Abstract
Background Fetal programming of diabetes might originate in early pregnancy when fingerprints are permanently established. The mean dermatoglyphic ridge count difference between fingertips 1 and 5 (‘Md15’) varies with the early prenatal environment. We hypothesized that Md15 would be associated with adult-onset diabetes.
Methods We obtained Md15 from 577 Dutch adults (aged 58.9 years, SD 1.1) whose births in 1943–47 were documented in maternity records and from 260 of their same-sex siblings for whom birth weights were not available. Of these 837 participants, complete anthropometry and diabetes status (from history or glucose tolerance test) were obtained for 819.
Results After adjustment for age, sex, parental diabetes and adult anthropometry, fingerprint Md15 was associated with prevalent diabetes [odds ratio (OR) = 1.37 per 1 SD (95% confidence interval 1.02–1.84)]. This relationship held [OR = 1.40 (1.03–1.92)] for diabetic cases restricted to those recently diagnosed (within 7 years). In the birth series restricted to recently diagnosed cases, the mutually adjusted ORs were 1.34 (1.00–1.79) per SD of Md15 and 0.83 (0.62–1.10) per SD of birth weight. Further adjustments for maternal smoking, conception season or prenatal famine exposure in 1944–45 did not alter these estimates. Among 42 sibling pairs discordant for diabetes, the diabetic sibling had higher Md15 by 3.5 (0.6–6.3) after multivariable adjustment.
Conclusions Diabetes diagnosed at age 50+ years was associated with a fingerprint marker established in early gestation, irrespective of birth weight. Fingerprints may provide a useful tool to investigate prenatal developmental plasticity.
Keywords: Birth weight, body weights and measures, dermatoglyphics, diabetes mellitus, Dutch famine, embryonic and fetal development, Netherlands, organogenesis
Background
Epidemiological studies suggest a role for the early-life, non-genetic environment in the aetiology of diabetes. This non-genetic role may be inferred from the associations of low birth weight,1–3 high birth weight4–6 or season of birth7–9 with subsequent diabetes. Birth weight data are widely available, but birth weights bear little relationship with the circumstances of early gestation—the time window in which organs are formed and metabolic potential may be established. Birth weights tend to describe circumstances limited to the last trimester of pregnancy, primarily factors associated with the late fetal deposition of subcutaneous adipose tissue.10 Birth weight is an inadequate marker for those early gestational factors that influence subsequent cardiometabolic risk.11,12 An easily accessible marker is needed that describes circumstances related specifically to early gestation.
We have recently described a quantitative characteristic in human fingerprints that is associated with seasonal, non-genetic aspects of the early prenatal environment.13 Our dermatoglyphic marker describes a contrast in the growth stimuli influencing the anterior and posterior aspects of the embryonic limb bud. Each fingertip's ridge count (RC) reflects the early fetal size of the fingertip's volar pad.14 We defined a continuous variable (Md15) as the mean RC of the fingerprints of both thumbs (digit 1, most anterior) minus the mean RC of both little fingers (digit 5, most posterior). This variable is a unitless number. The RC values on digits 1 and 5 appear to be minimally related to genetic control.15 Like all features of the human fingerprint, Md15 is permanently fixed by the 19th week of pregnancy,16 the gestational age described obstetrically as weeks elapsed since the onset of the mother's last menstrual period. Therefore, Md15 can only reflect circumstances that were present during or before the first half of a 40-week pregnancy.
Diabetes may result in part from early gestational factors such as a restricted number of embryogenic progenitor cells in the pancreas17 or from otherwise insufficient early growth of beta-cell mass.18 Constraints on pancreatic development may have a genetic basis,19 but epigenetic20 and environmental factors could also play a role. The endocrine pancreas is largely formed by gestational weeks 18–24.21 During early fetal development, hedgehog intercellular signalling proteins participate in the orderly differentiation of the foregut from which the pancreas is derived and developed.22,23 These same diffusible morphogens may have differential effects on the embryonic relative growth of forelimb digits 1 and 5.24–26 Thus, hedgehog morphogens appear to influence embryonic development of both the pancreas and of relative finger sizes (expressed as variation in Md15) during the first half of pregnancy.
