Abstract
The aims of the present study were to compare the levels of mRNA and protein expression of matrix metalloproteinase (MMP)-1, -3, -8 and -9 in human cervical tissue in preterm and term labor as well as not in labor and to determine if corticotropin-releasing hormone (CRH) has an effect on MMP-1, -3 and interleukin (IL)-8 secretion in both preterm and term cervical fibroblasts. Cervical biopsies were taken from 60 women: 18 at preterm labor, 7 at preterm not in labor, 18 at term labor and 17 at term not in labor. ELISA and Immulite were used for protein and real-time RT–PCR for mRNA analysis. Cervical fibroblast cultures were incubated for 18 h with different CRH concentrations (10−13–10−6 M). The mRNA expression of MMP-1, -3 and -9 was higher in laboring groups compared with term not in labor. Protein levels of MMP-8 and -9 were higher in term in labor group compared with non-laboring groups. There were no significant differences in mRNA and protein expression between the preterm and respective term control groups. CRH significantly increased secretion of IL-8 in preterm and term cervical fibroblasts compared with controls. The secretion of IL-8 and MMP-1 was significantly higher and MMP-3 secretion lower in preterm cervical fibroblasts. In conclusion, cervical ripening at preterm seems to be a similar inflammatory process as at term with CRH involved. However, preterm and term cervical fibroblasts might have different phenotypes based on different secretion patterns of IL-8, MMP-1 and MMP-3.
Keywords: cervical ripening, cytokines, extracellular matrix, matrix metalloproteinases, preterm birth
Introduction
Preterm birth (PTB) is the leading cause of neonatal morbidity and mortality (Saigal and Doyle, 2008). Despite the several strategies for treatment, the frequency of PTB has not changed significantly during the past three decades and the mechanisms underlying the initiation of both preterm and term labor remain largely unknown (Challis et al., 2000; Hertelendy and Zakar, 2004; Iams et al., 2008). Uterine contractions must be coordinated with a softening of the cervix for a successful delivery. Cervical softening involves intensive remodeling of the extracellular matrix (ECM), with extensive changes in concentration and composition of collagens (Uldbjerg et al., 1983; Ekman et al., 1986) and proteoglycans (Norman et al., 1993; Westergren-Thorsson et al., 1998). Cytokines and several other mediators, including estrogen, progesterone, prostaglandins and nitric oxide, participate in the remodeling of ECM and ripening of the human cervix (Stjernholm et al., 1997; Abelin Tornblom et al., 2002; Hertelendy and Zakar, 2004; Tornblom et al., 2005b). This process can be regarded as an inflammatory reaction associated with elevated levels of cytokines at the time of both preterm and time labor (Sennstrom et al., 2000; Tornblom et al., 2005a). Interleukin (IL)-6 and IL-8 increase at least 100-fold at term labor (Sennstrom et al., 2000). IL-8 promotes recruitment and activation of neutrophils, which in turn secrete proteolytic enzymes, such as matrix metalloproteinase (MMP)-8 and MMP-9 (Osmers et al., 1995; Stygar et al., 2002). The MMPs play a central role in the degradation of ECM components. The levels of MMPs in the cervix, lower uterine segment, amniotic fluid, fetal membranes and maternal plasma increase at labor time (Tu et al., 1998; Winkler et al., 1999; Stygar et al., 2002; Sennstrom et al., 2003; Weiss et al., 2007). Polymorphism in MMP-1 and MMP-9 genes has been shown to be associated with preterm premature rupture of membranes (Ferrand et al., 2002; Fujimoto et al., 2002).
Corticotropin-releasing hormone (CRH) is the principal regulator of the hypothalamic–pituitary–adrenal (HPA) axis. The HPA axis has been of interest in connection with labor since Liggins induced premature parturition in sheep with corticotropin and cortisol infusion (Liggins, 1968). CRH is produced by fetomaternal tissues and secreted into the maternal circulation, so that during pregnancy the maternal plasma levels of this hormone increase, while the corresponding binding protein (CRH-BP) levels are reduced (Linton et al., 1993; Hillhouse and Grammatopoulos, 2002). Patients at risk for PTB have significantly elevated plasma levels of CRH and lower CRH-BP levels (Hobel et al., 1999).
CRH, CRH-BP and the receptors for CRH, CRH-R1 and CRH-R2 are expressed in human placenta, deciduas, fetal membranes, endometrium and myometrium (Kalantaridou et al., 2007). Furthermore, CRH increases MMP-9 protein secretion by cultured cells from placenta and fetal membranes (Li and Challis, 2005). In our previous study we have localized CRH, CRH-BP, CRH-R1 and CRH-R2 in the cervix, which suggested that the cervix may be a target for CRH action (Klimaviciute et al., 2006). Therefore, our present aim was to determine if CRH has an effect on the secretion of IL-8, MMP-1 and MMP-3 in both preterm and term cervical fibroblasts. Furthermore, our aim was to compare the levels of mRNA and protein expression of MMP-1, MMP-3, MMP-8 and MMP-9 in human cervical tissue in preterm and term labor as well as not in labor.
