Abstract
Non-human primates are suitable models for preclinical research aimed at cell-replacement therapies. Recently, it has been reported that Rho-associated kinase inhibitor Y-27632 markedly reduced dissociation-induced apoptosis of human embryonic stem (hES) cells, and is expected as a novel supplement for hES cell maintenance or differentiation inductions; however, the effects of the chemical are still to be determined in model animals. Here, we demonstrated the effect of Y-27632 on cynomolgus monkey ES (cyES) cells. Also, in cyES cells, Y-27632 treatment dramatically improved the efficiency of colony formation from single cells without affecting the pluripotent state and karyotype. Y-27632 supplementation was also effective for feeder-free culture and differentiation induction. Neural stem cells directly induced from cyES cells could give rise to neurons, astrocytes and dopamine producing cells. The present result not only suggests that the chemical was effective for improving the culture system of primate ES cells, but also the similarity between cyES and hES cells regarding the reactions to the chemical, which might be further evidence that cyES cells are superior models for hES cells.
Keywords: primate, embryonic stem cells, pluripotency, differentiation
Introduction
Embryonic stem (ES) cell therapy is expected to be a highly promising method of treatment for seriously damaged or disordered tissues and organs (Hochedlinger and Jaenisch, 2003; Yang et al., 2007), since ES cells have high proliferative potency and the ability to differentiate into many kinds of somatic cells, including germ cells. In recent years, mouse ES (mES) cells have been used widely as a model system in cell transplantation and to explore ES cell biology, for example, signal pathways maintaining stem-ness. While the mouse model provides a foundation for studying stem cells, many differences between mES and human ES (hES) cells have been reported (Daheron et al., 2004; Ginis et al., 2004; Sumi et al., 2004). In particular, mechanisms of maintenance and the regulation of pluripotency are quite different between mice and primates. However, the use of hES cells in basic research has been confronted with major obstacles relating to ethical and legal restrictions in some countries.
Although hES cells significantly differ from mES cells, they closely resemble those of non-human primates. Furthermore, non-human primate models of some diseases are expected more closely to resemble human diseases than existing mouse models (Suemori and Nakatsuji, 2006). From these, it is reasonable to think that the non-human primate would be a more suitable model for preclinical research aimed at cell-replacement therapies (Haruta et al., 2004).
One of the important differences between mES and hES cells is the vulnerability of single cells to enzymatic digestion (Thomson et al., 1998; Amit et al., 2000; Watanabe et al., 2007). This characteristic restricted the application of the procedure for differentiation or introducing a transgene that has been developed in mES cells. Recently, it has been reported that Rho-associated kinase (ROCK) inhibitor Y-27632 markedly reduced dissociation-induced apoptosis of hES cells, and enabled expansion from single cell (low-density) culture (Watanabe et al., 2007). It was thought that the chemical might be the standard protocol in both basic science and the clinical use of hES cells; however, the effects of the chemical are still to be determined in other primates. To promote preclinical investigation of hES cells, it is indispensable to study differentiation and transplantation using non-human primate ES cells based on the same procedure as with hES cells.
Here, we demonstrated the effect of Y-27632 on cynomolgus monkey ES (cyES) cell cultures. Furthermore, we performed direct neural stem cell (NSC) induction from cyES cells using Y-27632, and examined the effectiveness for preparing transplantable cells through in vitro differentiation.
Materials and Methods
Establishment and culture of cyES cells
In the present study, we used an existing cell line (Cyk-1; Teramura et al., 2007) and a newly established cell line (Cyk-3). The method for cyES cell establishment and maintenance was previously reported. Briefly, superovulated oocytes were fertilized by ICSI using freeze–thawed sperm and cultured until the blastocyst stage in mCMRL medium at 37°C in 5% CO2, 5% O2 and 90% N2 until the blastocyst stage. After removal of zona pellucida by treatment with 0.05% Pronase (Roche Diagnostics, IN, USA), inner cell masses (ICMs) were collected by immunosurgery. Isolated ICMs were individually seeded onto culture dishes containing a layer of mouse embryonic feeder (MEF) cells in ES medium composed of Knockout-DMEM (Invitrogen Corporation, CA, USA), 1 mM l-glutamine, 0.1 mM β-mercaptoethanol (both from Sigma–Aldrich, Inc., MO, USA), and 0.1 mM MEM non-essential amino acid solution (Invitrogen), and supplemented with 4 ng/ml of human recombinant basic fibroblast growth factor (bFGF) (Upstate, NY, USA) 20% Knockout Serum Replacement (KSR, Invitrogen). After 10 days, ICM developed morphologically into ES cell-like colonies. After the first passage, colonies with an ES cell-like morphology were selected for propagation, characterization and storage. All manipulations related to sub-cloning or primary passage were performed by mechanical methods. The cyES cells were maintained in ES medium with mitotically inactivated MEF, and passaged by the hES cell protocol (Cameron et al., 2006).
All procedures related to animal treating were approved by the Institutional Animal Care and Use Committee at Kinki University, Japan.