We examined the association between fingerprint Md15 and diabetes in an adult study population from western Holland. This panel of research subjects had originally been assembled for a different primary purpose, that is, for the investigation of how maternal undernutrition (during the Dutch Winter Famine of 1944–45) might have affected the subsequent life course of the offspring.27
Methods
Population and setting
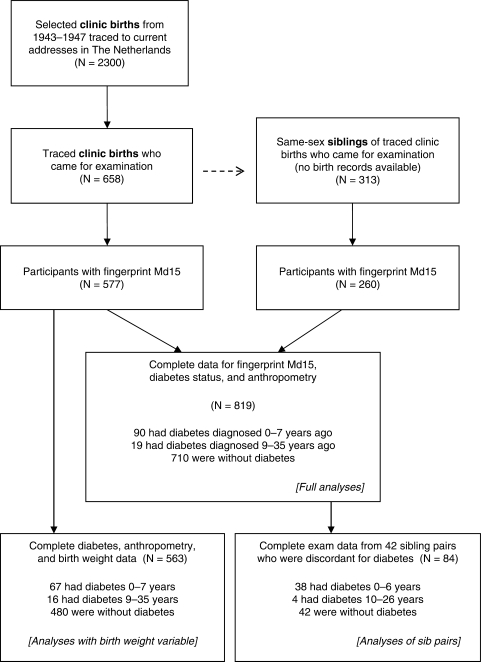
From the archived records of University Hospital in Leiden and midwifery training schools in Amsterdam and Rotterdam we identified for follow-up 3307 live-born, singleton births occurring in 1943–47. These were 2417 infants born between February 1945 and March 1946 who were exposed to famine during some portion of gestation and another 890 infants born in 1943 or 1947 who were unexposed to famine during gestation. With the assistance of population registers, we found current addresses in The Netherlands for 2300 (70%) of the identified clinic births. During 2003–05 we invited those located, together with a same-sex sibling whenever possible, for a telephone interview and a clinical examination at Leiden University Medical Center. Our study rationale, recruitment procedures and yields for the selected, traced births and the siblings (Figure 1) have been previously described.27,28 Study protocols were approved by human subjects committees of Columbia University and the collaborating Dutch institutions. All 971 participants in the clinical exam provided verbal consent at the start of their telephone interviews and written informed consent at the start of their examination visit.
Figure 1.
Origin of 819 participants in the full analyses, 563 in analyses with the birth weight variable and 84 who were in sibling pairs discordant for diabetes
For all the traced clinic births, we used the date of the mother's last menstrual period as noted in the original prenatal record to define the start of gestation unless this date was missing or implausible (12.4%). For these instances, we inferred the last menstrual date from the date of birth, an estimate of the gestational age recorded at birth and from gestational age estimates based on sex-, parity- and gestation-specific singleton birth weights established from a contemporaneous Dutch population.29 We assumed exposure to the 24-week Dutch Winter Famine during the first 20 weeks of gestation for those participants (55%) whose mother's last period occurred between July 9, 1944 and May 26, 1945.27 Consistent with the seasonal periodicity we previously reported for values of Md15,13 last menstrual dates occurring between January 12 and April 12 of any year (17.9% of the selected births) were designated as late-winter conceptions; last menstrual dates occurring between July 13 and October 11 of any year (24.9%) were designated as late-summer conceptions. The delivery records provided a birth weight for all but one traced birth. For participants recruited as same-sex siblings of our clinic population we had no documented information about their mother's last menstrual date or their birth weights. During scripted interviews, 24% of participants reported having at least one parent with diabetes. Based on the question ‘Did your mother smoke when you were a young child?’, we characterized 20% of the participants as probably exposed to maternal smoking during gestation.
Clinical assessment
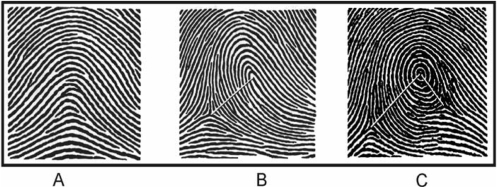
Fingertip RCs were obtained from ink-and-paper, rolled fingerprints. In accordance with standard dermatoglyphic practice, the RC for fingertips with a loop pattern equalled the number of ridges (range 1 to approximately 40) that intersected or touched a straight line drawn from the core of the loop to the delta (‘triradius’) point located on the radial or ulnar aspect of the finger; fingers with an arch pattern were assigned a RC of zero (see Figure 2 for fingerprint examples).30,31 For fingertips with whorls or double loops, we applied a summary RC formula that included half-unit values for those ridges located between the core and a second delta point or between multiple cores.13 All ridge counting was performed by one co-investigator (M.G.) who was blinded to the diabetic and anthropometric status of the participants.