Materials and Methods
Patients
A total of 51 women were included in the study for the analysis of MMP expression. Two preterm groups consisted of 14 women undergoing preterm labor (PTL) and 7 women delivered preterm by Caesarean section prior to the onset of labor (PTnotL). Preterm delivery was defined as delivery before the 37th week of gestation. Two control term groups included 13 women undergoing normal term labor (TL) and 17 women at term not in labor delivered by elective Caesarean section (TnotL). The labor groups (PTL and TL) were in active labor and demonstrated a ripe cervix, with dilatation more than 4 cm. They were either delivered vaginally or by emergency Caesarean section due to malpresentation (at preterm and at term) or due to threatening fetal asphyxia (at term). In all patients delivered by Caesarean section, the assessment of cervical dilatation was established immediately before surgery. Women not in labor (PTnotL and TnotL) had unripe cervices (with a Bishop score of <5 points) and were delivered by Caesarean section prior to the onset of labor. The preterm indications were suspected placental abruption or intrauterine growth retardation and the term indications were breech presentation, humanitarian or disproportion.
Cervical biopsies from an additional nine women (four PTL and five TL) were used for the cultures of cervical fibroblasts.
None of the women included in the study suffered from pre-eclampsia, diabetes or other systemic disease, nor did any of our subjects demonstrate clinical signs of infection in connection with parturition or during the post-partal period.
There were no significant differences between these groups of women with respect to maternal age, parity, previous PTBs and Caesarean sections. Moreover, there was no significant difference in gestational age between the groups PTL and PTnotL or TL and TnotL. Clinical data on the women is presented in Table I.
Table I.
Clinical data on women included in the study.
| Parameter | mRNA and protein analysis in the cervical tissue |
Cervical fibroblasts |
||||
|---|---|---|---|---|---|---|
| Preterm in labor (PTL) | Term in labor (TL) | Preterm not in labor (PTnotL) | Term not in labor (TnotL) | PTL | TL | |
| n | 14 | 13 | 7 | 17 | 4 | 5 |
| Age | 28 (18–37) | 28 (20–36) | 32 (27–35) | 30 (25–39) | 31 (30–36) | 29 (23–31) |
| Parity | 1 (0–3) | 1 (0–2) | 1 (0–2) | 0 (0–3) | 0 (0–1) | 1 (0–2) |
| Previous preterm births in the group | 1 | 0 | 1 | 2 | 0 | 0 |
| Previous Caesarian section | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–2) | 0 | 0 |
| Previous Caesarian sections in the group | 3 | 2 | 0 | 4 | 0 | 0 |
| Gestational age in fgwa | 33 (26–36) | 40 (37–42) | 27 (26–36) | 38 (37–39) | 30 (25–35) | 40 (38–40) |
| Gestational age in days | 238 (184–255) | 283 (259–294) | 193 (183–256) | 270 (260–278) | 214 (176–247) | 280 (272–282) |
| Treatment with corticosteroids | 4 | 0 | 4 | 0 | 2 | 0 |
Data are presented as median (range) if not otherwise stated. afull gestational weeks.
Prior to the performance of this study, the approval of the local Ethics Committee of Karolinska Institute (Ref. no. 97-089 and 04-637/4) and the informed consent of each subject was received.
Sampling procedure
Immediately following vaginal delivery and Caesarean section, a biopsy was taken transvaginally (at the 12 o'clock position) from the anterior cervical lip with scissors and tweezers. The samples to be utilized for mRNA or protein analysis were immediately frozen and stored thereafter at −70°C. The biopsies to be used for the cultures of cervical fibroblasts were immersed in GIBCO™ RPMI 1640 Medium (Invitrogen Corporation, Paisly, Scotland, UK) and stored in refrigerator for a maximum of 24 h before proceeding to the establishment of cell cultures.
The limited amount of tissue available from each woman did not allow performance of all of the different analyses on each individual sample.
Tissue homogenization and extraction of RNA
Tissue homogenization was carried out by the help of a dismembranation apparatus (Retsch KG, Haan, Germany) and followed by either RNA or protein extraction.
Total RNA was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) as described previously (Tornblom et al., 2005a).
Treatment with DNase and reverse transcription
The concentration of total RNA obtained was determined on the basis of the OD260 using a DU64® spectrophotometer (Beckman, Palo Alto, CA, USA) and only samples exhibiting an OD260/OD280 ratio higher than 1.5 were used for RT–PCR. 2 µg total RNA, pre-treated with 2 µl RQ1 RNase-Free DNase (Promega, Madison, WI, USA), was used for RT reaction, which was performed using SuperScript™ RNase H− Reverse Transcriptase (Invitrogen) as described elsewhere (Tornblom et al., 2005a). The cDNA was stored at −70°C prior to use.