Observation of the effect of ROCK inhibitor treatment for cyES cell culture
The cyES cells (Cyk-1) were dissociated into single cells by the treatment with 0.25% trypsin and 0.02% EDTA (Invitrogen) for 3 min, followed by the addition of fresh media to inhibit further digestion. Then, the cells were collected, resuspended in ES medium and filtered through 40-μm meshes (BD Biosciences Discovery Labware, MA, USA) in order to obtain a single cell suspension.
For 5-bromo-2′-deoxyuridine (BrdU) incorporation assays, dissociated cyES cells were seeded at a concentration of 500 cells/cm2 onto fresh feeder layers in the presence of the ROCK inhibitors Y-27632 (Wako Pure Chemical Industries, Tokyo, Japan) or Fasudil (Tocris Bioscience, MO, USA) during the first 24 h of culture. The BrdU incorporation assay was performed using colorimetric BrdU Cell proliferation Kit (Roche Diagnostics) according to the manufacturer's instructions. BrdU incorporations were also observed by immunocytochemical (ICC) staining with BrdU Labeling and Detection Kit I (Roche Diagnostics).
For live–dead assays, dissociated cyES cells were seeded at a concentration of 1500 cells/cm2 onto petri-dishes without feeder layers in the presence of the ROCK inhibitors Y-27632 or Fasudil. Induced apoptosis was determined by propidium iodide (PI) staining and by assessing the mitochondrial transmembrane potential (ΔΨm) using fluorescent probe Rhodamine123 (R123; Sigma–Aldrich). Briefly, total cells were collected after 24 h of culture, washed twice and stained with PI with PI/RNase Staining Buffer (BD Pharmingen, CA, USA) for 15 min or R123 at 10 µg/ml for 30 min. PI was excited by 488 laser light and the emission was detected at 650 nm (long pass filter). R123 was excited by 488 laser light and the emission was detected at 530 nm. Fluorescent intensities were observed with a FACS Caliber and the data were analyzed using Cell Quest (both from Becton Dickinson, CA, USA).
Colony-formation assay on feeder-free cultures
The efficiency of colony formation was determined as the percentage of cyES cell colonies formed and expressed alkaline phosphatase (ALP) activity per number of cyES cells seeded (500 cells per 35 mm-dish). After culture for 3 days, colonies were fixed with 10% formalin and used for ALP assays. For feeder-free cultures, cyES cells were maintained on gelatin-coated plates in the medium conditioned by contact with MEF, as previously reported (Khoo et al., 2005) with 4 ng/ml of human recombinant bFGF. Y-27632 was added to the culture medium at 10 µM only on the day of seeding the cyES cells by passaging, and the medium was changed with ES medium without Y-27632 after 24 h.
For ALP staining, we used an ALP kit (Sigma–Aldrich) according to the instructions provided. Colony forming assays were performed using both cell lines at passage 8–12. Enzymatically digested cyES cells were filtered through a 40-µm mesh to avoid the contamination of feeder cells or cell aggregates of cyES cells before seeding.
Immunocytochemistry for characterization of ES cells
The cyES cell cultures were fixed in 4% paraformaldehyde in PBS (4% PFA; pH 7.4). For ICC staining of OCT-4, fixed ES cells were then permiabilized in 0.5% Triton-X (Sigma–Aldrich) diluted in PBS (0.5% PBT) for 5 min, blocked by incubation in 5% skim milk (Sigma–Aldrich) diluted in PBS for 1 h, and then incubated with primary antibody overnight at 4°C. The antibodies were anti-OCT-4 rabbit IgG polyclonal antibody (Santa Cruz Biotechnology, Inc., CA, USA) diluted 1/100 in 0.1% PBT. For immunostaining of cell-surface molecules, such as SSEA-4, TRA1-60 and TRA1-81, fixed cells were washed with 0.5% BSA (Sigma–Aldrich) in PBS and blocked by incubation in 5% skim milk for 1 h. The antibodies were SSEA-4 mouse monoclonal IgG antibody, TRA1-60 and TRA1-81 mouse monoclonal IgM antibodies (both from Chemicon International, Inc,, CA, USA) diluted 1/100 in 0.5% BSA/PBS. For immunofluorescence microscopy of OCT-4 immunostained samples, sections were incubated for 1 h at room temperature with rhodamine-conjugated bovine anti-rabbit IgG antibody (Santa Cruz Biotechnology) diluted 1/1000 in 0.1% PBT to detect OCT-4. For observation of SSEA-4, samples were incubated for 1 h at room temperature with FITC-conjugated goat anti-mouse IgM antibody or FITC-conjugated goat anti-mouse IgM antibody (Santa Cruz Biotechnology), and for detection of TRA1-60 and TRA1-81, we used Cy3-conjugated goat anti-mouse IgM antibody (Chemicon International) as the secondary antibody. Samples were then washed three times for 10 min each in PBS, and incubated in DAPI (Vector Laboratories, Ltd, Peterborough, England) to counterstain chromosomes. These samples were mounted with VECTORSHIELD mounting medium (Vector Laboratories) to avoid signal reduction.