Figure 2.
Examples of common fingerprint patterns and the determination of summary RCs. (A) A minority of fingertips reveals an ‘arch’ pattern with neither a core nor a delta point (example RC = 0, suggests a very small volar pad). (B) Most fingertips show a ‘loop’ pattern containing one core and one delta point (example RC = 13, an average volar pad). (C) Also fairly common is the ‘whorl’ pattern with one core and two delta points (example summary RC = 21 [i.e. 17 + (½ × 8)], a large volar pad) and the ‘double loop’ pattern with two cores and two delta points (not shown). (Source: Holt SB. Br Med Bull 1961;17:247–50)
Among participants who provided adequate fingerprint images from both hands, the RCs for homologous fingers were highly correlated (r ∼ 0.8), but they tended to be higher on the right hand than the left. Consistent with the greater size of male fetuses in early pregnancy,32 the mean RCs for thumbs and little fingers were higher among men than women (P < 0.001). From the assessments of paired fingertips we estimated sex-specific, digit-specific average increments of RC values between homologous fingers of the right and left hands. Where a fingertip RC was unavailable for one side or the other, we used these average RC increments to provide homologous substitution values that were empirically defined for the missing RCs.13 We thereby obtained right and left RC values for 910 persons (digit 1) and 850 persons (digit 5). With these homologous substitution values we could compute Md15 for 837 participants.
Because of our focus on an aetiologic pathway that might be independent of diabetes risk factors operating in the late-gestational or postnatal time windows we controlled for adult anthropometric dimensions that commonly precede glucose intolerance: waist circumference,2,33–35 thigh circumference35–37 and leg length.38–40 Standing height was measured to the nearest 0.1 cm with a stadiometer, and trunk height (including the head and neck) was obtained from sitting height using the same stadiometer. We calculated leg length as the difference between standing height and trunk height. Circumferences of the waist (standing, at the level of iliac crest, intersection with midaxillary line) and right midthigh (supine, with hip flexed at 45°) were measured in duplicate to the nearest 0.1 cm with non-extendible tapes. If the circumference measurements at each site were not within 1 cm of each other, third and fourth measurements were taken and the three measurements that were closest together averaged.
The presence of diabetes was established by the initial telephone interview for 62 participants (6.4%, all of whom stated that their diagnosis was made by a physician). For another 69 participants (7.1%) diabetes was diagnosed by a 75-gm oral glucose tolerance test with either a fasting glucose concentration ≥7.0 mmol/l or a 2-h concentration ≥11.1 mmol/l. Glucose was assayed in promptly separated serum by the hexokinase reaction on a Modular P800 (Roche, Boehringer Mannheim, Germany); the interassay coefficient of variation was 1.3–1.8%. Among those participants who reported in their interview being free of diabetes, 15 did not complete their glucose tolerance test and were omitted from analyses.
Statistical analysis
Among 819 participants with complete examination data (Figure 1) we tested associations with diabetes using the GENMOD procedure with a linked logit function in SAS software (Release 9.1, SAS Institute, Cary, NC, USA). We adjusted for correlations within the same-sex sibling pairs. To assess the relative strengths of the associations of Md15 (our early-gestational marker) and birth weight (the conventional late-gestational marker) with diabetes we used the LOGISTIC procedure in models that excluded siblings because they lacked information on birth weight. In these models we standardized the continuous variables (with means and SDs calculated from only the traced births) so that their coefficients would be dimensionless and directly comparable.
We identified 227 pairs of same-sex siblings in which both members of the pair had Md15 values, established diabetes status and complete anthropometry. Among the 42 pairs that were discordant for diabetes we used the REG procedure to compare Md15 of the diabetic sibling with the non-diabetic sibling, adjusted for age and adult anthropometry.