Real-time RT–PCR
The levels of mRNA encoding MMP-1, MMP-3, MMP-8 and MMP-9 were quantified by real-time RT–PCR employing the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). All determinations were performed in triplicate in Taqman Universal PCR Master Mix (Applied Biosystems) on 96-well optical PCR plates. Appropriate primers and probes were purchased from commercially available Taqman® gene expression assays (Applied Biosystems). Two different Taqman® gene expression assays were used for mRNA detection of MMP-8. Primers and probes in these two assays detect different sequences. The second assay was used to confirm unexpected results from the first assay. Assay IDs and GenBank accession numbers are presented in Table II. For each reaction, 5 µl diluted cDNA (corresponding to 20 ng total RNA), 12.5 µl Universal Master Mix, 1.25 µl assay mixture and 18.75 µl sterile water was used. Real-time PCR reaction was carried out according to a standard manufacturer protocol involving 40–45 cycles of denaturation–annealing. The threshold cycles (CT), at which an increase in reporter fluorescence above the baseline signal could first be detected, were determined. 18s and β-actin were used as endogenous controls. The geometric mean of two endogenous controls was used for normalization of the mRNA levels for the gene of interest (Vandesompele et al., 2002). The geometric mean was subtracted from the respective gene, giving the ΔCT as a reflection of the relative mRNA expression. Since a higher ΔCT corresponds to a lower mRNA expression, the ΔCT values are presented inverted as 100/ΔCT. Serial dilutions of placental cDNA made from purchased total RNA (Ambion, Austin, TX, USA) were used for validation of the experiment.
Table II.
Assay IDs and GenBank accession number for gene expression assays used for real-time RT–PCR.
| Gene | Assay ID | GenBank accession number |
|---|---|---|
| MMP-1 | Hs00233958_m1 | NM_002421.2 |
| MMP-3 | Hs00233962_m1 | NM_002422.3 |
| MMP-8a | Hs01029057_m1 | NM_002424.1 |
| MMP-8a | Hs00233972_m1 | NM_002424.1 |
| MMP-9 | Hs00234579_m1 | NM_004994.2 |
| 18sb | 4319413E | X03205.1 |
| β-actinb | 4352935E | NM_001101.2 |
aTwo different expression assays were used for mRNA detection of MMP-8; b18s and β-actin were used as endogenous controls.
Protein extraction
Following the tissue homogenization, 1 ml of phosphate-buffered saline (PBS), including 0.01% Triton X-100, was added. After centrifugation at 10 000g, 4°C for 10 min, supernatant was retrieved and stored in aliquots at −70°C until analyzed.
Total protein concentration was determined using a BCA protein assay kit (Pierce Chemical Co., Rockford, IL, USA) according to the manufacturer's instructions.
Determination of the protein levels of MMPs
The concentrations of all MMPs in the supernatants were determined employing Amersham Matrix metalloproteinase Biotrak ELISA system (GE Healthcare, Amersham Place, Buckinghamshire, UK) according to the manufacturer's instructions. The assays detect the levels of total MMP-1, -3, -8 and -9. The results were interpolated from the standard reference curve provided with each kit. The sensitivity of kits was 1.7 ng/ml for MMP-1, 2.35 ng/ml for MMP-3, 0.032 ng/ml for MMP-8 and 0.6 ng/ml for MMP-9. The concentrations of MMP-1, -3, -8 and -9 were normalized against the total protein concentration.
Cell culture establishment
Cervical fibroblasts were established from the cervical biopsies obtained directly after vaginal delivery. The biopsies were rinsed in Earle's Minimal Essential Medium (EMEM), cut into pieces of ∼1 mm3, and allowed to adhere to the bottom of the culture chamber at 37°C and 5% CO2, for 4 h before addition of EMEM with penicillin (1%), streptomycin (1%), glutamine (1%) and donor calf serum (10%). Explants were incubated for 2–3 weeks until confluent fibroblast cultures were obtained. Sub-cultures of cells were done by trypsinization according to standard procedures giving rise to two separate but identical preparations for further experiments. All experiments were done with cells in passages between 3 and 5.
Phenotype of the fibroblasts was identified morphologically and employing three fibroblast markers: vimentin (sc53464, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-fibroblast surface protein antibody 1B10 (F4771, Sigma, St Louis, Missouri, USA) and prolyl-4-hydroxylase beta (AF0910-1, Acris Antibodies GmbH, Hiddenhausen, Germany). The cells were fixed with 4% formaldehyde for 10 min, permeabilized with 0.2% Triton X-100 in PBS for 10 min, and unspecific binding was blocked with a 2% solution of bovine serum albumin in PBS for 1 h. The cells were then incubated with the primary antibodies (1:100 in blocking buffer) overnight at 4°C. Alexa Fluor 488-conjugated or Alexa Flour 594-conjugated antibodies, raised in goat and directed against immunoglobulins of the species used to raise the primary antibody, were used for detection of the bound primary antibodies (1:200, 1 h). Between each step the cells were carefully rinsed with PBS. A light-scanning microscope was used to examine the cells.
Stimulation experiments
Cervical fibroblasts were seeded on 12-well cell culture plates and grown until confluence, usually after 3 days. Cells were stimulated for 18 h with CRH (Sigma) at concentrations ranging between 10−6 and 10−13 M and non-stimulated control cells were cultured in parallel. All concentrations were performed in duplicate. Media samples were collected and frozen until analysis. MMP-1, MMP-3 and MMP-9 were quantified using ELISA as described earlier. Quantification of IL-8 was performed employing IMMULITE Automated Analyser (Diagnostic Products Corp., Los Angeles, CA, USA), using the commercially available immulite chemiluminescent enzyme immunometric assay (Immulite®, Diagnostic Products Corp.) according to the manufacturer's instructions. The sensitivity of the assay was 2 pg/ml. The measured MMP-1, MMP-3 and IL-8 concentrations were normalized to the total amount of protein in corresponding cell layer, as determined using a BCA protein assay kit (Pierce Chemical Co.).