Chromosome analysis (karyotyping)
The cyES cells at 4 and 12 times passages were arrested in metaphase by addition of colcemid at 0.02 µg/ml (Sigma–Aldrich) to the culture medium for 25 min. the cells were treated with 0.25% trypsin and 0.02% EDTA for 3 min. After vigorous pipetting, the single cell suspension was centrifuged and resuspended in 0.075 M KCL solution at room temperature. After 20 min, the cells were fixed with methanol and acetic acid (3:1) solution. The cells were washed three times prior to spreading on slides. The chromosome slide were prepared by air-drying method, rinsed with distilled water and stained with 0.01 µg/ml Hoechst 33 258 (Sigma–Aldrich) and 5 µg/ml of quinacrine mustard. Stained slides were observed by fluorescent microscope (Leika Microsystems, Wetzlar, Germany).
Induction of teratoma formation
The cyES cells were injected into the peritoneal cavity of severe combined immunodeficient (SCID) mice (Charles River Laboratories Japan, Inc., Kanagawa, Japan). At 30 days post-injection, teratomas were recovered and embedded in Tissue-tek O.C.T freezing compound (Sakura Finetechnical. Co., Ltd, Tokyo, Japan). Cryo-sections of the teratomas were produced using a Cryostat CM1800 (Leica, Heidelberg, Germany) and differentiation capacity evaluated after staining with hematoxylin and eosin (both reagents from Sigma–Aldrich). Evaluations of pluripotency were performed using new cell line Cyk-3, which was established in present study, passaged 14 times by single cell digestion and treated 10 µM Y-27632 for first days of each seeding.
RNA isolation, reverse transcription and quantitative PCR
The cyES cell colonies passaged 8–12 times using 10 µM Y-27632 for first days of each seeding were retrieved mechanically using a glass capillary pipette to avoid the contamination of feeder cells, and stored in liquid nitrogen for analysis. Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. Single-strand cDNA was prepared from total RNA using an oligo-dT primer under standard conditions with Superscript III reverse transcriptase (Invitrogen). The cDNA from each sample was diluted and used for an RT-PCR-based assay for Nanog, Oct-4 and β-actin.
Quantitative real-time PCR with total cDNA was performed using Perfect Real-time SYBR Premix Ex-Taq™ (TAKARA BIO, Inc., Shiga, Japan) and following primer sets (listed 5′ to 3′ in the order of forward primer, reverse primer): Oct-4, ATTTGGGTAGGTGTTCAGCC, CGCCGCTTACTCATGTTCTT; Nanog, TCAGCTACAAACAGGTGAAG, TCACACCATTGCTATTCTTT. PCR amplifications were performed with the 7700 Real-time PCR System (Applied Biosystems, CA, USA) at 95°C for 10 s followed by 40 cycles of 95°C, 5 s; 60°C, 30 s. Reactions were replicated three times for two different samples independently differentiated and prepared. All experiments included negative controls consisting of no cDNA for each primer pair.
Fold changes were calculated relative to control treatment using the 2−delta–deltaCt method (Livak and Schmittgen, 2001). β-Actin expression was used as reference standard to obtain the delta Ct value, and delta–delta Ct values were calculated by subtracting the delta Ct of the calibrator (mean values of Y-27632 treated controls) from that of each sample.
Cryopreservation of cyES cells
Dissociated cyES cells were suspended in DMEM containing 20% KSR and 10% dimethyl sulfoxide (DMSO; Wako Pure Chemical Industries) and stored at −80°C for 2 weeks. As a control experiment, dissociated cyES cells were cryopreserved by vitrification method, i.e. cyES cells were dissociated, centrifuged and plunged directly into liquid nitrogen using DAP213 (2 M DMSO, 1 M acetamide and 3 M propylene glycol) as a cryoprotectant. Thawed cells were washed by ES medium, and cultured on feeder-layer in ES medium supplemented with 10 µM Y-27632. The medium was changed to ES medium without Y-27632 after 48 h. Colony-forming assay was performed by seeding cyES cells on feeder layer at a density of 500 cells per 35-mm dish. After culture for 3 days, colonies were fixed with 10% formalin and used for ALP assays.
Direct induction of NSCs
Dissociated cyES cells (Cyk-3) were placed in a bacterial dish and cultured in 10% fetal calf serum (FCS) supplemented DMEM to induce embryoid body (EB) for 3 days. Y-27632 was added to the culture medium at 10 µM only on the day of each seeding, and the medium was changed to ES medium without Y-27632 after 48 h. The EBs were then dissociated with 0.25% trypsin and 0.02% EDTA for 3 min, seeded on a bacterial dish and cultured in NSC induction medium (NSM) composed with DMEM/F-12 (Sigma), 10 ng/ml of bFGF, 20 ng/ml of epidermal growth factor and insulin transferrin selenium-A solution (ITS-A; Invitrogen) for 6 days (primary stage). After primary induction, the derivatives were dissociated again by collagenase and trypsin, washed and cultured in NSM with N2 supplement (Invitrogen) until aggregates formed (secondary stage). When enlarged spheres were observed, they were dispersed and cultured under the same conditions as outlined above. In order to purify the NSC fractions and prevent contamination of the undifferentiated cyES cells, the treatments were performed at least three times.
To induce neural cells, oligodendrocytes and dopamine producing cells, the spheres of NSCs were transplanted onto gelatin-coated dishes and cultured in IMDM (Invitrogen) supplemented with 20% FCS and 10 ng/ml of bFGF for 7 days.
