Abstract
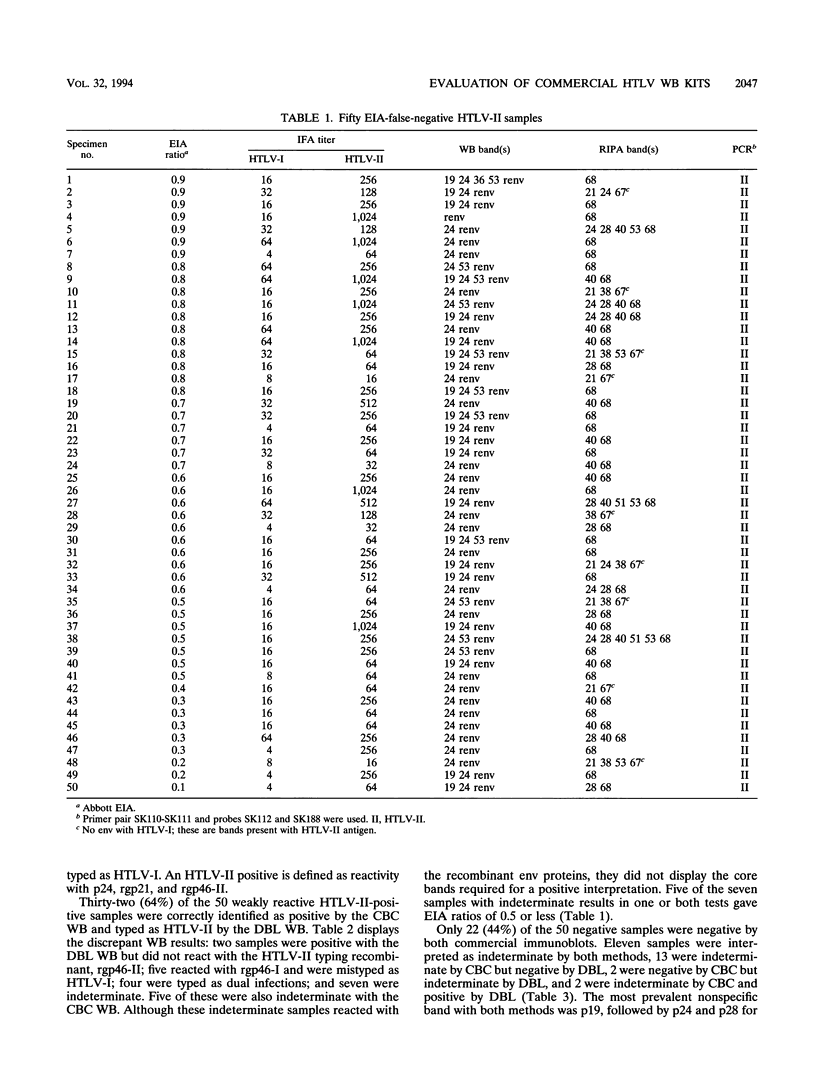
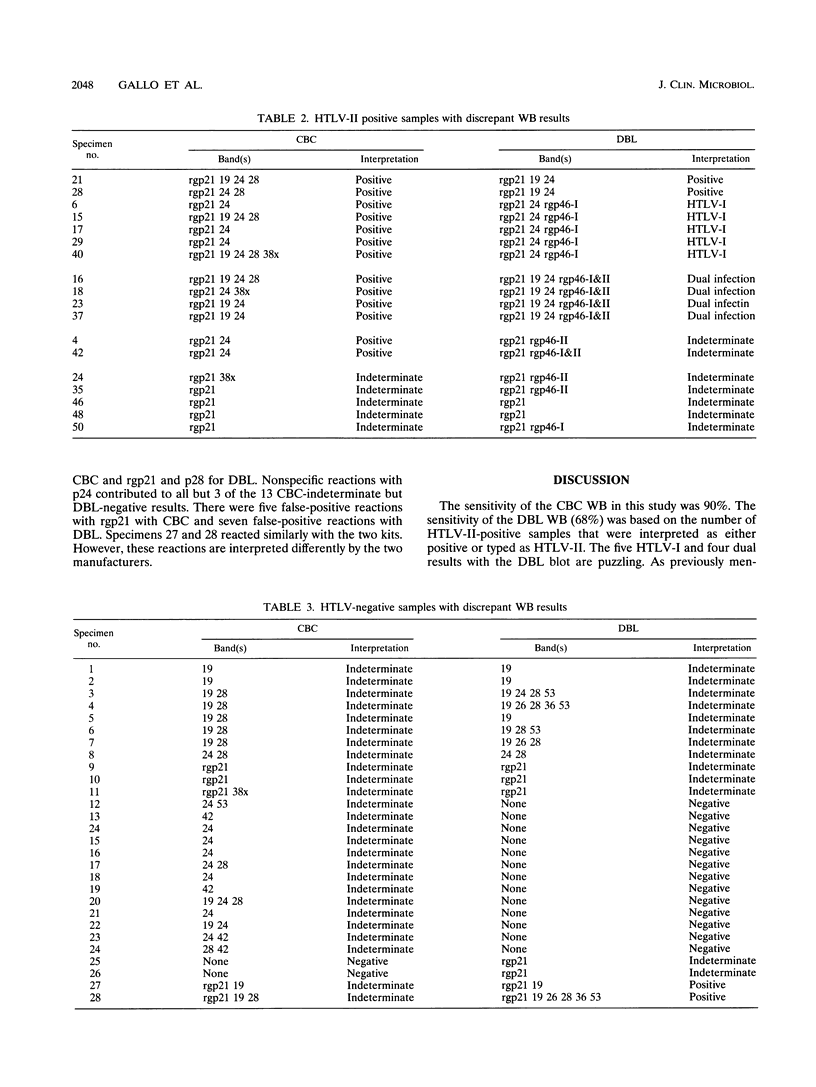
We evaluated two commercial human T-cell lymphotropic virus (HTLV) Western blot (WB; immunoblot) kits, Cambridge Biotech Corp. (CBC) and Diagnostic Biotechnology Ltd. (DBL). Both methods employ HTLV type I (HTLV-I) viral lysate and rgp21. The DBL WB kit also distinguishes between HTLV-I and HTLV-II antibodies, using an HTLV-I-specific and an HTLV-II-specific recombinant. Fifty weakly reactive HTLV-II-positive plasma specimens which were falsely negative with the Abbott enzyme immunoassay (EIA) and 50 Ortho EIA false-positive samples were selected to determine sensitivity and specificity. The sensitivities of the CBC and the DBL WB kits were 90 and 68%, respectively. All positive samples reacted with rgp21 in both kits, but some did not display core bands. Five samples were typed as HTLV-I and four were typed as dual infection by the DBL WB kit. The specificities of the CBC and DBL kits were 48 and 70%, respectively. The most prevalent WB reaction with the negative samples was with the core protein, p19, followed by p24 and p28 for CBC and rgp21 and p28 for DBL. DBL had two false-positive interpretations, and CBC had none, rgp21 was the most sensitive antigen in both kits for the weakly reactive HTLV-II samples. If all samples not reacting with this protein were interpreted as WB negative, regardless of other bands, the specificity would improve to 90% for CBC and 86% for DBL.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cossen C., Hagens S., Fukuchi R., Forghani B., Gallo D., Ascher M. Comparison of six commercial human T-cell lymphotropic virus type I (HTLV-I) enzyme immunoassay kits for detection of antibody to HTLV-I and -II. J Clin Microbiol. 