Statistical analysis
Comparisons between two independent groups were performed utilizing the Mann–Whitney U-test. When more than two groups were compared, the Kruskal–Wallis test was applied, followed by multiple comparison with Bonferroni–Dunn correction. For the comparison of the results in the stimulation experiments, the Friedman ANOVA and Kendall's concordance and the Wilcoxon matched pair test were employed. In all cases, a P-value of <0.05 was considered to be statistically significant. All calculations were performed with the STATISTICA 8.0 software (StatSoft Inc, Tulsa, OK, USA).
Results
The mRNA expression of MMP-1, -3, -8 and -9 in the cervical tissue
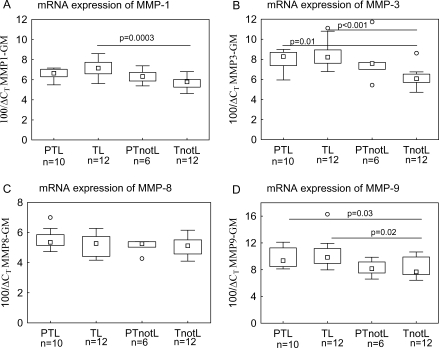
The mRNA expression of MMP-1, MMP-3 and MMP-9 was significantly higher in the term labor compared with term not in labor group (Fig. 1A, B and D). The preterm labor group showed a similar pattern and this difference reached statistical significance for MMP-3 and MMP-9. There were no significant differences between the preterm and respective term control groups. There were no differences between the groups in MMP-8 mRNA expression and the results were the same with two different Taqman® gene expression assays (Fig. 1C).
Figure 1:
The mRNA expression of MMP-1 (A), MMP-3 (B), MMP-8 (C) and MMP-9 (D) in the cervical tissue.
CT, the threshold cycle at which an increase in reporter fluorescence above the baseline signal is first detected. The mRNA levels are normalized using geometric mean (GM) of two endogenous controls (18s and β-actin). The studied groups are: preterm labor (PTL), preterm not in labor (PTnotL), term labor (TL), term not in labor (TnotL). The box represents median value with 25–75% of all data falling within the box. The whiskers extend to the non-outlier range. The outliers are marked as circles. The number of samples analyzed in each group is shown under the group name. Statistically significant differences are indicated above the plots.
Protein levels of MMPs in the cervical tissue
MMP-8 and MMP-9 were detected in all the samples. The laboring groups had higher concentrations of MMP-8 and MMP-9, where the difference was statistically significant between the term in labor and not in labor groups (Fig. 2). There were no significant differences between the preterm and respective term control groups.
Figure 2:
Protein levels of MMP-8 (A) and MMP-9 (B) in the cervical tissue.
The protein levels are normalized to the total amount of protein. The studied groups are: preterm labor (PTL), preterm not in labor (PTnotL), term labor (TL), term not in labor (TnotL). The box represents median value with 25–75% of all data falling within the box. The whiskers extend to the non-outlier range. The outliers are marked as circles. The number of samples analyzed in each group is shown under the group name. Statistically significant differences are indicated above the plots.
The concentrations of MMP-1 and MMP-3 were generally low in all the samples. MMP-1 was below detection limit in 25 samples and MMP-3 was undetected in 9 samples. There were no significant differences in MMP-1 and MMP-3 concentrations between the groups (data not shown).
Stimulation of cervical fibroblasts
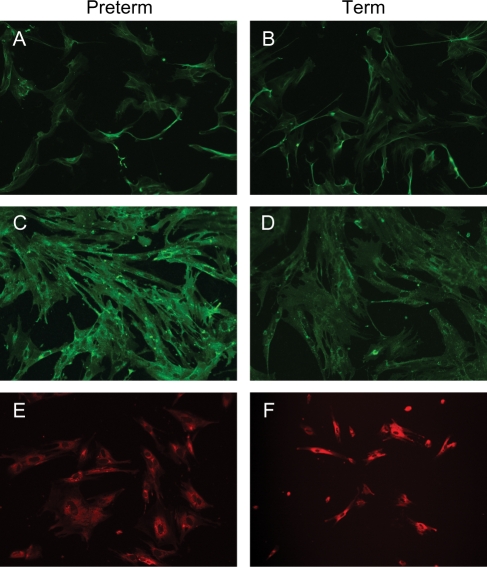
Cell cultures from preterm and term cervical biopsies stained positively for all three fibroblast markers (Fig. 3).
Figure 3:
Immunostaining of cell cultures for fibroblast markers.
Cell cultures from preterm (left column) and term (right column) cervical biopsies were immunostained using antibodies directed against vimentin (A and B), fibroblast surface marker 1B10 (C and D) and prolyl-4-hydroxylase beta (E and F).