RT-PCR analysis of Musashi-1 was performed using the following primer sets (listed 5′–3′ in the order of forward primer, reverse primer): Musashi-1, CGAGCTCGACTCCAAAACAATTGACC, TCTACACGGAATTCGGGGAACTGGTA.
ICC staining was performed using anti-Musashi-1 rabbit IgG polyclonal antibody (Abcam, Tokyo, Japan), anti-neuronal Class III β-tublin mouse monoclonal IgG antibody (TuJ; Abcam), anti-O4 rabbit IgG polyclonal antibody (Abcam) and anti-tyrosine hydroxylase rabbit IgG polyclonal antibody (TH; Abcam). Samples were then observed after reaction with FITC-conjugated anti-rabbit IgG or rhodamine-conjugated anti-mouse IgG secondary antibody (both from Santa Cruz Biotechnology). Differentiation studies were performed using both cell lines maintained as above at passage 14.
Western blot analysis
Cultured cells were collected by scraping, homogenized in SDS buffer (4% SDS, 125 mM tris–glycine, 10% β-mercaptoethanol, 2% bromophenol blue in 30% glycerol) then centrifuged at 10 000×g for 10 min at 4°C to remove debris. Aliquots were subjected to polyacrylamide gel electrophoresis in the presence of SDS (SDS–PAGE) followed by electrotransfer onto PVDF membrane (Hybond-P; Amersham Pharmacia Biotech, Buckinghamshire, UK). Molecular size was calibrated with Precision plus protein™ all blue standards (Bio-Rad; Mississauga, ON, Canada). The blotted membranes were blocked overnight with Block ace (Dainippon Sumitomo Pharma, Osaka, Japan), then immuned with objective primary antibody for over-night at 4°C. Antibody incubations and washes were performed in 0.1%Tween-20 in PBS throughout. Detection was realized by enhanced chemiluminescence with an ECL plus western blotting detection system (Amersham Pharmacia Biotech) and horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology). The lumino-labeled membranes were developed using X-Ray Film Processor. Relative expression levels in each sample were quantified by normalizing western blot signals to housekeeping protein β-actin as a ubiquitous component of cytoskeleton.
Statistical analysis of the data
Significant difference was detected by one-way ANOVA analysis followed by Tukey–Kramer multiple comparison tests. A value of P< 0.05 was considered significant.
Results
cyES cell preparation for the experiment
We obtained one female cell line (Cyk-3) from four embryos, and performed further experiments using two cell lines. These cell lines maintained pluripotency, and expressed pluripotent primate ES cell markers such as ALP, Oct-4, SSEA-4, TRA1-60 and TRA1-81.
ROCK inhibitors promote cyES cell survival after single cell digestion, but did not cause cell proliferation
First, we demonstrated the effect of ROCK inhibitors Y-27632 and Fasudil on cultures of cyES cells.
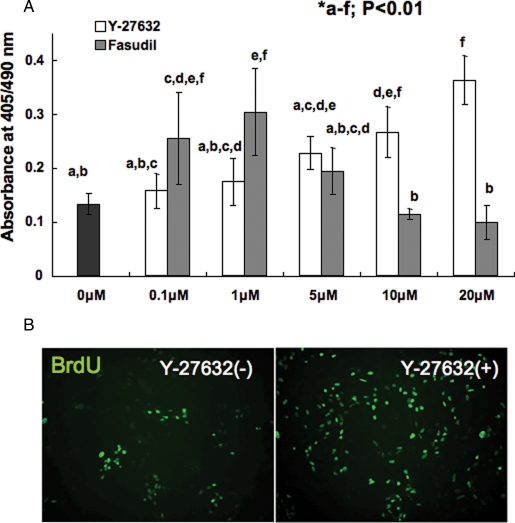
Y-27632 promote the numbers of cells proliferated after passage by single-cell digestion in a dose-dependent manner when observed by BrdU incorporation assay. BrdU-positive cells increased significantly when 10 or 20 µM Y-27632 was added. In contrast, high amounts (5–20 µM) of Fasudil treatment resulted in dose-dependent decrease in proliferated cells (Fig. 1), although Y-27632 did not show toxic effect even when it was added at 60 µM (data not shown). From the experiment, the positive effect of ROCK inhibitors on cyES cell viability and the toxicity of high-dose Fasudil were clearly demonstrated.
Figure 1:
Effect of ROCK inhibitor for cyES cell repopulation after single-cell digestion.
(A) Dose-dependent effect of two kind of ROCK inhibitors Y-27632 and Fasudil. Different characters indicate statistically significant difference (P < 0.01) determined by ANOVA and Tukey–Kramer multiple comparison tests. Data represent the mean ± SD of six experiments. (B) Immunocytochemical observation of BrdU incorporation to cyES cells maintained with/without 10 µM Y-27632.
