Abstract
Dushinsky et al. left a great gift to human beings with the discovery of 5-fluorouracil (5-FU). Approximately 50 years have elapsed from that discovery to the development of S-1 (TS-1®). The concept of developing an anticancer agent that simultaneously possesses both efficacy-enhancing and adverse reaction-reducing effects could be achieved only with a three-component combination drug. S-1 is an oral anticancer agent containing two biochemical modulators for 5-FU and tegafur (FT), a metabolically activated prodrug of 5-FU. The first modulator, 5-chloro-2,4-dihydroxypyridine (CDHP), enhances the pharmacological actions of 5-FU by potently inhibiting its degradation. The second modulator, potassium oxonate (Oxo), localizing in mucosal cells of the gastrointestinal (GI) tract after oral administration, reduces the incidence of GI toxicities by suppressing the activation of 5-FU in the GI tract. Thus, S-1 combines FT, CDHP and Oxo at a molar ratio of 1:0.4:1. In 1999–2007, S-1 was approved for the treatment of the following seven cancers: gastric, head and neck, colorectal, non-small cell lung, breast, pancreatic and biliary tract cancers. ‘S-1 and low-dose cisplatin therapy’ without provoking Grade 3 non-hematologic toxicities was proposed to enhance its clinical usefulness. Furthermore, ‘alternate-day S-1 regimen’ may improve the dosing schedule for 5-FU by utilizing its strongly time-dependent mode of action; the former is characterized by the low incidences of myelotoxicity and non-hematologic toxicities (e.g. ≤Grade 1 anorexia, fatigue, stomatitis, nausea, vomiting and taste alteration). These two approaches are considered to allow long-lasting therapy with S-1.
Key words: 5-FU (5-fluorouracil), S-1 (TS-1®), balancing efficacy and toxicity, S-1 and low-dose CDDP therapy, alternate-day S-1 regimen
INTRODUCTION
The history of cancer chemotherapy started with the use of nitrogen mustards, derivatives of poisonous gas (Yperite), in 1943 during World War II as a therapeutic drug for Hodgkin's disease and leukemias by Goodman et al. (1). Treatment with nitrogen mustards aimed to utilize the antitumor activity of the drugs through their toxicities (e.g. leukopenia, diarrhea and stomatitis) in human beings. Numerous novel compounds entered the field of cancer chemotherapy for solid tumors since then, including mitomycin discovered by Hata et al. (2) and 5-fluorouracil (5-FU) discovered by Dushinsky et al. (3). In 1970–90s, when multidrug combined consolidation therapy was conducted, combined anticancer agents differing in mechanisms of action without definite theoretical evidence and easily provoking adverse reactions were developed, therefore, consolidation therapy may be considered as a short-term decisive therapeutic modality. Especially, such therapy not only provides relatively high response rates but also presents proportionally increased incidences of adverse reactions, resulting in failure of long-lasting treatment and making it difficult to eventually provide survival benefit to patients.
Therefore, none of the entities completely satisfied our demands, and concern about the efficacy of cancer chemotherapy emerged. In consequence, an increasing need emerged for a therapeutic modality that is patient-friendly and beneficial, and a broad array of explorations has been made for a novel drug or combination therapy with a new mechanism of action or a new therapeutic concept in response to the relevant need.
Acceptable international standard regimens for gastrointestinal (GI) cancers were established as follows: in 1980, 5-FU, doxorubicin and mitomycin (FAM) (4) therapy for gastric cancer; and in 1989, continuous venous infusion (CVI) of 5-FU therapy for colorectal cancer (5). All these treatments combined three or more anticancer agents, and 5-FU has been used as the core drug in all the regimens except etoposide, doxorubicin and cisplatin (EAP) therapy (6).
Approximately 50 years have elapsed since the discovery of 5-FU in 1957 before eventually elucidating the mechanisms by which the drug exerts its pharmacological actions and provokes its adverse reactions.
Since 1990, however, we expanded the conventional concept of using a single anticancer agent to overcome a contradictory issue of exerting high anticancer activity and provoking less adverse reactions in a simultaneous manner, and finally concretized a novel, patient-friendly, long-lasting therapeutic modality that would replace the conventional concept. I describe here the following two topics on the background of biochemical research on the efficacy and toxicity of 5-FU over the last 50 years and balancing them.
History covering: (a) the synthesis of 5-FU and the discovery of its antitumor activity, (b) the developments of the first masked form oral anticancer agent of 5-FU and tegafur (FT) and of a combination between FT and uracil—UFT® and (c) the development of a combination of FT, 5-chloro-2,4-dihydroxypyridine (CDHP) and potassium oxonate (Oxo)—S-1 (TS-1®).
Clinical usefulness and future vistas of S-1.
HISTORY COVERING THE SYNTHESIS OF 5-FU AND DISCOVERY OF ITS ANTITUMOR ACTIVITY
Timeline: from the Discovery of 5-FU to the Development of S-1 (TS-1®)
Dushinsky et al. (3) left a great gift to human beings. I refer to the timeline covering an ∼50-year history from the discovery of 5-FU to the development of S-1 (TS-1®).
As shown in Table 1, there were four major inventions and encounters from the discovery of 5-FU to the development of S-1.
Table 1.
Timeline: from the discovery of 5-FU to the development of S-1 (TS-1®)
| Years | Events |
|---|---|
| 1957 | (i) Synthesis of 5-FU and discovery of its antitumor activity by Dushinsky et al. (3). |
| 1967 | (ii) Synthesis of FT by Hiller et al. (11) at the Latvian Institute of Synthesis, USSR |
| June 1969 | Encounter with FT (Futraful®): Y. Kobayashi, the President of Taiho met with Blokhin the President Cancer Research Center, USSR Academy of Medical Science |
| December 1969 | Introduction of FT by Taiho Pharmaceutical Co., Ltd |
| 1970 | Development of FT from the intravenous to oral agent by Kimura, Fujii and Taguchi |
| 1976 | (iii) Invention by Fujii et al. (16) of UFT® (oral combination drug), FT:Ura =1:4 |
| 1984 | Discovery of an inhibitor for 5-FU catabolic enzyme, CDHP by Tatsumi et al. (17) |
| May 1987 | Discovery of Oxo that reduces 5-FU-induced gastrointestinal toxicities by Shirasaka et al. (18) |
| November 1990 | Establishment of the S-1 project by Y. Kobayashi |
| January 1991 | (iv) Invention of S-1 by Shirasaka et al. (19) S-1, FT:CDHP:Oxo =1:0.4:1 |
| March 1991 | Onset of preclinical studies |
| November 1992 | Onset of Phase I clinical trials |
| March 1994 | Onset of Phase II clinical trials |
| November 1997 | NDA of S-1 for gastric cancer |
| January 1999 | Approval of S-1 (TS-1®) for gastric cancer through the priority review system (20,21) |
| April 2001 | Approval of S-1 for head and neck cancer (22) |
| December 2003 | Approval of S-1 for colorectal cancer (23,24) |
| December 2004 | Approval of S-1 for non-small cell lung cancer (25) |
| November 2005 | Approval of S-1 for breast cancer (26) |
| August 2006 | Approval of S-1 for pancreatic cancer (27) |
| August 2007 | Approval of S-1 for biliary tract cancer (28) |
| 2008 | Phase III studies of S-1 (29,30) |
FT, tegafur; CDHP, 5-chloro-2,4-dihydroxypyridine; Oxo, potassium oxonate; NDA, new drug application.