IL-8, MMP-1 and MMP-3 were detected in all culture medium samples of cervical fibroblasts. MMP-9 was not detected in any of the samples.
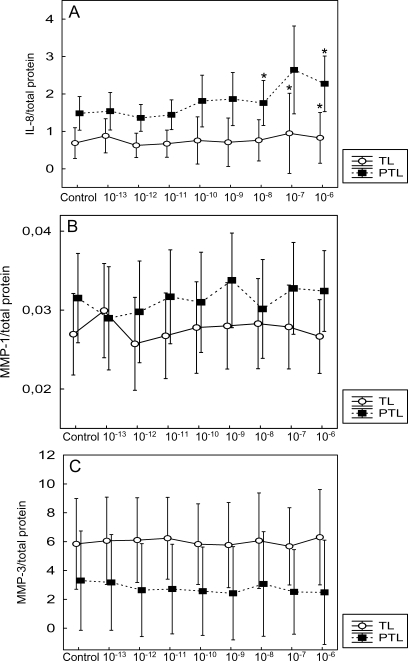
CRH significantly increased the secretion of IL-8 from the term and preterm cervical fibroblasts at concentrations 10−7–10−6 and 10−8, 10−6 M, respectively, compared with controls (Fig. 4A). The levels of MMP-1 and MMP-3 were not significantly changed by CRH (Fig. 4B and C).
Figure 4:
The effect of CRH on secretion of IL-8 (A), MMP-1 (B) and MMP-3 (C) by preterm (PTL) and term (TL) cervical fibroblasts.
The cervical fibroblasts were incubated with different CRH concentrations for 18h and the levels of IL-8, MMP-1 and MMP-3 determined in the culture medium. The protein levels are normalized to the total amount of protein in the corresponding cell layer. Different concentrations (M) of CRH are indicated on the x-axis. Data is presented as mean ± SD. *P < 0.05 (compared with control). The secretion of IL-8 and MMP-1 was higher in preterm cervical fibroblasts (P < 0.001 and P < 0.05, respectively). MMP-3 secretion was higher in term cervical fibroblasts (P < 0.001).
Furthermore, preterm and term cervical fibroblasts showed different secretion patterns of IL-8, MMP-1 and MMP-3. This difference was statistically significant when the whole preterm group was compared with the term group. The secretion of IL-8 and MMP-1 was higher in the preterm cervical fibroblasts (P < 0.001 and <0.05, respectively). However, MMP-3 secretion was higher in the term cervical fibroblasts (P < 0.001) (Fig. 4A–C).
Discussion
In the present study, we have demonstrated for the first time, that the mRNA and protein expression of several MMPs increase in cervix both at preterm and term labor. This finding together with our earlier studies on CRH and cytokines in preterm cervix (Tornblom et al., 2005b; Klimaviciute et al., 2006) indicates that preterm cervical ripening is the same inflammatory process as the cervical ripening at term.
In this study, we also analyzed the effect of CRH on the secretion of MMPs and IL-8 in the cervical fibroblasts. To our knowledge, this is the first such kind of study on the preterm and term cervical fibroblasts. We have shown that CRH increased the secretion of IL-8 in both preterm and term cervical fibroblasts, but did not affect the secretion of MMP-1 and MMP-3. MMP-9 was not detected in the culture medium, which confirms the earlier studies that MMP-9 is not produced by cervical fibroblasts (Ledingham et al., 1999; Stygar et al., 2002). IL-8 is an important mediator of cervical ripening (Osmers et al., 1995; Sennstrom et al., 1997). The fact that CRH and its receptors are localized in the cervix (Klimaviciute et al., 2006) and that it has an effect on IL-8 secretion from cervical fibroblasts points towards the possible role of CRH in the process of the cervical ripening. Several studies have shown that CRH can stimulate the production of proinflammatory cytokines in different cells (Angioni et al., 1993; Yang et al., 2005; Wang et al., 2007), although some studies present contradicting results (Angioni et al., 1993; Sehringer et al., 2000). The possible mechanism by which CRH induces IL-8 production in the cervical fibroblasts could be a selective activation of the p38/MAPK signaling pathway (Wang et al., 2007). However, CRH could be involved in several different signaling pathways, like reactive oxygen intermediates (Yang et al., 2005) or the NF-κB signaling pathway (Zhao and Karalis, 2002; Zbytek et al., 2004).
The results on the secretion of MMP-1 and MMP-3 from cervical fibroblasts contradict the findings by Li and Challis (2005). Li and Challis showed an induced secretion of MMP-9 by CRH in the cell cultures from human placenta and fetal membranes. The explanation for this contradiction could be that the cell cultures were established from different tissues. Furthermore, different MMPs were analyzed. However, cytokines are known to stimulate the production of MMPs (Ito et al., 1991; Imada et al., 1997a, b; Oner et al., 2008), so it would be logical to expect an elevation of MMP-1 and MMP-3 levels when there is an increased secretion of IL-8. One possible explanation could be that even if the levels of IL-8 increase under the action of CRH, the concentration might still be too low to induce secretion of MMPs. Another explanation may be that IL-8 can induce the activity of MMPs (Thirumangalakudi et al., 2007) with no effect on the total concentration of MMPs.