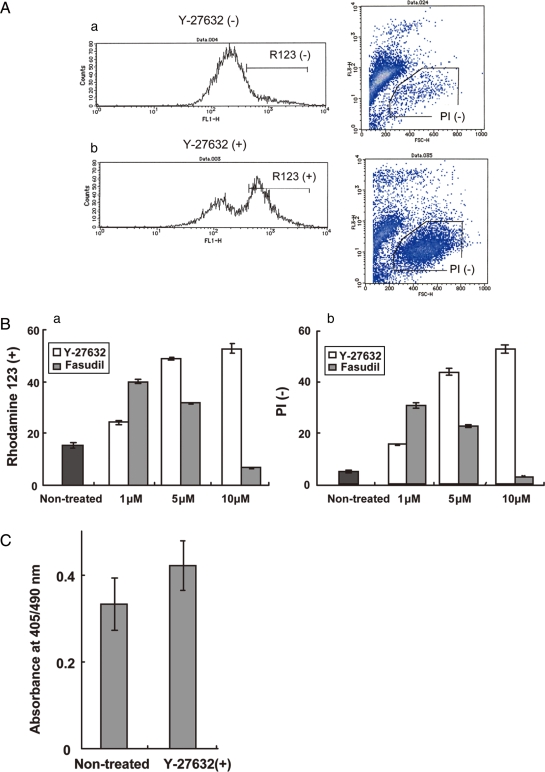
To determine whether the effect of ROCK inhibitors prevent cell death of digested cells or promote proliferation after seeding, we performed live–dead staining and BrdU assay following conventional passages and ROCK inhibitor treatment. The surviving cells were increased at conditions of 1, 5, 10 µM Y-27632 or 1, 5 µM Fasdil additions (Fig. 2A and B). On the other hand, the results of BrdU assay following Y-27632 treatment to attached cyES cells elucidated that the inhibitor did not result promoting cell proliferation but protecting cyES cells from cell death after single cell digestion (Fig. 2C).
Figure 2:
Determination of the effect of ROCK inhibitors for cell surviving and proliferation.
(A) Flow cytometric observation after single-cell digestion and culture without (a) or with (b) Y-27632. Live/dead evaluations were performed using Rhodamine 123 (R123; left) and propidium iodide (PI; right). (B) Percent of live cells on the FACS-based assay using R123 (a) and PI (b). Values represented the percentage of R123 positive or PI negative cells in total cells. Significant differences were observed among all experiment groups (P < 0.01). Data represent the means ± SD of six experiments. (C) BrdU incorporation assay for attached cyES cells passaged by mechanical method. Significant difference was not observed (P < 0.05). Data represent the means ± SD of six experiments.
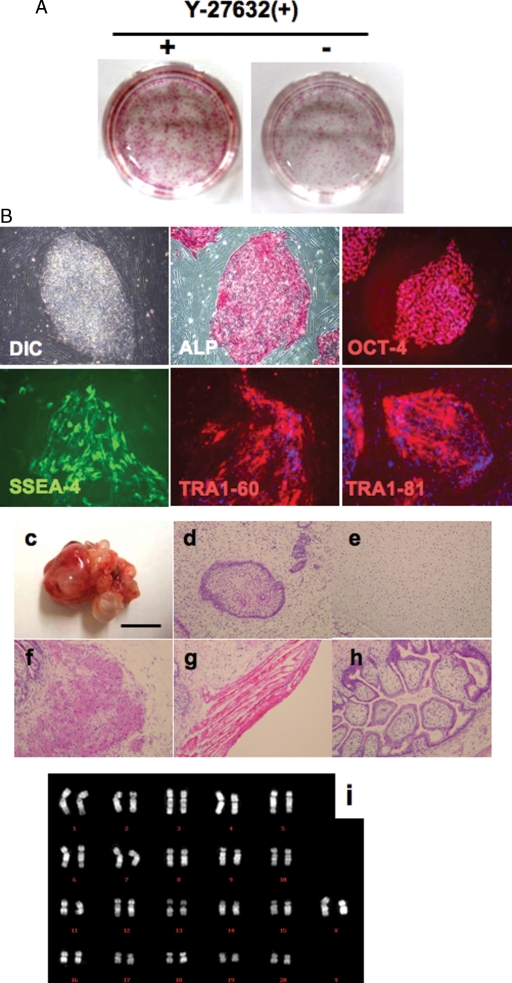
Inhibition of ROCK for a day does not affect the pluripotency of cyES cells
In order to examine whether the undifferentiated state of cyES cells was maintained when they were expanded by single-cell digestion using ROCK inhibitor Y-27632, we determined the expression of primate ES cell markers ALP, OCT-4, SSEA-4, TRA1-60 and TRA1-81. As shown in Fig. 3A and B, these pluripotent state-specific markers were expressed. To further examine the pluripotency of cyES cells treated with Y-27632, we injected the cyES cells into SCID mice. These cells generated teratomas in 4 weeks; histological examination revealed that they consisted of all three germ layers derivatives, including neural tissue, cartilage, muscular tissue and intestine-like structure (Fig. 3C–H). Furthermore, 92% of total cells maintained normal female karyotype for 40XX, and any chromosomal abnormalities such as translocations or inversions were not observed after eight single-cell digestion passages (Fig. 3I). These results suggest that Y-27632 treatment enhances cell survival without affecting their pluripotency and chromosomal normality.