(i) In 1957, Dushinsky et al. (3) synthesized 5-FU and discovered its antitumor activity. In many studies conducted by them, they discovered that uracil and 5-FU more preferably concentrate into tumor cells than other pyrimidine bases. Therefore, they synthesized 5-FU in which hydrogen at five positions in uracil was substituted with fluorine. This finding is very important to establish the optimal schedule for 5-FU administration. Therefore, it is easy to predict that the long-term exposure of 5-FU to tumor cells induces high cytotoxicity and totally kills them.
Wilkoff et al. (7), Skipper et al. (8) and Shimoyama and Kimura (9) concluded, based on the results from their in vitro studies, that 5-FU is a typical antimetabolite with a strongly time-dependent mode of action. Skipper et al. (8) also suggested that a longer interval among doses than the S-phase of the cell cycle is preferable for agents, e.g. 5-FU, which are S-phase-specific but self-limiting with respect to cytotoxicity. Shimoyama and Kimura examined the features of cell death induced by 5-FU and discovered that cytotoxicity during long-term exposure at a low concentration induces marked reproductive or clonal death. Namely, cell death was observed while microscopic small colonies were formed during four or five cell divisions. Furthermore, they stressed that long-term repeated exposure to cancer cells is a very important factor for the dosing schedule for 5-FU (9). Until ∼2000, CVI was considered to be the best schedule for 5-FU administration (5,10).
However, the adverse reactions of 5-FU differ largely in nature between the bolus and CVI regimens. The dose-limiting toxicities of 5-FU are represented by myelotoxicity in the bolus regimen, and by GI toxicities (e.g. diarrhea and stomatitis) and hand–foot syndrome (HFS) in the CVI regimen (5,10). To establish a highly useful therapeutic modality with an aim to conduct long-lasting treatment with 5-FU, therefore, it is crucially important to devise a regimen that provides a good balancing of its adverse reactions, e.g. GI toxicities and HFS causing great discomfort for patients, and its antitumor activity in cancer chemotherapy.
(ii) In 1967, Hiller et al. (11) synthesized the first anticancer prodrug of 5-FU, FT. A historical encounter with FT occurred in June 1969 when Dr Y. Kobayashi (Taiho Pharmaceutical Co., Ltd, Tokyo, Japan) paid a visit to President Dr N.N. Blokhin at the Cancer Research Center USSR, Academy of Medical Science, Moscow, USSR. Dr Kobayashi looked at an ampule on the table and asked him the following question, ‘What is this ampule?’. Dr Blokhin then replied, ‘This is a derivative of 5-FU that was synthesized at the Latvian Institute of Organic Synthesis, USSR.’ Dr Kobayashi, strongly attracted by the derivative, received a sample for preclinical studies and introduced FT into Japan in December of that year to initiate the joint development of the drug. This encounter really triggered the development of FT to UFT, and then to S-1.
In 1970, FT was initially introduced as an injectable drug. However, the drug provoked adverse reactions, e.g. nausea, vomiting, eruption and central nervous system (CNS) disturbances, prior to exerting its clinical effects, making long-lasting treatment difficult. Therefore, FT failed to provide clinical effects in most patients. Since FT had been found to release 5-FU gradually in the liver microsomal fraction in the presence of NADPH by Toide et al. (12), Drs K. Kimura, S. Fujii and T. Taguchi considered that its oral administration would not adversely affect the GI tract in a direct manner and proposed the change of its route of administration, i.e. from the intravenous to the oral route. That was the moment when FT marked the first step as an oral anticancer agent.
FT is the first masked form oral anticancer agent that gradually releases 5-FU by the action of a liver microsomal P450 enzyme CYP2A6 (12,13). So far, CYP2A6 has been clarified to show racial differences in enzyme activity among black, Caucasian and yellow populations and to have higher activity in the Caucasian population than in the yellow population (14). It would not be an exaggeration to say that such change to the oral route made it possible to develop S-1 from UFT subsequently. In fact, the injection of FT was developed up to Phase II in the USA. However, its development was discontinued due to insufficient effects and to the high incidences of CNS disturbances (15).
Nevertheless, plasma concentrations of 5-FU after oral administration of FT were lower than those obtained by CVI, a regimen that was considered best for 5-FU administration, and failed to reach the effective blood drug concentration. Therefore, improvements were attempted to elevate plasma concentrations of 5-FU in order to enhance further the antitumor activity of FT.
(iii) In 1976, Fujii et al. (16) invented an oral anticancer agent, UFT®, and verified that uracil inhibits the degradation of 5-FU and determined to combine uracil with FT. Subsequently, Fujii et al. found the optimal molar ratio for UFT, i.e. 4 mol of uracil and 1 mol of FT, and thus successfully elevated the initial plasma concentrations of 5-FU after oral administration of UFT. The combination of uracil allowed UFT to exhibit more potent antitumor activity than does FT. Nevertheless, UFT, an improvement from FT, also failed to sustain the effective plasma concentration of 5-FU for as a long time as the CVI regimen could provide. Hence, improvements were attempted to sustain plasma concentrations of 5-FU at equal to or greater than its plasma concentrations that could be obtained by the CVI regimen. We then initiated the exploration of an inhibitor of dihydroxy pyrimidine dehydrogenase (DPD), a 5-FU-degrading enzyme, that was more potent than uracil.
In 1984, consequently, we discovered a potent 5-FU- degrading enzyme inhibitor CDHP, a pyridine derivative (17). Long-lasting high plasma concentrations of 5-FU were thus obtained.
High plasma concentrations of 5-FU in the long-term were predicted to induce strong myelotoxicity and GI toxicities. Therefore, we initiated the exploration of a substance that might reduce GI toxicities. In 1987, we discovered Oxo that has reducing activity principally on GI toxicities induced by 5-FU (18).
In those days, I had already invented a combination drug among FT, CDHP and Oxo.
In November 1990, Dr Kobayashi proposed the establishment of the S-1 Project (leader: Shirasaka) that comprised 26 members. At that time, the following negative views arose on the project inside and outside the company: (a) 5-FU is obsolete, (b) it is difficult to obtain approval for a three-component combination drug and (c) enormous costs and much time are required to perform the preclinical safety studies of a three-component combination drug. Despite these negative views, in-house consent was obtained because of the established project. Thereafter, S-1 was developed smoothly up to its clinical trials.
(iv) In 1991, we invented a three-component combination drug, S-1 (TS-1®) (19). S-1 is an oral anticancer agent that combines FT, CDHP and Oxo at a molar ratio of 1:0.4:1 and that concurrently has dual actions, i.e. effect-enhancing activity and adverse reaction-reducing activity.
Preclinical concepts built in basic research were thus also proved in clinical trials. Therefore, the new drug application (NDA) for S-1 was filed in November 1997 and was then approved for gastric cancer in January 1999 through the priority review system (20,21). By that year, ∼30 years elapsed since the historical encounter of Dr Kobayashi with FT in Moscow. Subsequently, S-1 was approved for the following six cancers from 2001 to 2007: head and neck (22), colorectal (23,24), non-small cell lung (25), breast (26), pancreatic (27) and biliary tract cancers (28). S-1 is currently applied to acquire its expanded indications to treat cervical and prostate cancers. The Phase III clinical trials of S-1 have reported the usefulness of S-1 as postsurgical adjuvant chemotherapy for gastric cancer (29) and its combination with cisplatin (CDDP) for advanced gastric cancer (30).