One interesting finding of this study is that preterm and term cervical fibroblasts show different secretion patterns of IL-8, MMP-1 and MMP-3, where IL-8 and MMP-1 are secreted at higher levels in preterm and MMP-3 in term cervical fibroblasts. In our unpublished study, we were able to show the differences in proteoglycan production in preterm and term cervical fibroblasts. These findings indicate that cervical fibroblasts at preterm and term vaginal delivery may have different phenotypes. However, in our studies, we were not able to show any differences in the concentrations of cytokines or MMPs in the preterm cervical tissue compared with term. One explanation for this could be that we have many different cells producing MMPs and IL-8 in the cervical tissue, like leukocytes, cervical smooth muscle cells and macrophages. The clinical significance of different secretion patterns of IL-8, MMP-1 and MMP3 in preterm and term cervical fibroblasts still needs to be elucidated.
A notable observation is that MMP-1 was higher in preterm cervical fibroblasts, whereas MMP-3 was higher at term. MMP-1 and MMP-3 have different substrates in the tissue: MMP-1 breaks down fibrillar and non-fibrillar collagens, whereas MMP-3 cleaves proteoglycans, fibronectine, gelatins, collagen IV and V (Hulboy et al., 1997). Earlier studies have shown some differences between MMP-1 and MMP-3 in amniotic fluid. MMP-1, but not MMP-3, concentrations increase in amniotic fluid with advancing gestational age. Spontaneous parturition at term and preterm is associated with significant increase in amniotic fluid concentrations of MMP-3, but not MMP-1 (Maymon et al., 2000; Park et al., 2003).
The main strength of this study is that human material was used. We have well-defined patient groups both in labor and not in labor at preterm and at term. Cervical biopsies are extremely hard to obtain especially in relation to preterm delivery, which is the reason why so few studies are conducted on the human preterm cervix. Our group has applied this sampling procedure for almost 30 years to obtain cervical tissue samples including squamous and cylindrical epithelium, vessels, glands and ECM. The study also includes both analysis in the cervical tissue and experiments on the cervical fibroblasts in vitro.
A weakness of the study could be the relatively small size of the groups investigated. This could be explained by the invasive sampling procedure, which is extremely hard to coordinate with delivery and renders it difficult to get a large number of samples. Nevertheless, important differences between the groups are still possible to detect as we have seen in our earlier studies (Sennstrom et al., 1997, 2003). Secondly, the use of different passages of cervical fibroblasts could be seen as disadvantage. However, we have seen a stable phenotype until passage 8 in our earlier studies of cervical fibroblasts (Malmstrom et al., 2007). In this study, we have determined the levels of total MMPs. It is possible that the results could have been different if we had examined the active forms of MMPs. Prostaglandin E2, for instance, induces activation of MMP-3, but does not affect MMP-3 total protein in the rat cervix (Chien et al., 2005). However, proteolytic activity of MMP-1 and MMP-3 follows the same pattern as total protein levels in human decidual cells (Oner et al., 2008). We have chosen to perform analysis of the total MMPs, as it was not possible to perform analysis of both total and active forms in this study because of the shortage of material. Moreover, the lower levels of active MMPs could be undetectable (Li and Challis, 2005).
In conclusion, the current study shows that spontaneous parturition at preterm and at term is associated with elevated mRNA and protein levels of MMPs in the cervical tissue. However, different secretion patterns of IL-8, MMP-1 and MMP-3 in preterm and term cervical fibroblasts indicate that they might have different phenotypes. Furthermore, CRH stimulates the secretion of IL-8 both in preterm and term cervical fibroblasts, which suggests that CRH could be involved in the process of cervical ripening. Further studies are still needed to elucidate the significance of these findings.
Funding
Swedish Research Council (K2006-73X-14 612-04-3 to G.E.O.); ALF funds to G.E.O.; Karolinska Institute Funds to G.E.O.
Acknowledgements
The authors would like to thank Yvonne Pierre for her excellent work with Immulite and ELISA analyses.