Figure 3:
Evaluation of pluripotency of Y-27632 treated cyES cells (cyES cells).
(A) Low-density culture of dissociated cyES cells in the presence or absence of 10 µM Y-27632 on MEF cells. Almost all colonies were positive for ALP. (B) Characteristics of Y-27632-treated cyES cells (Cyk-3) in undifferentiated state. These figures include immunocytochemical staining of Y-27632-treated cyES cell colonies with anti-OCT-4, anti-SSEA-4, anti-TRA1-60 and anti-TRA1-81 antibodies. (C) Teratoma derived from Y-27632 treated cyES cells. Cells were transplanted into kidney capsule of SCID mouse. Bar = 1 cm. (D–H) Hematoxylin and eosin staining of teratoma derived from cyES cells (Cyk-3). (D) Neural tissue (ectoderm), (E) Cartilage (mesoderm), (F) smooth muscle(mesoderm), (G) skeletal muscle (mesoderm) and (H) intestine-like structure (endoderm). (I) Karyotyping of cyES cells (Cyk-3) passaged after 12 times single-cell digestion passages using Y-27632. DIC, differential interference contrast; ALP, alkaline phosphatase.
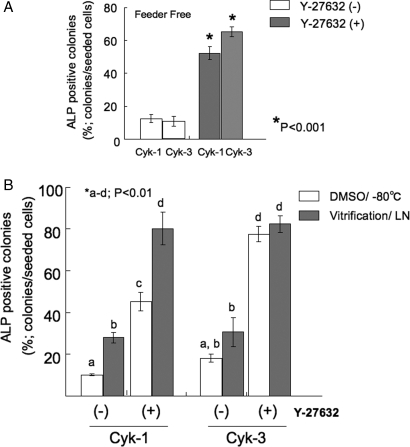
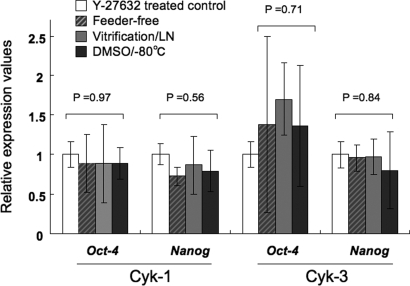
The numbers of colonies were significantly increased in feeder-free cultures by the addition of Y-27632 (Fig. 4A). Same results were observed in different two cell lines Cyk-1 and Cyk-3, Oct-4 and Nanog mRNA expressions were also maintained after five times passaging without feeder cells (Fig. 5).
Figure 4:
Effect of Y-27632 treatment to cyES cell colony formation.
(A) Number of ALP-positive colonies cultured in the absence/presence of 10 µM Y-27632 without feeder cells (*P < 0.001, n = 3). (B) Number of ALP-positive colonies cultured in the absence/presence of 10 µM Y-27632 after cryopreservation by DMSO-added cryoprotectant or vitrification method using DAP213 (*P < 0.01, n = 3).
Figure 5:
Quantitative RT-PCR-based evaluation of pluripotency-related genes Oct-4 and Nanog.
The relative expression values were calculated by delta–delta Ct method using the mean value of Y-27632 treated controls as a calibrator (control). The between group differences were evaluated by ANOVA. cyES cells cultured in Y-27632 supplemented ES medium, white rectangles; cyES cells cultured in Y-27632 supplemented and feeder-free condition, striped rectangles; cyES cells expanded after cryopreservation by vitrification method and stored in liquid nitrogen (LN), shaded rectangles; cyES cells expanded after cryopreservation using DMSO and stored at −80°C.
cyES cells were successfully cryopreserved by the addition of Y-27632
The addition of Y-27632 enables cyES cells to survive after cryopreservation with DMSO and −80°C conditions. Approximately 45–80% of cryopreserved cells survived in Cyk-1 and Cyk-3. No significant differences were observed between DMSO/−80°C storage and vitrification in the case of Cyk-3. In contrast, significantly fewer colonies formed when the cells treated without Y-27632. In total, 10–30% cells survived and formed colonies (Fig. 4B).
Real-time PCR analysis revealed that the expression of pluripotency-related genes, Nanog and Oct-4, were maintained in both cases (Fig. 5).
NSC induction from cyES cells
We induced NSCs using Cyk-3 maintained in Y-27632-added medium. In some cases, ES-cell differentiation induction was performed through EB formation, which is a spontaneously aggregated structure mimicking the developing embryo. In mice, EBs were induced by non-adherent culture of dispersed cyES cells.