In exploratory analyses, we divided the diabetic participants into two subgroups distinguished by the duration of their disease: relatively short (0–7 years, mean duration 1.4 years; mean diagnostic age 57 years, range 50–64 years) and long (9–35 years, mean duration 17 years; mean diagnostic age 42 years, range 24–53 years). This division was prompted by our concern that long-time survivors of diabetes might have different disease characteristics from diabetic cases whose disease had been recently diagnosed. We thereby addressed the potentials for survivorship bias and for distinct diabetic typologies. The choice of a cut point, while somewhat arbitrary, was suggested by a recent report showing an increased hazard of death commencing ∼8 years after diagnosis among diabetic patients with abnormal birth weights.41 The 8-year cut point was also consistent with a natural break in the distribution of disease durations among diabetic cases who had Md15 values and complete anthropometry.
Results
Demographic characteristics and study outcomes for the traced births and their siblings are presented in Table 1. Fingerprint Md15 had a mean value of 6.6 (SD 7.4) and a range from −20.0 (a non-diabetic man) to +28.6 (a man recently diagnosed with diabetes). Md15 and the mean RCs for each finger were similar when comparing the participants whose gestations occurred within and outside the wartime famine interval.13
Table 1.
Characteristics of the Dutch adults examined in 2003–2005
| All participants, including siblings |
Clinic births 1943–47, excluding siblingsa |
|||||
|---|---|---|---|---|---|---|
| Variable | N | Mean or categorical distribution | SD | N | Mean or categorical distribution | SD |
| Age (years) | 971 | 58.4 | 3.8 | 658 | 58.9 | 1.1 |
| Sex (men/women) | 971 | 437/534 | – | 658 | 304/354 | – |
| Birth weight (kg) | (available only for birth sample) | 657 | 3.368 | 0.510 | ||
| Fingerprint Md15 | 837 | 6.6 | 7.4 | 577 | 6.9 | 7.3 |
| Waist circumference (cm) | 970 | 98.0 | 11.5 | 657 | 98.8 | 11.6 |
| Midthigh circumference (cm) | 969 | 52.3 | 5.1 | 657 | 52.4 | 5.2 |
| Leg length (cm) | 966 | 81.6 | 5.4 | 654 | 81.5 | 5.4 |
| Diabetes (yes/no/unknown) | 971 | 131/825/15 | – | 658 | 95/550/13 | – |
| Years since diabetes diagnosis (<1/1–7/8–35) | 131 | 71/38/22 | – | 95 | 44/33/18 | – |
| Parent with diabetes (yes/no or unknown) | 971 | 233/738 | – | 658 | 159/499 | – |
| Gestational smoking exposure (yes/no or unknown) | 971 | 196/775 | – | 658 | 137/521 | – |
| Early fetal famine exposure (yes/no) | 971 | 365/606 | 658 | 365/293 | – | |
| Season of mother's last menstrual period (late winter/late summer/other) | (available only for birth sample) | 658 | 118/164/376 | – | ||
aNo birth records are available for siblings.
With adjustments only for age and sex, Md15 (per increment of 10) had an odds ratio (OR) for identifying prevalent diabetes of 1.22 (95% CI 0.93–1.59) (Table 2, Model 1). With subsequent adjustments for parental diabetes and for adult anthropometry the OR was 1.26 and 1.37 (95% CI 1.02–1.84) (Table 2, Models 2 and 3). There was no interaction (P > 0.9) between the terms for Md15 and sex. A quadratic term in Md15 was not significant (P > 0.5), suggesting a linear relation of Md15 to diabetes. The association persisted [OR = 1.40 (95% CI 1.03–1.92)] in the subgroup of the 90 diabetes cases of short duration (diagnosed <8 years ago; model not shown). For the 19 cases with disease duration 9–35 years the OR for the association of Md15 with diabetes was 1.34 (95% CI 0.67–2.66); P > 0.4, model not shown.
Table 2.