Metabolism of 5-FU
I provide a succinct overview of the metabolism of 5-FU, the core drug of this review.
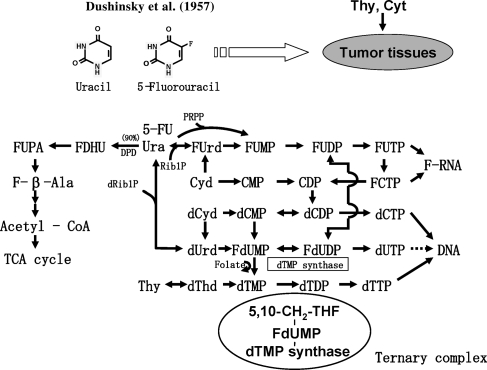
Figure 1 shows the metabolic pathways of pyrimidine nucleotides and 5-FU.
Figure 1.
Metabolic pathways of pyrimidine nucleotides and 5-FU. Main sites of action of 5-FU. 5-FU, 5-fluorouracil; FdUMP, 5-fluoro-2′- deoxyuridine 5′-monophosphate; F-β-Ala, alpha-fluoro-beta-alanine.
Main Sites of Action of 5-FU
Approximately 90% of 5-FU that was administered to the patient is degraded in F-β-Ala by DPD in the liver and excreted as F-β-Ala in the urine within 24 h after i.v. bolus administration (31) and ∼10% of 5-FU is converted to F-RNA and FdUMP. 5-FU is so similar to uracil in chemical structure that it is recognized and metabolized by all enzymes that are involved in the uracil metabolism except for one, dTMP synthase. Therefore, 5-FU can be converted through the intermediates FUrd, FUMP, FUDP and FUTP to F-RNA that induces the metabolic abnormality in RNA and the inhibition of the RNA synthesis and that exhibits cytotoxicity. 5-FU can also be converted into FdUDP from FUDP by ribonucleotide reductase and then to FdUMP. Furthermore, dTMP synthase binds to FdUMP and forms a ternary complex with reduced folate (Fig. 1). However, the complex cannot perform its enzyme reaction and remains irreversibly bound to FdUMP. Consequently, FdUMP inhibits dTMP synthase activity, inhibits DNA synthesis, and exhibits cytotoxicity. Figure 2 summarizes the biological actions of Ura, CDHP, Oxo on the metabolic pathways of 5-FU and the conversion of FT to 5-FU.
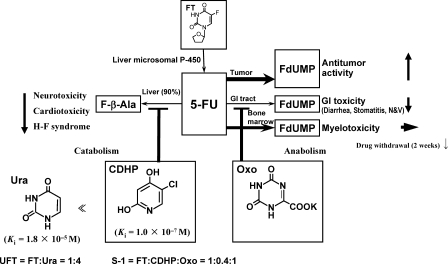
Figure 2.
Biological actions of Ura, CDHP and Oxo on the metabolic pathways of 5-FU, as well as the conversion of FT to 5-FU. FT, futraful; CDHP, 5-chloro-2,4-dihydroxypyridine; Oxo, potassium oxonate.
5-FU is anabolized to FdUMP in various tissues. FdUMP in tumor cells, the GI tract and bone marrow shows antitumor activity, GI toxicities (32) and myelotoxicity (33), respectively.
F-β-Ala induces all sorts of toxicities because of its long blood retention time (31). In fact, F-β-Ala is known to cause cardio (34) and neurotoxicities (35). Recently, the possible development of HFS due to the degradation product of 5-FU was reported by Yen-Revollo et al. (36).
INVENTION OF S-1
Discovery of CDHP
To utilize 5-FU efficaciously, it is indispensable to inhibit the degradation of 5-FU by DPD. Hence, we conducted enzymological studies on DPD inhibitors. In 1984, consequently, we discovered CDHP as a novel compound with potent DPD-inhibitory activity and whose enzymological patterns show reversible competitive inhibition (17).
Only part (10%) of 5-FU is anabolized and converted to FdUMP and F-RNA that exhibit antitumor activity and provoke adverse reactions, e.g. myelotoxicity and GI toxicities. For the efficacious use of 5-FU, therefore, it is necessary to first and strongly inhibit the 5-FU-degrading enzyme in an attempt to elevate its antitumor activity. Regarding the synthesis of a DPD inhibitor, we initiated the synthesis of pyrimidine derivatives containing uracil and expanded our research to the synthesis of barbituric acid and pyridine derivatives before finally reaching the discovery of a potent DPD inhibitor CDHP. The Ki values of CDHP and Ura for DPD-inhibitory activity were 1 × 10−7 and 1.8 × 10−5 M, respectively (Fig. 2). DPD-inhibitory activity intensified by ∼200-fold from pyrimidine derivatives (e.g. Ura) to pyridine derivatives (e.g. CDHP). Pyridine derivatives exhibited the most potent DPD-inhibitory activity. The fact that a compound has potent DPD-inhibitory activity is an important factor. However, the facts that a compound is a competitive inhibitor of DPD and that it is safe are more important than its efficacy. Out of these derivatives, CDHP—a novel highly safe pyridine derivative with a competitive inhibition pattern and the fourth potency—was selected as a component of S-1 (17).
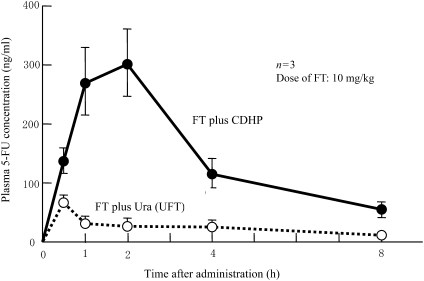
FT plus CDHP whose inhibitory activity is 200-fold more potent than uracil and UFT were administered orally to rats, and plasma 5-FU concentrations were examined. As shown in Fig. 3, plasma 5-FU concentrations increased markedly in the combination of FT plus CDHP than in the combination of UFT.
Figure 3.
Potent inhibitory activity of CDHP that is translated into increased plasma 5-FU concentrations after oral administration of FT and CDHP to rats as compared with FT and Ura (UFT) (19,39).
The FT plus CDHP combination group showed the Cmax value of 300 ng/ml or higher; the values were markedly higher than those in the UFT group (37).
The FT plus CDHP combination group allowed us to expect high anticancer activity. However, increased plasma concentrations of 5-FU induced higher incidences of GI toxicities and myelotoxicity. Therefore, the exertion of its potent antitumor effect could not be expected unless devising a scheme to reduce these toxicities.
Subsequently, we initiated the examination of the mechanism to reduce the degradation of 5-FU in the liver by CDHP, resulting in (i) enhancement of the antitumor activity of 5-FU and (ii) reduced incidences of HFS, cardiotoxicity and neurotoxicity induced by the drug.
Discovery of Oxo
In 1965, Grant et al. administered Oxo intravenously to cancer patients as an anticancer agent that inhibits orotate phosphoribosyltransferase (OPRT) resulting from the de novo biosynthesis of pyrimidine. However, Oxo showed insufficient antitumor activity (38). Furthermore, they did not notice that Oxo reduces the incidences of GI toxicities induced by 5-FU, presumably due to the route of administration they selected—intravenous. Consequently, Oxo has never been used as an anticancer agent thereafter.