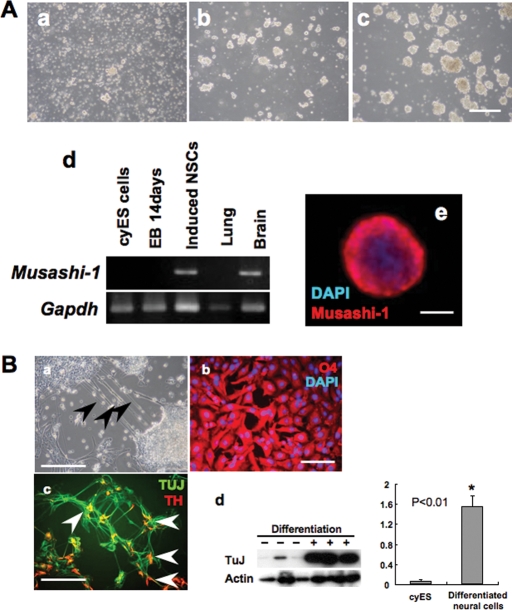
In the present study, Y-27632 dramatically improved cell aggregation and enlargement in the primary stage. On the other hand, almost no aggregates were obtained if the cells were induced differentiation without Y-27632 (data not shown). The induced NSCs expressed NSC marker Musashi-1 in the undifferentiated state. Furthermore, multiple differentiation potentials of cells were determined by differentiation induction to neural cells, oligodendrocytes and dopamine-producing cells. The differentiated cells expressed TuJ as a neural cell marker, TH as a dopamine-producing cell marker, and O4 as an oligodendrocyte marker. Significant increase in the expression of TuJ was also determined observed by western blot analysis (Fig. 6B-d).
Figure 6:
Neural cell determination by neurosphere method from Y-27632-treated cyES cells and EBs. (A) Differentiation induction of neural stem cells by neurosphere method. (a) Dispersed cells were cultured in NSM. On Day 3, formation of cell aggregates was observed (b). These aggregates were enlarged on Day 6. Bar = 200 µm (c). Note that the sphere was positive for the neuronal marker Musashi-1 (d). (e) Immunostaining of the neurosphere with anti-Musashi-1 antibody. Bar = 50 µm. (B) Immunostaining of cyES cell-derived neural cells following attachment of the neurosphere. (a) Phase-contrast imaging of neural cells. Arrows, neurofilament extended from the ES-derived neurosphere. Bar = 200 µm. (b,c) Induced neural cells derived oligodendrocytes (evaluated with lineage-specific marker O4, bar = 50 µm) and neuron (evaluated with lineage-specific marker TUJ, bar = 100 µm). The function of neural cells was determined by catecholamine biosynthesizing enzyme TH (arrows). (d) Western blot-based quantification of TuJ expression and its relative expression ratio to β-actin (C).
Discussion
Here, we demonstrated that Y-27632 promotes survival of cyES cells, and it enabled expansion from single cells without losing pluripotent characteristics. The cyES cells maintained the expression of undifferentiated markers ALP, OCT-4, SSEA-4, TRA1-60 and TRA1-81, and represented the potential of teratoma formation, which consisted with all three embryonic germ layers. These results were consistent with the results of hES cells, suggesting that reactions to the chemical might be preserved in primates. Furthermore, Y-27632 supplementation enables cyES cell expansion also in feeder-free culture. The cyES cells are traditionally cultured on feeder cells derived from mouse fetus. Feeder cells provide secreted factors, extracellular matrix, and cellular contacts for the maintenance of ES cells in the undifferentiated state without losing pluripotency; however, feeder cells may possess a risk of cross-contamination, such as passing animal pathogens to hES cells. Feeder-free stem cell culture is a system for maintaining stem cells in the undifferentiated state without the need for direct contact with feeder cells, and is expected as a prominent method for hES cell-based therapy. The result, that chemical additions dramatically improved the rate of colony formation in feeder-free culture suggests that primate ES cell adhesion and proliferations, which were brought about by animal sources, might be alternatively complemented by the chemical, and it might be an important factor to realize the establishment of fully chemically defined culture systems.
To apply ES cell technology to practical medicine, passaging or differentiation induction procedures should be simple and easily reproducible, since all procedures relating to medicine are needed to be performed in accordance with the international cGMP (Current Good Manufacturing Practice) and GTP (Good Tissue Practice) guidelines. However, some differentiation protocols were performed through EB formation, and primate EBs are ordinarily induced by hanging drop (Galli et al., 2003; Fujioka et al., 2004), which includes handling procedures, and may induce the risk of contamination. In the present study, we examined direct differentiation induction of NSCs by single-cell digestion and suspension culture in a bacterial dish using Y-27632.
NSCs are self-renewing, multipotent cells that generate neurons, astrocytes, and oligodendrocytes (Gage, 2000; Geschwind et al., 2001). They have great potential as a therapeutic tool for the repair of a number of central nervous system (CNS) disorders. Potential targets of NSC transplants include stroke, spinal cord injury, and neurodegenerative diseases, such as Parkinson's disease (Imitola et al., 2004; Takagi et al., 2005; Sharma et al., 2007). However, the technical and ethical difficulties in obtaining sufficient and appropriate donor tissue have limited the application of this therapy, and they are an inadequate source of dopamine-synthesizing neurons because their ability to generate these neurons is unstable. Neural precursor cells from ES cells are expected to be candidates for use as potential donor cells for transplantation (Barberi et al., 2003; Ben-Hur et al., 2004; Keirstead et al., 2005; Takagi et al., 2005; Fukuda et al., 2006). Y-27632 supplementation was effective for cyES cell survival also under differentiation conditions, and the number of aggregates dramatically increased. NSCs were obtained in the secondary induction stage, in which floating cell clusters called neurospheres were found. These spheres expressed undifferentiated NSC marker Musashi-1 in the unattached state. They differentiated by adhesion to a gelatin-coated dish, giving rise to neurons, oligodendrocytes and dopamine-producing cells. Spheres passaged five times also retained multilineage potential (data not shown). These results suggest that the NSCs that induced the present systems were functional, and Y-27632 was a superior supplement, not only for the maintenance of pluripotent ES cells, but also for the induction of EBs.