Odds ratios (ORs) from logistic regression models for the identification of prevalent diabetic cases (n = 109) among all participants (n = 819) with values for fingerprint Md15, waist circumference, midthigh circumference and leg length
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Md15 (10 units of RC difference) | 1.22 | (0.93–1.59) | 0.14 | 1.26 | (0.97–1.65) | 0.088 | 1.37 | (1.02–1.84) | 0.037 |
| Age (year) | 1.09 | (1.03–1.16) | 0.002 | 1.10 | (1.03–1.17) | 0.002 | 1.08 | (1.02–1.15) | 0.012 |
| Female sex | 0.68 | (0.45–1.03) | 0.068 | 0.62 | (0.40–0.97) | 0.035 | 0.50 | (0.29–0.88) | 0.016 |
| Diabetic parent | – | 2.48 | (1.57–3.91) | <0.001 | 2.19 | (1.39–3.45) | <0.001 | ||
| Waist circumference (cm) | – | – | 1.08 | (1.06–1.11) | <0.001 | ||||
| Midthigh circumference (cm) | – | – | 0.93 | (0.88–0.98) | 0.004 | ||||
| Leg length (cm) | – | – | 0.91 | (0.87–0.96) | 0.001 | ||||
To address the simultaneous contributions of Md15 and birth weight toward predicting diabetes, we restricted analyses to the traced clinic births. In this model with 83 diabetic cases and 480 non-diabetic participants, siblings were necessarily excluded because they had no birth weight information. With adjustment for sex, having a diabetic parent, and three anthropometric dimensions, the ORs for diabetes were 1.24 (95% CI 0.95–1.61) per SD of Md15 and 0.89 (95% CI 0.68–1.15) per SD of birth weight.
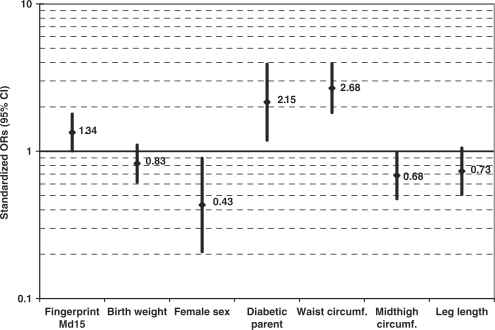
In the subgroup of the 67 diabetic cases of short duration (diagnosed <8 years ago) the ORs for diabetes were 1.34 (95% CI 1.00–1.79) per SD of Md15 and 0.83 (95% CI 0.62–1.10) per SD of birth weight (Figure 3). For the 16 diabetic cases with disease duration of 9–35 years, the ORs were 0.95 (95% CI 0.53–1.69) per SD of Md15 and 1.20 (95% CI 0.70–2.04) per SD of birth weight. For this long-duration diabetic subgroup, in contrast to the short-duration subgroup presented in Figure 3, there were stronger associations with having a diabetic parent [OR = 7.81 (95% CI 2.58–23.6)] and with leg length [OR = 0.36 (95% CI 0.17–0.77) per SD of leg length]. In these models, additional adjustments for maternal smoking, season of conception, exposure to wartime famine or age (range 55.7–65.1 years among the clinic births) did not alter the OR estimates for Md15 or birth weight.
Figure 3.
Mutually adjusted ORs (95% CI) for diabetes per SD of Md15, birth weight, waist circumference, midthigh circumference and leg length as well as sex and parental diabetes in a birth sample examined at age 58.9 years (SD 1.1). The logistic regression model includes 67 cases whose diabetes was diagnosed <8 years ago and 480 participants free of diabetes. (Siblings were excluded from this model because they lacked birth weight documentation)
Among the 42 sibling pairs who were discordant for disease status the sibling with diabetes was older by a mean 3.4 years (SD 6.5). With adjustment for the within-sibship differences in age, the diabetic sibling had a larger value of Md15 by 2.8 (95% CI −0.1 to +5.7). If we restricted the analysis to diabetes cases of short duration, the 38 diabetic siblings had a larger value of Md15 by 3.4 (95% CI 0.3–6.5). With further adjustments for within-sibship differences in waist circumference, midthigh circumference and leg length, the 42 diabetic siblings had a larger Md15 by 3.5 (95% CI 0.6–6.3). Restricted to diabetes cases of short duration, the 38 diabetic siblings had a larger value of Md15 by 3.4 (95% CI 0.3–6.5).
In sex-adjusted correlational analyses, Md15 was not significantly associated with birth weight (P > 0.5) or with any of the anthropometric dimensions used in our models (P > 0.1).