In 1987, we administered Oxo orally to Yoshida sarcoma-bearing rats in combination with 5-FU as part of preclinical studies to seek a substance that reduces the incidences of the GI toxicities induced by 5-FU. Consequently, we found that: (i) Oxo inhibits OPRT in the small intestine not in tumor and bone marrow tissues and (ii) orally administered Oxo accumulates more intensively in GI tissues than in other tissues (e.g. blood and tumor) of tumor-bearing rats. Oxo is preferably distributed in the GI mucosa after oral administration and inhibits 5-FU-induced GI toxicities (18).
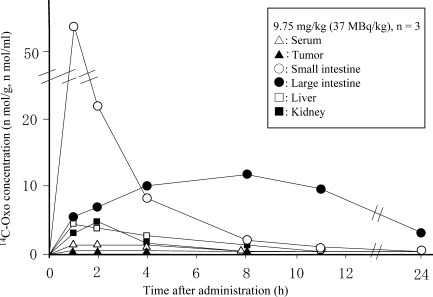
Figure 4 shows the tissue distributions of 14C-Oxo after oral administration to Yoshida sarcoma-bearing rats. High concentrations of Oxo were distributed in the small and large intestines in the long-term, and a very low concentration of Oxo was distributed in the tumor.
Figure 4.
14C-oxonic acid concentrations in blood and tissues of Yoshida sarcoma-bearing rats after oral administration of 14C-Oxo (potassium oxonate) (18).
5-FU is anabolized to FdUMP in actively dividing cells, i.e. tumor cells, mucosal cells of the GI tract and bone marrow cells. Therefore, we attempted to reduce the incidences of adverse reactions of 5-FU, especially GI toxicities, for the purpose of improving patient compliance.
The usefulness of Oxo to suppress vomiting and diarrhea was examined in beagle dogs that have high susceptibility to 5-FU. As shown in Table 2, the group in which Oxo was removed from S-1 showed the high incidences of vomiting and diarrhea [7 of 11 (63.6%) and 10 of 11 (90.9%), respectively], whereas the Oxo-containing S-1 group showed marked improvements [1 of 11 (0.9%) and 1 of 11 (0.9%), respectively] (37,39). This result led us to consider the potential usefulness of S-1 in alleviating vomiting and diarrhea also in clinical settings, thus allowing the patient to take a meal normally and contributing to the improvement of his/her quality of life (QOL).
Table 2.
Incidences of vomiting and diarrhea reduced by Oxo after oral administration of FT and CDHP to beagle dogs (Refs 37,39)
| Drug | Dose (mg/kg) | Duration (day) | Animal (n) | Vomiting [n (%)] | Diarrhea [n (%)] |
|---|---|---|---|---|---|
| FT plus CDHP (1:0.4) | 6 | 5 | 11 | 7/11 (63.6) | 10/11 (90.9) |
| S-1 [FT plus CDHP plus Oxo, (1:0.4:1)] | 6 | 5 | 11 | 1/11 (0.9) | 1/11 (0.9) |
Study of the Molar Ratios among FT, CDHP and Oxo
To determine the best molar ratio among FT, CDHP and Oxo, it was of primary importance to consider balancing efficacy and toxicity. First, different moles (0, 0.2, 0.4, 0.6 and 0.8) of CDHP were combined with 1 mol of FT. The suspension was administered orally for 7 consecutive days to Yoshida sarcoma-bearing rats in which the tumor had been transplanted 24 h previously. Animals were sacrificed on Day 10 after the onset of oral administration to examine the antitumor activity and toxicities of the combinations based on tumor weight and body weight decreases against the control group. Furthermore, the combinations at the above molar ratios were administered orally to normal rats in a concurrent manner to measure plasma concentrations of 5-FU on a time-course basis. Consequently, antitumor activity approximated the peak at molar ratios of ≥1:0.4 between FT and CDHP, and body weight decrease was least at a molar ratio of 1:0.4 between FT and CDHP. Further increases in molar ratios between FT and CDHP failed to enhance the antitumor activity of 5-FU, and body weight decrease intensified. Furthermore, increases in the molar ratios between FT and CDHP did not indicate increases in plasma 5-FU concentrations. The above results led us to determine the molar ratio of 1:0.4 between FT and CDHP. Second, different molar ratios (0, 0.5, 1 and 2) of Oxo were examined while maintaining the fixed molar ratio of 1:0.4 between FT and CDHP. The antitumor activity of 5-FU did not reduce when 1 mol of Oxo was combined with FT and CDHP (1:0.4) but markedly decreased when >1 mol of Oxo was combined. Therefore, we determined the molar ratio of 1:0.4:1 for the combination of FT, CDHP and Oxo. Concurrently with this study, a study to examine the antitumor activity of 5-FU similar to the above was conducted using different timings to administer these three compounds. Consequently, balancing efficacy and toxicity was found best, especially when Oxo, FT and CDHP were administered concurrently. Therefore, these three compounds were formulated to combination drugs in capsules to allow their simultaneous oral administration in clinical trials (19,37).
Preclinical Studies of S-1
Preclinical studies to examine the anticancer activity of S-1 were conducted mainly in rats because rats precisely reflect the effects and adverse reactions of 5-FU due to the sustainment of plasma 5-FU concentrations for a longer time after oral administration of S-1 than do mice.
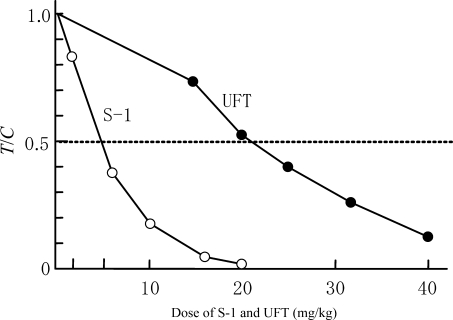
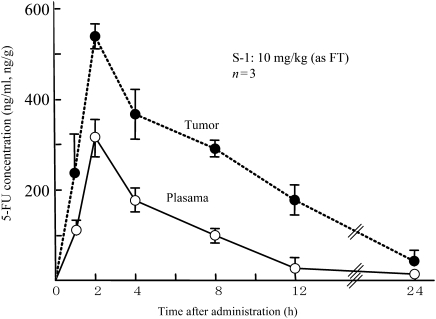
We used an anticancer experiment system with Yoshida sarcoma and administered S-1 orally for 7 consecutive days to examine its antitumor effect and the tumor and blood concentrations of 5-FU. As shown in Fig. 5, S-1 showed a more potent antitumor effect than UFT and indicated absolutely no tumor proliferation at an FT dose of 20 mg/kg. Furthermore, we administered S-1 orally at a dose level of 10 mg/kg, an FT dose level showing a treatment/control (T/C) value (≥0.5), to measure the tumor and plasma concentrations of 5-FU on a time-course basis. As shown in Fig. 6, the Tmax of 5-FU was 2 h in both the plasma and tumor. Plasma 5-FU concentrations persisted at 100 ng/ml or higher up to 8 h after administration, while tumor concentrations of 5-FU persisted at 200 ng/g or higher up to 12 h after administration. The results of these pharmacological studies validated the potent antitumor activity of S-1 (19,37).
Figure 5.
Antitumor activity of S-1 and UFT in Yoshida sarcoma-bearing rats (19,37). T/C, treatment/control; UFT, FU and uracil.
Figure 6.
5-FU concentrations in plasma of and tumor in Yoshida sarcoma-bearing rats after oral administration of S-1 (19,37).