1992 Mar;30(3):724–725. doi: 10.1128/jcm.30.3.724-725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo D., Diggs J. L., Hanson C. V. Comparison of Western immunoblot antigens and interpretive criteria for detection of antibody to human T-lymphotropic virus types I and II. J Clin Microbiol. 1990 Sep;28(9):2045–2050. doi: 10.1128/jcm.28.9.2045-2050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo D., Hoffman M. N., Cossen C. K., Diggs J. L., Hurst J. W., Penning L. M. Comparison of immunofluorescence, enzyme immunoassay, and Western blot (immunoblot) methods for detection of antibody to human T-cell leukemia virus type I. J Clin Microbiol. 1988 Aug;26(8):1487–1491. doi: 10.1128/jcm.26.8.1487-1491.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo D., Penning L. M., Hanson C. V. Detection and differentiation of antibodies to human T-cell lymphotropic virus types I and II by the immunofluorescence method. J Clin Microbiol. 1991 Oct;29(10):2345–2347. doi: 10.1128/jcm.29.10.2345-2347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok S., Gallo D., Hanson C., McKinney N., Poiesz B., Sninsky J. J. High prevalence of HTLV-II among intravenous drug abusers: PCR confirmation and typing. AIDS Res Hum Retroviruses. 1990 Apr;6(4):561–565. doi: 10.1089/aid.1990.6.561. [DOI] [PubMed] [Google Scholar]
- Lal R. B., Brodine S., Kazura J., Mbidde-Katonga E., Yanagihara R., Roberts C. Sensitivity and specificity of a recombinant transmembrane glycoprotein (rgp21)-spiked western immunoblot for serological confirmation of human T-cell lymphotropic virus type I and type II infections. J Clin Microbiol. 1992 Feb;30(2):296–299. doi: 10.1128/jcm.30.2.296-299.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