Discussion
We here provide evidence that diabetes diagnosed in adulthood is associated with a quantitative fingerprint characteristic known to be fixed in early pregnancy.16 Our earlier work demonstrated that the Md15 characteristic was related to the intrauterine environment during early gestation or the periconceptional circumstances of perhaps either parent.13 In this study of the association between Md15 and diabetes, we adjusted initially for correlations within sibling pairs, age and sex. We then further controlled for a diabetes risk factor associated with genetic circumstances before conception (having a diabetic parent) and for risk factors likely to reflect circumstances of late gestation or postnatal life (waist circumference, midthigh circumference and leg length). In this way our fully controlled analysis (Model 3 in Table 2) presented the clearest quantitative estimate of how Md15 might represent the developmental influences operating exclusively or primarily in an early gestational time window. Among these adult study participants those with higher values of Md15 were more likely to have diabetes. In a subset analysis restricted to same-sex sibling pairs who were discordant for diabetes status we also found that the diabetic sibling had a larger Md15 compared with the non-diabetic sibling.
One may think of Md15 as a permanent, dermatoglyphic marker of fetal morphogenic activity that occurs within an early gestational time window. This early morphogenic activity has plausible relevance to pancreatic development and perhaps to other factors that precede diabetes.
It is worth noting that severe maternal undernutrition in early pregnancy appears not to decrease offspring birth weight.42 Nevertheless, some environmental feature during an early gestational time window may influence the offspring's developmental plasticity,43 and it may lead to ‘functional teratogenesis’ through pathophysiological mechanisms yet unknown.44 If the association between a permanent fingerprint characteristic and diabetes can be confirmed in other populations, then fingerprints could be employed at any age to enhance aetiologic investigations of diabetes or possibly to improve the prediction of diabetes before its clinical diagnosis.
Our study's traced births benefited from high quality prenatal and delivery data preserved in a clinical archive, but maternity records of that era did not systematically collect information regarding gestational diabetes or parental habits such as smoking or diet. The generalizability of our study results may be limited by the narrow range of the participants’ race–ethnicity and age, and by the fact that our selected births included many pregnancies occurring in an unusual circumstance of urban famine.
In this study of older adults, the association of Md15 with prevalent diabetes persisted in the subgroup of cases with short-duration disease. These patients were initially diagnosed at the age of 50 years or more, and we therefore feel most confident that they represent the conventional category of type 2 diabetes. These older-onset cases are of special interest to us as they would likely be similar to diabetes cases shown elsewhere to be associated with reduced birth weight.1–3 Other categories such as type 1 diabetes or maturity-onset diabetes of the young may have been included in the small subgroup of diabetic cases with disease duration longer than 7 years, but we have neither immunologic nor genetic evidence to confirm these typological classifications. The available numbers in this long-duration subgroup were too small, we feel, for separate comparisons. Analyses of the long-duration cases could also have been subject to survivorship bias, especially if there was excess mortality specific to diabetic patients who were born after abnormal gestations.41
These epidemiologic findings do not establish the prenatal mechanisms that underlie RC differences between fingers, nor do they explain with certainty how fingerprint Md15 might be related to a diabetes diagnosis that occurs decades after birth. It is unlikely that Md15 represents a purely genetic expression, since Md15 was associated with the calendar season of conception.13 Learning the environmental details and the precise time windows that influence Md15 might bring a new understanding of early prenatal plasticity as it applies to organ growth and tissue metabolism. The ultimate benefit could be improved strategies for the primary prevention of adult diabetes.
Acknowledgements
We thank the Vroedvrouwenscholen of Amsterdam and Rotterdam and Leiden University Medical Center for access to their archives. Patient tracing and follow-up were carried out at TNO-Quality of Life, Leiden, The Netherlands (Dr Karin M. van der Pal-de Bruin). Clinical examinations were carried out at the Study Center of Gerontology & Geriatrics, Leiden University Medical Center under the supervision of Ms Liesbeth de Man. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. This study was supported by National Institutes of Health (HL067914 to L.H.L.)
Conflicts of interest: None declared.
KEY MESSAGES.
Aspects of developmental plasticity are likely to be operating in early gestation.
Fingerprints—permanent features that are established before the midpoint of a normal pregnancy—may carry information associated with early fetal variations in organ anatomy or metabolic programming.
A quantitative fingerprint marker (the ridge-count contrast between digits 1 and 5) was associated with diabetes at age ∼58 years.
Fingerprints obtained at any age may facilitate research into early fetal metabolic programming or contribute to the prediction of diabetes in later adulthood.