Pharmacokinetic Clinical Trials of S-1 in Patients with Gastric Cancer, Colorectal Cancer and Breast Cancer
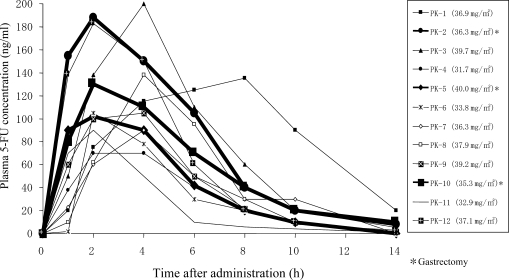
The early Phase II clinical trials of S-1 were completed. These trials were conducted according to the recommended regimen for S-1, i.e. S-1 80 mg/m2/day, given orally twice daily after breakfast and dinner for 28 consecutive days. The initial dose of S-1 for each patient was determined according to body surface area (BSA) as follows: for BSA of <1.25 m2, 80 mg/day; for BSA of ≥1.25<1.5 m2, 100 mg/day; and for BSA of ≥1.5 m2, 120 mg/day. Twelve patients were recruited to these trials: five with gastric cancer (three of whom underwent total gastrectomy), four with colorectal cancer and three with breast cancer. As shown in Fig. 7, the pharmacokinetic parameters of 5-FU in plasma were as follows: Cmax, 128.5 ± 41.5 ng/ml; Tmax, 3.5 ± 1.7 h; AUC0–14, 723.9 ± 272.7 ng h/ml; and T1/2, 1.9 ± 0.4 h. The 28-day consecutive oral regimen indicated neither pharmacokinetic fluctuations nor drug accumulation (40). All the 12 patients showed plasma 5-FU concentrations of 60–200 ng/ml. The pharmacokinetics of orally administered S-1 constantly provided plasma 5-FU concentrations that were almost equivalent to or higher than those obtained by CVI of 5-FU (41). S-1 improves patient’s QOL because of the following advantages: (i) being an oral agent, thus releasing the patient from the restriction of CVI and (ii) being administrable on outpatient settings. Furthermore, Tmax for 5-FU in all the three patients who underwent total gastrectomy (thick solid line) was 2 h, being faster than 4 h in non-gastrectomized patients. However, the AUC0–14h values for 5-FU were 691 and 822.8 ng h/ml in patients who did not have total gastrectomy and patients who underwent the surgery, respectively. Therefore, no significant difference was found in AUC for 5-FU between these patient groups.
Figure 7.
Plasma 5-FU concentrations in cancer patients after oral administration of S-1. Bold lines represent three patients who underwent total gastrectomy (40).
In addition, a recent study (42) measured plasma 5-FU concentrations after oral administration of S-1 to six patients with gastric cancer before and after total gastrectomy. Consequently, Tmax significantly decreased from 4 h before surgery to 3.3 h after surgery, and AUC0–10h for 5-FU significantly increased from 680 ng h/ml before surgery to 1030 ng h/ml after surgery. Studies (40,42) provided a concordant interpretation that Tmax for 5-FU is decreased by gastrectomy. AUC for 5-FU seems to tend to increase.
The above results suggested the usefulness of S-1 as postsurgical adjuvant therapy for patients with gastric cancer. Recently, its usefulness as postgastrectomy adjuvant therapy for gastric cancer was demonstrated in a Phase III clinical trial (29).
Late Phase II Clinical Trials of S-1
Table 3 summarizes the results from the late Phase II clinical trials of S-1 in patients with gastric, head and neck, colorectal, NSCL, breast, pancreatic or biliary tract cancers at the time of NDA. S-1 alone showed high overall response rates for advanced and recurrent gastric, colorectal, pancreatic and biliary tract cancers. S-1 is superior to the first-line drug for pancreatic cancer, gemcitabine (GEM) whose response rate was 23.8% and whose median survival time (MST) was 170 days. A randomized clinical trial of 5-FU alone in patients with advanced and recurrent pancreatic cancer has revealed its usefulness in survival rate and clinical benefit (43). S-1, when combined with CDDP, showed a high response rate for non-small cell lung cancer (NSCLC) and was effective also for patients with taxane-resistant breast cancer. The possible reasons to explain why S-1 has shown high response rates for the six cancers are the following: (i) S-1 presents a high rate of reaching cancer tissue through the long duration of high plasma 5-FU concentrations and (ii) long-lasting treatment with S-1, by which the cycles of 4-week consecutive administration and 2-week drug withdrawal are repeated.
Table 3.
Summary results from the late Phase II clinical trials of S-1 in Japan
| Cancers | Phases | Response rate (CR plus PR/patient) | Regimens | MST (days) | References |
|---|---|---|---|---|---|
| Gastric | Late P II | 49.0% (25/51) | S-1 | 250 | 20 |
| Late P II | 40.0% (20/50) | S-1 | 207 | 21 | |
| Head and neck | Late P II | 28.8% (17/59) | S-1 | 344 | 22 |
| Colorectal | Late P II | 35.5% (22/62) | S-1 | 378 | 23 |
| Late P II | 39.5% (15/38) | S-1 | 358 | 24 | |
| NSCL | Late P II | 47.3% (26/55) | S-1 plus CDDP | 334 | 25 |
| Breast (taxane-resistant) | Late P II | 21.8% (12/55) | S-1 | 470 | 26 |
| Pancreatic | Late P II | 37.5% (15/40) | S-1 | 269 | 27 |
| Biliary tract | Late P II | 35.0% (14/40) | S-1 | 286 | 28 |
Seven indications were approved in 1999–2007.
MST, median survival time; NSCL, non-small cell lung cancer; CDDP, cisplatin.
As shown in Table 4, the incidences of ≥Grade 3 adverse reactions of S-1 in a total of 449 patients were not >10% in the Phase II clinical trials, except for neutropenia (11.1%). The incidences of ≥Grade 3 non-hematologic adverse reactions, stomatitis, nausea, vomiting, diarrhea and anorexia, were low because of the Oxo contained in S-1. HFS, although not life-threatening, can severely disrupt the patient’s daily life. Therefore, the fact that HFS was not observed at all because of CDHP is noteworthy.
Table 4.
| Grade |
≥G3 (%) (n = 449) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Leukopenia | 114 | 95 | 12 | 4 | 3.6 |
| Neutropenia | 71 | 91 | 46 | 4 | 11.1 |
| Thrombocytopenia | 87 | 19 | 3 | 5 | 1.8 |
| Anemia | 82 | 102 | 32 | 5 | 8.2 |
| Stomatitis | 84 | 17 | 1 | 0 | 0.2 |
| Nausea | 102 | 20 | 10 | 0 | 2.2 |
| Vomiting | 40 | 27 | 9 | 0 | 2.0 |
| Diarrhea | 61 | 31 | 14 | 0 | 3.1 |
| Anorexia | 107 | 60 | 20 | 1 | 4.7 |
| Hand–foot syndrome | 12 | 7 | 0 | 0 | 0 |
Hand–foot syndrome, causing a great discomfort of patients, was not observed.
