Am. 1986;68:1218–1224. [PubMed] [Google Scholar]
- 21.Reis ND, Better OS. Mechanical muscle-crush injury and acute muscle-crush compartment syndrome: with special reference to earthquake casualties. J Bone Joint Surg Br. 2005;87:450–453. doi: 10.1302/0301-620X.87B4.15334. [DOI] [PubMed] [Google Scholar]
- 22.Abassi ZA, Gurbanov K, Mulroney SE, et al. Impaired nitric oxide-mediated renal vasodilation in rats with experimental heart failure: role of angiotensin II. Circulation. 1997;96:3655–3664. doi: 10.1161/01.cir.96.10.3655. [DOI] [PubMed] [Google Scholar]
- 23.Brodsky S, Gurbanov K, Abassi Z, et al. Effects of eprosartan on renal function and cardiac hypertrophy in rats with experimental heart failure. Hypertension. 1998;32:746–752. doi: 10.1161/01.hyp.32.4.746. [DOI] [PubMed] [Google Scholar]
- 24.Mashiach E, Sela S, Winaver J, et al. Renal ischemia-reperfusion injury: contribution of nitric oxide and renal blood flow. Nephron. 1998;80:458–467. doi: 10.1159/000045220. [DOI] [PubMed] [Google Scholar]
- 25.Fuhr J, Kaczmarczyk J, Kruttgen CD. Eine einfache colorimetrische methode zur inulinbestimmung. Klin Wochenschr. 1955;33:729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto Y, Niki E, Eguchi J, et al. Oxidation of biological membranes and its inhibition. Free radical chain oxidation of erythrocyte ghost membranes by oxygen. Biochim Biophys Acta. 1985;819:29–36. doi: 10.1016/0005-2736(85)90192-0. [DOI] [PubMed] [Google Scholar]
- 27.Koster JF, Slee RG. Lipid peroxidation of human erythrocyte ghosts induced by organic hydroperoxides. Biochim Biophys Acta. 1983;752:233–239. doi: 10.1016/0005-2760(83)90117-0. [DOI] [PubMed] [Google Scholar]
- 28.Schnackenberg CG. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. Am J Physiol Regul Integr Comp Physiol. 2002;282:R335–R342. doi: 10.1152/ajpregu.00605.2001. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Jittikanont S, Falk SA, et al. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol Renal Physiol. 2003;284:F532–F537. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- 30.Leach M, Frank S, Olbrich A, et al. Decline in the expression of copper/zinc superoxide dismutase in the kidney of rats with endotoxic shock: effects of the superoxide anion radical scavenger, tempol, on organ injury. Br J Pharmacol. 1998;125:817–825. doi: 10.1038/sj.bjp.0702123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int. 1995;47:927–934. doi: 10.1038/ki.1995.138. [DOI] [PubMed] [Google Scholar]
- 32.Doi K, Suzuki Y, Nakao A, et al. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;65:1714–1723. doi: 10.1111/j.1523-1755.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 33.Ferraz FF, Kos AG, Janino P, et al. Effects of melatonin administration to rats with glycerol-induced acute renal failure. Ren Fail. 2002;24:735–746. doi: 10.1081/jdi-120015677. [DOI] [PubMed] [Google Scholar]
- 34.Yasar M, Yildiz S, Mas R, et al. The effect of hyperbaric oxygen treatment on oxidative stress in experimental acute necrotizing pancreatitis. Physiol Res. 2003;52:111–116. [PubMed] [Google Scholar]
- 35.Thom SR, Fisher D, Zhang J, et al. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol. 2003;284:H1230–H1239. doi: 10.1152/ajpheart.01043.2002. [DOI] [PubMed] [Google Scholar]
- 36.Ansari KA, Wilson M, Slater GE, et al. Hyperbaric oxygenation and erythrocyte antioxidant enzymes in multiple sclerosis patients. Acta Neurol Scand. 1986;74:156–160. doi: 10.1111/j.1600-0404.1986.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 37.Elayan IM, Axley MJ, Prasad PV, et al. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J Neurophysiol. 2000;83:2022–2029. doi: 10.1152/jn.2000.83.4.2022. [DOI] [PubMed] [Google Scholar]
- 38.Atochin DN, Demchenko IT, Astern J, et al. Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia. J Cereb Blood Flow Metab. 2003;23:1219–1226. doi: 10.1097/01.WCB.0000089601.87125.E4. [DOI] [PubMed] [Google Scholar]
- 39.Cabigas BP, Su J, Hutchins W, et al. Hyperoxic and hyperbaric-induced cardioprotection: role of nitric oxide synthase 3. Cardiovasc Res. 2006;72:143–151. doi: 10.1016/j.cardiores.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 40.Torbati D, Parolla D, Lavy S. Organ blood flow, cardiac output, arterial blood pressure, and vascular resistance in rats exposed to various oxygen pressures. Aviat Space Environ Med. 1979;50:256–263. [PubMed] [Google Scholar]