References
- 1.Burke JP, Forsgren J, Palumbo PJ, et al. Association of birth weight and type 2 diabetes in Rochester, Minnesota. Diabetes Care. 2004;27:2512–13. doi: 10.2337/diacare.27.10.2512. [DOI] [PubMed] [Google Scholar]
- 2.Tian JY, Cheng Q, Song XM, et al. Birth weight and risk of type 2 diabetes, abdominal obesity and hypertension among Chinese adults. Eur J Endocrinol. 2006;155:601–7. doi: 10.1530/eje.1.02265. [DOI] [PubMed] [Google Scholar]
- 3.Lawlor DA, Davey Smith G, Clark H, Leon DA. The associations of birth weight, gestational age and childhood BMI with type 2 diabetes: findings from the Aberdeen Children of the 1950s cohort. Diabetologia. 2006;49:2614–17. doi: 10.1007/s00125-006-0408-z. [DOI] [PubMed] [Google Scholar]
- 4.Innes KE, Byers TE, Marshall JA, Baron A, Orleans M, Hamman RF. Association of a woman's own birth weight with subsequent risk for gestational diabetes. JAMA. 2002;287:2534–41. doi: 10.1001/jama.287.19.2534. [DOI] [PubMed] [Google Scholar]
- 5.Wei JN, Sung FC, Li CY, et al. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care. 2003;26:343–48. doi: 10.2337/diacare.26.2.343. [DOI] [PubMed] [Google Scholar]
- 6.Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165:849–57. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- 7.Doblhammer G. The Late Life Legacy of Very Early Life. Berlin: Springer; 2004. [Google Scholar]
- 8.McKinney PA. Seasonality of birth in patients with childhood type I diabetes in 19 European regions. Diabetologia. 2001;44(Suppl 3):B67–74. doi: 10.1007/pl00002957. [DOI] [PubMed] [Google Scholar]
- 9.Vaiserman AM, Carstensen B, Voitenko VP, et al. Seasonality of birth in children and young adults (0–29 years) with type 1 diabetes in Ukraine. Diabetologia. 2007;50:32–35. doi: 10.1007/s00125-006-0456-4. [DOI] [PubMed] [Google Scholar]
- 10.Larciprete G, Valensise H, Di PG, et al. Intrauterine growth restriction and fetal body composition. Ultrasound Obstet Gynecol. 2005;26:258–62. doi: 10.1002/uog.1980. [DOI] [PubMed] [Google Scholar]
- 11.Gillman MW. Epidemiological challenges in studying the fetal origins of adult chronic disease. Int J Epidemiol. 2002;31:294–99. [PubMed] [Google Scholar]
- 12.Wells J. Commentary: Games people play – birth weight. Int J Epidemiol. 2006;35:277–79. doi: 10.1093/ije/dyl021. [DOI] [PubMed] [Google Scholar]
- 13.Kahn HS, Graff M, Stein AD, Zybert PA, McKeague IW, Lumey LH. A fingerprint characteristic associated with the early prenatal environment. Am J Hum Biol. 2008;20:59–65. doi: 10.1002/ajhb.20672. [DOI] [PubMed] [Google Scholar]
- 14.Babler WJ. Embryologic development of epidermal ridges and their configurations. Birth Defects. 1991;27:95–112. [PubMed] [Google Scholar]
- 15.Medland SE, Loesch DZ, Mdzewski B, Zhu G, Montgomery GW, Martin NG. Linkage analysis of a model quantitative trait in humans: finger ridge count shows significant multivariate linkage to 5q14.1. PLoS Genet. 2007;3:1736–44. doi: 10.1371/journal.pgen.0030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babler WJ. Quantitative differences in morphogenesis of human epidermal ridges. Birth Defects. 1979;15:199–208. [PubMed] [Google Scholar]
- 17.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–91. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 18.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758–68. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- 19.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–41. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008;118:2316–24. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scharfmann R. Control of early development of the pancreas in rodents and humans: implications of signals from the mesenchyme. Diabetologia. 2000;43:1083–92. doi: 10.1007/s001250051498. [DOI] [PubMed] [Google Scholar]