References
- Abelin Tornblom S, Ostlund E, Granstrom L, Ekman G. Pre-term cervical ripening and labor induction. Eur J Obstet Gynecol Reprod Biol. 2002;104:120–123. doi: 10.1016/s0301-2115(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Angioni S, Petraglia F, Gallinelli A, Cossarizza A, Franceschi C, Muscettola M, Genazzani AD, Surico N, Genazzani AR. Corticotropin-releasing hormone modulates cytokines release in cultured human peripheral blood mononuclear cells. Life Sci. 1993;53:1735–1742. doi: 10.1016/0024-3205(93)90160-5. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Chien EK, Ji H, Feltovich H, Clark K. Expression of matrix metalloproteinase-3 in the rat cervix during pregnancy and in response to prostaglandin E2. Am J Obstet Gynecol. 2005;192:309–317. doi: 10.1016/j.ajog.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Ekman G, Malmstrom A, Uldbjerg N, Ulmsten U. Cervical collagen: an important regulator of cervical function in term labor. Obstet Gynecol. 1986;67:633–636. [PubMed] [Google Scholar]
- Ferrand PE, Parry S, Sammel M, Macones GA, Kuivaniemi H, Romero R, Strauss JF., 3rd A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8:494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Parry S, Urbanek M, Sammel M, Macones G, Kuivaniemi H, Romero R, Strauss JF., 3rd A single nucleotide polymorphism in the matrix metalloproteinase-1 (MMP-1) promoter influences amnion cell MMP-1 expression and risk for preterm premature rupture of the fetal membranes. J Biol Chem. 2002;277:6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Zakar T. Prostaglandins and the myometrium and cervix. Prostaglandins Leukot Essent Fatty Acids. 2004;70:207–222. doi: 10.1016/j.plefa.2003.04.009. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. Role of stress peptides during human pregnancy and labour. Reproduction. 2002;124:323–329. doi: 10.1530/rep.0.1240323. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Arora CP, Korst LM. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Ann N Y Acad Sci. 1999;897:54–65. doi: 10.1111/j.1749-6632.1999.tb07878.x. [DOI] [PubMed] [Google Scholar]
- Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- Imada K, Ito A, Kanayama N, Terao T, Mori Y. Urinary trypsin inhibitor suppresses the production of interstitial procollagenase/proMMP-1 and prostromelysin 1/proMMP-3 in human uterine cervical fibroblasts and chorionic cells. FEBS Lett. 1997;a 417:337–340. doi: 10.1016/s0014-5793(97)01319-7. [DOI] [PubMed] [Google Scholar]
- Imada K, Ito A, Sato T, Namiki M, Nagase H, Mori Y. Hormonal regulation of matrix metalloproteinase 9/gelatinase B gene expression in rabbit uterine cervical fibroblasts. Biol Reprod. 1997;b 56:575–580. doi: 10.1095/biolreprod56.3.575. [DOI] [PubMed] [Google Scholar]
- Ito A, Sato T, Ojima Y, Chen LC, Nagase H, Mori Y. Calmodulin differentially modulates the interleukin 1-induced biosynthesis of tissue inhibitor of metalloproteinases and matrix metalloproteinases in human uterine cervical fibroblasts. J Biol Chem. 1991;266:13598–13601. [PubMed] [Google Scholar]
- Kalantaridou S, Makrigiannakis A, Zoumakis E, Chrousos GP. Peripheral corticotropin-releasing hormone is produced in the immune and reproductive systems: actions, potential roles and clinical implications. Front Biosci. 2007;12:572–580. doi: 10.2741/2083. [DOI] [PubMed] [Google Scholar]
- Klimaviciute A, Calciolari J, Bertucci E, Abelin Tornblom S, Stjernholm-Vladic Y, Bystrom B, Petraglia F, Ekman-Ordeberg G. Corticotropin-releasing hormone, its binding protein and receptors in human cervical tissue at preterm and term labor in comparison to non-pregnant state. Reprod Biol Endocrinol. 2006;4:29. doi: 10.1186/1477-7827-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledingham MA, Denison FC, Riley SC, Norman JE. Matrix metalloproteinases-2 and -9 and their inhibitors are produced by the human uterine cervix but their secretion is not regulated by nitric oxide donors. Hum Reprod. 1999;14:2089–2096. doi: 10.1093/humrep/14.8.2089. [DOI] [PubMed] [Google Scholar]
- Li W, Challis JR. Corticotropin-releasing hormone and urocortin induce secretion of matrix metalloproteinase-9 (MMP-9) without change in tissue inhibitors of MMP-1 by cultured cells from human placenta and fetal membranes. J Clin Endocrinol Metab. 2005;90:6569–6574. doi: 10.1210/jc.2005-1445. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Premature parturition after infusion of corticotrophin or cortisol into foetal lambs. J Endocrinol. 1968;42:323–329. doi: 10.1677/joe.0.0420323. [DOI] [PubMed] [Google Scholar]
- Linton EA, Perkins AV, Woods RJ, Eben F, Wolfe CD, Behan DP, Potter E, Vale WW, Lowry PJ. Corticotropin releasing hormone-binding protein (CRH-BP): plasma levels decrease during the third trimester of normal human pregnancy. J Clin Endocrinol Metab. 1993;76:260–262. doi: 10.1210/jcem.76.1.8421097. [DOI] [PubMed] [Google Scholar]
- Malmstrom E, Sennstrom M, Holmberg A, Frielingsdorf H, Eklund E, Malmstrom L, Tufvesson E, Gomez MF, Westergren-Thorsson G, Ekman-Ordeberg G, et al. The importance of fibroblasts in remodelling of the human uterine cervix during pregnancy and parturition. Mol Hum Reprod. 2007 doi: 10.1093/molehr/gal117. [DOI] [PubMed] [Google Scholar]
- Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, Yoon BH. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–920. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- Norman M, Ekman G, Malmstrom A. Changed proteoglycan metabolism in human cervix immediately after spontaneous vaginal delivery. Obstet Gynecol. 1993;81:217–223. [PubMed] [Google Scholar]
- Oner C, Schatz F, Kizilay G, Murk W, Buchwalder LF, Kayisli UA, Arici A, Lockwood CJ. Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and -3 expression in term decidual cells: implications for treatment of chorioamnionitis-induced preterm delivery. J Clin Endocrinol Metab. 2008;93:252–259. doi: 10.1210/jc.2007-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstet Gynecol. 1995;86:223–229. doi: 10.1016/0029-7844(95)93704-4. [DOI] [PubMed] [Google Scholar]
- Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, Gomez R, Yoon BH, Romero R. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12–22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Sehringer B, Schafer WR, Wetzka B, Deppert WR, Brunner-Spahr R, Benedek E, Zahradnik HP. Formation of proinflammatory cytokines in human term myometrium is stimulated by lipopolysaccharide but not by corticotropin-releasing hormone. J Clin Endocrinol Metab. 2000;85:4859–4865. doi: 10.1210/jcem.85.12.7006. [DOI] [PubMed] [Google Scholar]
- Sennstrom MK, Brauner A, Lu Y, Granstrom LM, Malmstrom AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol. 1997;74:89–92. doi: 10.1016/s0301-2115(97)02757-7. [DOI] [PubMed] [Google Scholar]
- Sennstrom MB, Ekman G, Westergren-Thorsson G, Malmstrom A, Bystrom B, Endresen U, Mlambo N, Norman M, Stabi B, Brauner A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6:375–381. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- Sennstrom MB, Brauner A, Bystrom B, Malmstrom A, Ekman G. Matrix metalloproteinase-8 correlates with the cervical ripening process in humans. Acta Obstet Gynecol Scand. 2003;82:904–911. doi: 10.1080/j.1600-0412.2003.00249.x. [DOI] [PubMed] [Google Scholar]
- Stjernholm Y, Sahlin L, Malmstrom A, Barchan K, Eriksson HA, Ekman G. Potential roles for gonadal steroids and insulin-like growth factor I during final cervical ripening. Obstet Gynecol. 1997;90:375–380. doi: 10.1016/s0029-7844(97)00245-7. [DOI] [PubMed] [Google Scholar]
- Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–894. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- Thirumangalakudi L, Yin L, Rao HV, Grammas P. IL-8 induces expression of matrix metalloproteinases, cell cycle and pro-apoptotic proteins, and cell death in cultured neurons. J Alzheimers Dis. 2007;11:305–311. doi: 10.3233/jad-2007-11307. [DOI] [PubMed] [Google Scholar]
- Tornblom SA, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol. 2005;a 3:39. doi: 10.1186/1477-7827-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornblom SA, Maul H, Klimaviciute A, Garfield RE, Bystrom B, Malmstrom A, Ekman-Ordeberg G. mRNA expression and localization of bNOS, eNOS and iNOS in human cervix at preterm and term labour. Reprod Biol Endocrinol. 2005;b 3:33. doi: 10.1186/1477-7827-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu FF, Goldenberg RL, Tamura T, Drews M, Zucker SJ, Voss HF. Prenatal plasma matrix metalloproteinase-9 levels to predict spontaneous preterm birth. Obstet Gynecol. 1998;92:446–449. doi: 10.1016/s0029-7844(98)00222-1. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147:662–666. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Nan X, Ji P, Dow KE. Corticotropin releasing hormone modulates endotoxin-induced inflammatory cytokine expression in human trophoblast cells. Placenta. 2007;28:1032–1038. doi: 10.1016/j.placenta.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Weiss A, Goldman S, Shalev E. The matrix metalloproteinases (MMPS) in the decidua and fetal membranes. Front Biosci. 2007;12:649–659. doi: 10.2741/2089. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G, Norman M, Bjornsson S, Endresen U, Stjernholm Y, Ekman G, Malmstrom A. Differential expressions of mRNA for proteoglycans, collagens and transforming growth factor-beta in the human cervix during pregnancy and involution. Biochim Biophys Acta. 1998;1406:203–213. doi: 10.1016/s0925-4439(98)00005-2. [DOI] [PubMed] [Google Scholar]
- Winkler M, Fischer DC, Ruck P, Marx T, Kaiserling E, Oberpichler A, Tschesche H, Rath W. Parturition at term: parallel increases in interleukin-8 and proteinase concentrations and neutrophil count in the lower uterine segment. Hum Reprod. 1999;14:1096–1100. doi: 10.1093/humrep/14.4.1096. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hahm E, Kim Y, Kang J, Lee W, Han I, Myung P, Kang H, Park H, Cho D. Regulation of IL-18 expression by CRH in mouse microglial cells. Immunol Lett. 2005;98:291–296. doi: 10.1016/j.imlet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Pfeffer LM, Slominski AT. Corticotropin-releasing hormone stimulates NF-kappaB in human epidermal keratinocytes. J Endocrinol. 2004;181:R1–R7. doi: 10.1677/joe.0.181r001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Karalis KP. Regulation of nuclear factor-kappaB by corticotropin-releasing hormone in mouse thymocytes. Mol Endocrinol. 2002;16:2561–2570. doi: 10.1210/me.2001-0334. [DOI] [PubMed] [Google Scholar]