CLINICAL USEFULNESS AND FUTURE VISTAS OF S-1
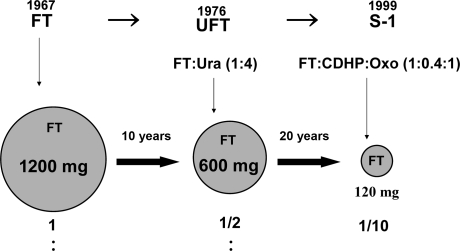
Approximately 30 years elapsed from the discovery of FT to the development of S-1 through UFT, thus indicating the evolution of drug development (UFT rather than FT and S-1 rather than UFT). As shown in Fig. 8, FT is used as an effector in all these drugs. Namely, the daily doses of FT in routine medical care are 1200 mg/day (FT), 600 mg/day (UFT) and 120 mg/day (S-1). Therefore, we successfully reduced the total daily doses of FT to 1/10th the initial value in 30 years. This extensive reduction in the total daily dose of FT has allowed the efficacious use of 5-FU to be extracted from FT, the elevation of 5-FU efficacy, and the reduction in the incidences of adverse reactions of 5-FU. Therefore, S-1 may be considered patient-friendly drug.
Figure 8.
Reductions in total daily doses of FT in an attempt to establish patient-friendly formulations.
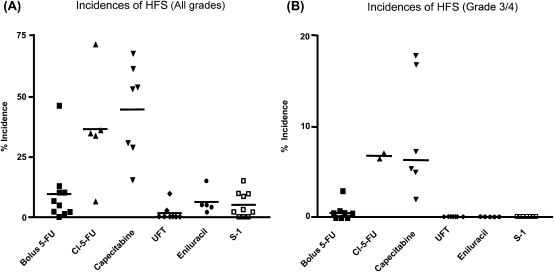
As shown in Fig. 9, Yen-Revollo et al. (36) recently reported in their review article that patients treated with 5-FU given by CVI or oral capecitabine are at greater risk of developing HFS of all grades than patients who undergo treatment with DPD inhibitor-containing fluoropyrimidines. The incidence of Grade 3/4 HFS was high when 5-FU was given by CVI and oral capecitabine, while HFS did not develop when UFT, S-1 and eniluracil were administered. These results suggest that the decreased incidence of HFS is presumably due to the limited production of degradation products of 5-FU because CDHP potently inhibits the degradation of 5-FU in the liver.
Figure 9.
Incidences of hand–foot syndrome (HFS) in patients during treatment with different fluoropyrimidines. (A) Patients treated by continuous infusion of 5-FU or capecitabine are at significantly higher risk of developing all grades of HFS as compared with patients on bolus 5-FU or combination therapy containing a DPD inhibitor (UFT, S-1, or 5-FU/eniluracil). (B) Grade 3 or 4 symptoms were extremely rare in patients who received UFT, S-1, or 5-FU/eniluracil (36).
They concluded as follows: with the accumulating findings from clinical trials that show the benefits of DPD inhibition in decreasing the risk of HFS, consideration should be given to changing the recommendations for the treatment of cancer patients with fluoropyrimidines to include DPD inhibitor components as standard therapy.
S-1 and Low- or High-Dose CDDP Therapy
Scanlon et al. (44) discovered a synergic cytotoxic effect in 1983 when combining 5-FU with CDDP in in vitro studies, while we did it in in vivo studies in 1993 (45) and 2000 (46). In low-dose FP therapy, CDDP is used not as an effector but as a modulator for 5-FU; the frequent dosing of CDDP at a not greater than 1/10th the usual dose markedly reduces the incidences of its adverse reactions while sustaining its effects, allowing long-lasting treatment with 5-FU. Since around 1990, the therapy has widely been accepted and performed in routine medical care in Japan (47,48). Recently, low-dose FP therapy in which S-1 is used instead of 5-FU given by CVI has come into wide use as a patient-friendly therapeutic modality for outpatients with cancer (49,50). This regimen requires no hydration, enables treatment in outpatient settings at low medical costs.
There are two published articles for the combination therapy of S-1 and CDDP in Japan. One of them has described a randomized Phase III clinical trial of S-1 and high-dose CDDP [S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (the SPIRITS trial)] in patients with advanced gastric cancer, in which CDDP was used as the effector for 5-FU (50,51). Another has described a retrospective study of low-dose therapy in outpatients with advanced and recurrent gastric cancer, in which CDDP was used as the modulator for 5-FU (52). In the former study, S-1 80 mg/m2 was administered orally twice daily for 3 consecutive weeks followed by 2-week drug withdrawal and CDDP 60 mg/m2 was given by the intravenous drip infusion on Day 8 within a 5-week cycle, and S-1 alone 80 mg/m2 was administered orally for 3 weeks followed by 2-week drug withdrawal within a 5-week cycle. In the latter study, S-1 80 mg/m2 was administered orally for 4 weeks followed by 2-week drug withdrawal within a 6-week cycle. CDDP 6 mg/m2 was administered by intravenous drip infusion for 30 min twice a week (Days 1 and 4) within the 6-week cycle. These two clinical trials cannot be compared directly, especially for efficacy, because the former study is the SPIRITS trial in which the S-1 and high-dose CDDP group enrolled 148 patients and the latter is a retrospective study in which the S-1 and low-dose CDDP group enrolled 32 patients. Table 5 indicates that the combination therapy of S-1 and low-dose CDDP is at least equivalent to that of S-1 and high-dose CDDP in efficacy and MST. These two clinical trials can be compared to a certain extent with respect to the overall incidences of ≥Grade 3 adverse reactions that cause the greatest distress to the patient. Namely, 15.6% (5 of 32) in the S-1 and low-dose CDDP group; 66.9% (99 of 148) in the S-1 and high-dose CDDP group; and 24.7% (37 of 150) in the S-1 alone group. The incidences of ≥Grade 3 non-hematologic toxicities (i.e. anorexia and nausea/vomiting) in these groups were 0 and 0%, 30.4 and 15.6% and 6 and 3%, respectively. S-1 and high-dose CDDP elicited slight concern because they presented an ∼70% incidence of ≥Grade 3 non-hematologic adverse reactions that constituted great factors leading the patient to deny the further ingestion of the drugs and required the hydration of the patient by hospitalization, while S-1 and low-dose CDDP therapy did not provoke ≥Grade 3 non-hematologic adverse reactions at all and required no hydration, although the patient needed to visit the hospital twice a week to receive low-dose CDDP. Therefore, S-1 and low-dose CDDP therapy allows both long-lasting treatment in outpatient settings and survival benefit at low medical costs.
Table 5.
Efficacies and adverse reactions of S-1 alone and of S-1 in combination with low- or high-dose CDDP
| A retrospective study (Ref. 52) |
SPIRITS trial (Phase III) (Refs 50,51) |
||
|---|---|---|---|
| S-1 and low-dose CDDP, n = 32 | S-1 and high-dose CDDP, n = 148 | S-1 alone, n = 150 | |
| Efficacy | |||
| Response rate | 78.1% (25/32) | 54.0% (47/87) | 31.1% (33/106) |
| Prior chemotherapy | |||
| Absent | 80.0% (16/20) | 54.0% (47/87) | 31.1% (32/106) |
| Present | 75.1% (9/12) | — | — |
| MST | 12 months | 13 months | 11 months |
| 1-year survival | 48.1% | 54.1% | 46.7% |
| 2-year survival | 23.0% | 23.6% | 15.3% |
| Adverse events | |||
| Total (≥G3, %) | 15.6 (5/32) | 66.9 (99/148) | 24.7 (37/150) |
| Hematologic | |||
| Leukocytopenia | 0 | 11.5 | 2.0 |
| Anemia | 0 | 25.7 | 4.0 |
| Thrombocytopenia | 12.5 | 5.4 | 0 |
| Non-hematologic | |||
| Nausea/vomiting | 0 | 15.6 | 3.3 |
| Anorexia | 0 | 30.4 | 6.0 |
| Fatigue | 0 | 4.1 | 1.3 |
| HFS | 0 | 0 | 0 |
| Renal dysfunction | 3.1 | 0 | 0 |
| Convenience and quality of life (QOL) | Hydration (−) Outpatient |
Hydration (+) Hospitalization (3–4 days/5 weeks) |
Hydration (−) Outpatient |
SPIRITS, S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer; CDDP, cisplatin; MST, median survival time; HFS, hand–foot syndrome.