- 22.Hebrok M. Hedgehog signaling in pancreas development. Mech Dev. 2003;120:45–57. doi: 10.1016/s0925-4773(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 23.Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–21. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Tickle C. Patterning systems – from one end of the limb to the other. Dev Cell. 2003;4:449–58. doi: 10.1016/s1534-5807(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 25.Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–16. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–28. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Lumey LH, Stein AD, Kahn HS, et al. Cohort Profile: The Dutch Hunger winter families study. Int J Epidemiol. 2007;36:1196–204. doi: 10.1093/ije/dym126. [DOI] [PubMed] [Google Scholar]
- 28.Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85:869–76. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- 29.Kloosterman GJ. On intrauterine growth. Int J Gynaecol Obstet. 1970;8:895–912. [Google Scholar]
- 30.Penrose LS. Memorandum on dermatoglyphic nomenclature. Birth Defects. 1968;4:1–13. [PubMed] [Google Scholar]
- 31.Loesch DZ. Quantitative Dermatoglyphics: Classification, Genetics, and Pathology. Oxford: Oxford University Press; 1983. [Google Scholar]
- 32.Bukowski R, Smith GCS, Malone FD, et al. Human sexual size dimorphism in early pregnancy. Am J Epidemiol. 2007;165:1216–18. doi: 10.1093/aje/kwm024. [DOI] [PubMed] [Google Scholar]
- 33.Wei M, Gaskill SP, Haffner SM, Stern MP. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans – a 7-year prospective study. Obes Res. 1997;5:16–23. doi: 10.1002/j.1550-8528.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 34.Katzmarzyk PT, Craig CL, Gauvin L. Adiposity, physical fitness and incident diabetes: the physical activity longitudinal study. Diabetologia. 2007;50:538–44. doi: 10.1007/s00125-006-0554-3. [DOI] [PubMed] [Google Scholar]
- 35.Chuang YC, Hsu KH, Hwang CJ, Hu PM, Lin TM, Chiou WK. Waist-to-thigh ratio can also be a better indicator associated with type 2 diabetes than traditional anthropometrical measurements in Taiwan population. Ann Epidemiol. 2006;16:321–31. doi: 10.1016/j.annepidem.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–77. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 37.Sakai Y, Ito H, Egami Y, et al. Favourable association of leg fat with cardiovascular risk factors. J Intern Med. 2005;257:194–200. doi: 10.1111/j.1365-2796.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 38.Lawlor DA, Ebrahim S, Davey Smith G. The association between components of adult height and Type II diabetes and insulin resistance: British women's heart and health study. Diabetologia. 2002;45:1097–106. doi: 10.1007/s00125-002-0887-5. [DOI] [PubMed] [Google Scholar]
- 39.Moses RG, Mackay MT. Gestational diabetes: Is there a relationship between leg length and glucose tolerance? Diabetes Care. 2004;27:1033–35. doi: 10.2337/diacare.27.5.1033. [DOI] [PubMed] [Google Scholar]
- 40.Asao K, Kao WHL, Baptiste-Roberts K, Bandeen-Roche K, Erlinger TP, Brancati FL. Short stature and the risk of adiposity, insulin resistance, and type 2 diabetes in middle age: the third national health and nutrition examination survey (NHANES III), 1988–1994. Diabetes Care. 2006;29:1632–37. doi: 10.2337/dc05-1997. [DOI] [PubMed] [Google Scholar]
- 41.Leibson CL, Burke JP, Ransom JE, et al. Relative risk of mortality associated with diabetes as a function of birth weight. Diabetes Care. 2005;28:2839–43. doi: 10.2337/diacare.28.12.2839. [DOI] [PubMed] [Google Scholar]
- 42.Stein AD, Zybert PA, van de Bor M, Lumey LH. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J Epidemiol. 2004;33:831–36. doi: 10.1093/ije/dyh083. [DOI] [PubMed] [Google Scholar]
- 43.Gluckman PD, Hanson MA. Developmental plasticity and human disease: research directions. J Intern Med. 2007;261:461–71. doi: 10.1111/j.1365-2796.2007.01802.x. [DOI] [PubMed] [Google Scholar]
- 44.Plagemann A. ‘Fetal programming’ and ‘functional teratogenesis’: on epigenetic mechanisms and prevention of perinatally acquired lasting health risks. J Perinat Med. 2004;32:297–305. doi: 10.1515/JPM.2004.055. [DOI] [PubMed] [Google Scholar]