Another feasible result of Y-27632 supplementation was that it enabled cryostorage by the conventional method using DMSO in −80°C conditions, as for mES cells. Ordinarily, primate ES cells could be cryopreserved when treated as cell clumps and stored by vitrification in liquid nitrogen. On the other hand, only 0.4% of cells survived if stored by standard slow-rate cooling methods using DMSO (Fujioka et al., 2004). Interestingly, significant improvement was observed in the rates of colony formation from cryopreserved cells when cells were treated with Y-27632. In the case of Cyk-3, a significant difference was not observed between the experimental group using DMSO and vitrification when supplemented with Y-27632, and almost all cells survived and formed colonies (∼80% of total cells) in both methods. Cryostorage using DMSO and Y-27632 enables easier and safer shipping of primate ES cells between research facilities. Furthermore, it may avoid the many risks considered in a original method. Liquid nitrogen storage was problematic in itself, because tank insulation was inefficient and there was a potential risk for microbial contamination such as hepatitis B when stored together with contaminated samples (Chen et al., 2006).
In conclusion, we demonstrated the effectiveness of Y-27632 supplementation to primate ES cell culture, differentiation induction and cryostrage. The present result that cyES cells showed the same reactions to the chemical as hES cells might provide further evidence that cyES cells would be superior models for hES cells. This technology may accelerate translation of the method developed using mES cells to preclinical stages.
Acknowledgements
We thank Dr Miki Sugimoto, Kyoto University, for histological assessment of HE stained sections of teratomas. We also thank Dr Kazuo Goto, Central Institute for Experimental Animals for karyotyping. And we gratefully acknowledge Ms Naomi Backes Kamimura, Department of Biology Oriented Science and Technology, Kinki University, for English editing.
References
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Idelson M, Khaner H, Pera M, Reinhartz E, Itzik A, Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- Cameron CM, Hu WS, Kaufman DS. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94:938–948. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- Chen HI, Tsai CD, Wang HT, Hwang SM. Cryovial with partial membrane sealing can prevent liquid nitrogen penetration in submerged storage. Cryobiology. 2006;53:283–287. doi: 10.1016/j.cryobiol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Daheron L, Opitz SL, Zaehres H, Lensch WM, Andrews PW, Itskovitz-Eldor J, Daley GQ. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Yasuchika K, Nakamura Y, Nakatsuji N, Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2004;48:1149–1154. doi: 10.1387/ijdb.041852tf. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Takahashi J, Watanabe K, Hayashi H, Morizane A, Koyanagi M, Sasai Y, Hashimoto N. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006;24:763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells: an overview. Circ Res. 2003;92:598–608. doi: 10.1161/01.RES.0000065580.02404.F4. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Ou J, Easterday MC, Dougherty JD, Jackson RL, Chen Z, Antoine H, Terskikh A, Weissman IL, Nelson SF, et al. A genetic analysis of neural progenitor differentiation. Neuron. 2001;29:325–339. doi: 10.1016/s0896-6273(01)00209-4. [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, et al. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269:360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Haruta M, Sasai Y, Kawasaki H, Amemiya K, Ooto S, Kitada M, Suemori H, Nakatsuji N, Ide C, Honda Y, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci. 2004;45:1020–1025. doi: 10.1167/iovs.03-1034. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R. Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N Engl J Med. 2003;349:275–286. doi: 10.1056/NEJMra035397. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplantsremyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo ML, McQuade LR, Smith MS, Lees JG, Sidhu KS, Tuch BE. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: effect of glucose and basic fibroblast growth factor. Biol Reprod. 2005;73:1147–1156. doi: 10.1095/biolreprod.104.036673. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Sharma R, McMillan CR, Niles LP. Neural stem cell transplantation and melatonin treatment in a 6-hydroxydopamine model of Parkinson's disease. J Pineal Res. 2007;43:245–254. doi: 10.1111/j.1600-079X.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Suemori H, Nakatsuji N. Generation and characterization of monkey embryonic stem cells. Methods Mol Biol. 2006;329:81–89. doi: 10.1385/1-59745-037-5:81. [DOI] [PubMed] [Google Scholar]
- Sumi T, Fujimoto Y, Nakatsuji N, Suemori H. STAT3 is dispensable for maintenance of self-renewal in nonhuman primate embryonic stem cells. Stem Cells. 2004;22:861–872. doi: 10.1634/stemcells.22-5-861. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramura T, Takehara T, Kawata N, Fujinami N, Mitani T, Takenoshita M, Matsumoto K, Saeki K, Iritani A, Sagawa N, et al. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem Cells. 2007;9:144–156. doi: 10.1089/clo.2006.0070. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet. 2007;39:295–302. doi: 10.1038/ng1973. [DOI] [PubMed] [Google Scholar]