Biological Theory of the Alternate-day S-1 Regimen
In 1963 and later, Lipkin et al. (53), Clarkson et al. (54) and Cronkite et al. (55) reported differences in cell cycles between cancer cells and normal cells (bone marrow cells, GI mucosal cells and cells with rapid cell division). As shown in Table 6, the generation time of normal cells (bone marrow and GI mucosal cells) lasts for as very shortly as 0.42–1.32 days. Therefore, 1-day drug withdrawal allows the emergence of unexposed normal cells by ∼50%. On the other hand, the generation time of cancer cells was 3.8–5 days, and the duration of the S-phase during which 5-FU acts predominantly was one or more days (17–60 h) in most cancer cells. Therefore, the repetition of 1-day drug withdrawal leads to the sustainment of the anticancer activity of 5-FU. By making the use of this large difference in cell cycles, we invented a new dosing schedule for 5-FU, an antimetabolic agent with the strong time-dependent mode of action.
Table 6.
Comparisons of generation time (TG) and of the durations of the S-, G2-, M- and G1-phases between cancer and non-cancer cells
| TG (days) | S (h) | G2 (h) | M (h) | G1 (h) | |
|---|---|---|---|---|---|
| Cancer cells (diagnosis) (53) | |||||
| Endometrial cancer | 4.6 | 60 | 4 | >1 | 48 |
| Ovarian cancer | 5 | 28 | 8 | 1 | 84 |
| Ovarian cancer | 5 | 34 | 6 | 1.4 | 72 |
| Gastric cancer | 3 | 20 | 3 | >1 | 48 |
| Gastric cancer | 4.6 | 32 | 5 | 1.1 | 72 |
| Lymphosarcoma | 3.8 | 17 | 2 | >1 | 72 |
| Non-cancer cell (52) | |||||
| Stomach | 0.42 | 9–14 | 2 | 1 | 1 |
| Ileum | 0.63 | 11 | 2 | 1 | 2 |
| Colon | 0.67 | 14 | 1–2 | 1 | 1–2 |
| Rectum | 0.42 | 9–10 | 2 | 1 | 2 |
| Myeloblast (54) | 1.32 | — | — | — | — |
TG, generation time; S, synthesis phase; G2, gap 2 phase; M, mitosis phase; G1, gap 1 phase.
We then examined the alternate-day S-1 regimen (alternate-day dosing or dosing on Monday, Wednesday and Friday per week). We have reported these theories and practices in in vitro studies (56,57) in vivo studies (57) and a retrospective clinical trial (58). Based on the above theories, alternate-day exposure to 5-FU did not reduce its cytocidal activity in the in vitro studies (56,58). In the in vivo study using nude mice, the anticancer activity of S-1 given by the alternate-day regimen was equivalent to or better than that of daily dosing. Furthermore, the alternate-day S-1 regimen markedly reduced myelotoxicities and GI toxicities (58). The fundamental regimen in clinical settings was started with the recommended daily dosing of S-1 for 4 weeks followed by 2 weeks of drug withdrawal. S-1 was administered orally at 40 mg/m2 twice daily after a meal. Grade 2 or higher adverse reactions and Grade 1 non-hematologic toxicities made patients unwilling to undergo chemotherapy continuously, and the daily regimen was converted to the alternate-day regimen (40 mg/m2 twice daily on an alternate-day basis) based on the above theory.
The alternate-day regimen was equivalent to or better than the daily regimen in patients with inoperable or advanced gastric cancer in a clinical trial (58). A fact of particular note is that the incidences of adverse reactions remarkably differ between these regimens. As shown in Table 7, the daily regimen was continued in 20 among 92 patients; the regimen was discontinued in 72 among 92 patients due to ≥Grade 1 adverse reactions and was converted to the alternate-day regimen after about 1 week of drug withdrawal. The incidences of adverse reactions that developed in these 72 patients were compared before and after the conversion. After the conversion, Grade 1 or 2 leukopenia/neutropenia and ≥Grade 3 diarrhea and pigmentation did not develop. The total numbers of Grade 3, 2 and 1 adverse reactions markedly reduced from 3 to 0, 33 to 2 and 36 to 5 cases, respectively. Furthermore, the mean duration of treatment was 47 days in 92 patients who underwent the daily regimen and was 272 days in 72 patients who underwent the alternate-day regimen (57). The disappearance of hematologic toxicities allows long-lasting treatment while sustaining the efficacy of 5-FU. The elimination of Grade 1/2 adverse reactions, e.g. fatigue, diarrhea, pigmentation/dermatitis, nausea/vomiting, appetite loss, stomatitis and taste alteration, permits patient compliance and makes long-lasting treatment possible. The alternate-day S-1 regimen was at least equivalent to its daily regimen with respect to efficacy. Thirty-five of 42 curability C patients underwent the alternate-day S-1 regimen and had an MST of 383 days. On the other hand, six of 23 curability C patients underwent the daily regimen of S-1 and had an MST of 274 days (58).
Table 7.
The incidences of adverse reactions in patients with advanced gastric cancer who were treated by the daily regimen of S-1 (n = 92) and then by the alternate-day regimen of S-1 (n = 72) (Ref. 58)
| Adverse reactions | Daily regimen (n = 92)a |
Alternate-day regimen (n = 72)b |
||||||
|---|---|---|---|---|---|---|---|---|
| G1, 36 (50%); G2, 33 (46%); G3, 3 (4%) |
G1, 5 (7%); G2, 2 (3%) |
|||||||
| G1 | G2 | G3 | G4 | G1 | G2 | G3 | G4 | |
| Leukopenia/neutropenia | 5 | 11 | 0 | 0 | 1 | 0 | 0 | 0 |
| Anemia | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver dysfunction | 5 | 4 | 1 | 0 | 0 | 0 | 0 | 0 |
| Renal dysfunction | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| General fatigue | 14 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 12 | 7 | 1 | 0 | 1 | 1 | 0 | 0 |
| Pigmentation/dermatitis | 12 | 5 | 1 | 0 | 1 | 1 | 0 | 0 |
| Nausea/vomiting | 11 | 4 | 0 | 0 | 2 | 0 | 0 | 0 |
| Appetite loss | 9 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stomatitis | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Taste alteration | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Herpes zoster | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Others | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
G, grade. aMean duration of treatment 47 days, b272 days. Overlapped toxicities developed in some patients.
Retrospective clinical studies on the alternate-day S-1 regimen, on the twice-a-week administration of CDDP, and on the once-a-week administration of paclitaxel (TXL) were conducted in patients with advanced pancreatic (59), colorectal (60) and gastric (61) cancers and in patients with early gastric cancer (62). MST was extended considerably in all these cancers, and the incidences of ≥Grade 1 non-hematologic adverse reactions were very low. Consequently, long-lasting treatment was possible.
Clinical Importance of GI Toxicities
The GI tract, especially the small intestine, has drawn much attention as an organ that plays a major role in the immune function of the human body since 1997. Furthermore, enteral nutrition through the small intestine has been evidenced to be superior to central venous nutrition in sustaining intestinal functionality for patients who are no longer capable of ingestion. Animals fed by total parenteral nutrition only had a significantly fewer number of gut-associated lymphoid tissue lymphocytes compared with that of chow-fed control animals. The number of Peyer’s patches increased after a single day of refeeding, returning to their normal account by 48 h thereafter (63,64). Therefore, the alleviation of GI toxicities to a level at which treatment can be conducted while the patient continues to have a meal not only means the suppression of diarrhea or vomiting, but is also crucially important in improving immune function of the human body and conducting long-lasting treatment. Most anticancer drugs induce immunosuppression as an adverse reaction. We have reported on 5-FU-induced immunosuppression in a preclinical study (65). Body weight of rats treated with S-1 (FT plus CDHP plus Oxo) for seven consecutive days was significantly higher than that of rats treated with FT plus CDHP for a similar period. The number of peripheral leukocytes was significantly higher in S-1-treated rats than that FT plus CDHP-treated rats. This suggests that Oxo in S-1 may reduce the suppression of antitumor immunity induced by 5-FU.
CONCLUSION
5-FU is currently used as a core drug in the widely accepted international standard regimes to treat GI cancers. All these therapeutic modalities are the combinations of three or more existing anticancer agents and their combinations with recently developed molecular target agents. Combination therapy is now a predominant approach in cancer chemotherapy. Most recent combination studies of S-1 with CDDP, CPT-11, TXL and other anticancer agents indicate the crucial importance of exploring the combination between the best partner drug and S-1: (i) allows long-lasting combination therapy in an attempt to validate a therapeutic modality that is useful for cancer patients, (ii) prolongs the treatment period without increasing the incidences of non-hematologic toxicities that are most distressful for them and (iii) confers survival benefit to cancer patients.
‘S-1 and low-dose CDDP therapy’ and ‘alternate-day S-1 regimen’ may be considered as the most patient-friendly therapies available to date that allow long-lasting treatment that provokes no, or little, hematologic toxicities and induces only ≤Grade 1 non-hematologic toxicities. I am fully confident that ‘balancing between the efficacy and toxicity of an anticancer agent, conferring survival benefit to cancer patients’ will definitely contribute to their routine medical care.
Funding
Taiho Pharmaceutical Co., Ltd and Otsuka Pharmaceutical Co., Ltd. Funding to pay the Open Access publication charges for this article was provided by Taiho Pharmaceutical Co., Ltd.
Conflict of interest statement
The author is a consultant of Taiho Pharmaceutical Co., Ltd and receives a consulting fee.
Acknowledgements
The author manifests his gratitude to Dr Setsuro Fujii (deceased), Dr Kiyoji Kimura (deceased), Professor Tetsuo Taguchi and Dr Yukio Kobayashi for their contributions to the preclinical and clinical research of S-1. The author is also grateful to all investigators and personnel who have been involved in the preclinical and clinical developments of S-1 in Japan.
References
- 1.Goodman LS, Wintrobe MM, Dameshek W, Goodman MJ, Gilman A, McLennan MT. Nitrogen mustard therapy. Use of methyl-bis(beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl)amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia, and certain allied and miscellaneous disorders. J Am Med Assoc. 1946;132:126–32. doi: 10.1001/jama.1946.02870380008004. Reprinted in J Am Med Assoc 1984;251:2255-61. [DOI] [PubMed] [Google Scholar]
- 2.Hata T, Sano R, Sugawara R, Matsumae A, Kanamori K, Shima T, et al. Mitomycin, a new antibiotic from Streptomyces. J Antib Ser A. 1956;9:141–6. [PubMed] [Google Scholar]
- 3.Dushinsky R, Pleven E, Heidelberger C. The synthesis of 5-fluoropyrimidines. J Am Chem Soc. 1957;79:4559–60. [Google Scholar]
- 4.MacDonald JS, Schein P, Woolley PV, Smythe T, Ueno W, Hoth D, et al. 5-Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med. 1980;93:533–6. doi: 10.7326/0003-4819-93-4-533. [DOI] [PubMed] [Google Scholar]
- 5.Lokich JJ, Ahlgren JD, Gullo JJ, Philips JA, Fryer JG. A prospective randomized comparison of continuous infusion fluorouracil with conventional bolus schedule in metastatic colorectal carcinoma: A Mid-Atlantic Oncology Program Study. J Clin Oncol. 1989;7:425–32. doi: 10.1200/JCO.1989.7.4.425. [DOI] [PubMed] [Google Scholar]
- 6.Preusser P, Wilke H, Achterrath W, Fink U, Lenaz L, Heiinicke A. Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol. 1989;7:1310–7. doi: 10.1200/JCO.1989.7.9.1310. [DOI] [PubMed] [Google Scholar]
- 7.Wilkoff LJ, Wilcox WS, Burdeshaw JA, Dixon GJ, Dulmadge EA. Effect of antimetabolites on kinetic behavior of proliferating cultured L1210 leukemia cells. J Natl Cancer Inst. 1967;39:965–75. [PubMed] [Google Scholar]
- 8.Skipper HE, Schabel FM, Jr, Melllet LB, Montgomery JA, Wilkoff LJ, Lloyd HH, et al. Implications of biochemical, cytokinetics, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemother Rep. 1970;54:431–50. [PubMed] [Google Scholar]
- 9.Shimoyama M, Kimura K. Quantitative study on cytocidal action of anticancer agents. Saisin Igaku. 1973;28:1024–40. (in Japanese) [Google Scholar]
- 10.The Meta-analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal caner. J Clin Oncol. 1998;16:301–8. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 11.Hiller SA, Zhuk RA, Lidak MY. Pyrimidine nucleoside analogues. N1-(a-furanidyl) derivative of natural pyrimidine bases and their antimetabolites. Dokl Akad Nauk SSSR. 1967;176:332–5. (Chem Abstrt 1968;69:29664j) [PubMed] [Google Scholar]
- 12.Toide H, Akiyoshi H, Minato Y, Okuda H, Fujii S. Comparative studies on the metabolism of 2-(tetrahydrofuryl)-5-fluorouracil and 5-fluorouracil. Gann (Jpn J Cancer Res) 1977;68:553–60. [PubMed] [Google Scholar]
- 13.Ikeda K, Yoshisue K, Matsushima E, Nagayama S, Kobayashi K, Tyson CA, et al. Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin Cancer Res. 2000;6:4409–15. [PubMed] [Google Scholar]
- 14.Shimada T, Yamazaki H, Guengerich FP. Ethnic differences in coumarin 7-hydroxylation activities catalyzed by cytochrome P4502A6 in liver microsomes of Japanese and caucasian population. Xenobiotica. 1996;26:395–403. doi: 10.3109/00498259609046718. [DOI] [PubMed] [Google Scholar]
- 15.Buroker T, Miller A, Baker L, McKenzie M, Somson M, Vaitkevicius VK. Phase II clinical trial of ftorafur in 5-fluorouracil refractory colorectal cancer. Cancer Treat Pep. 1977;61:1579–80. [PubMed] [Google Scholar]
- 16.Fujii S, Kitano S, Ikenaka K, Shirasaka T. Effect of coadministration of uracil or cytosine on the anti-tumor activity of clinical doses of 1-(2-tetrahydrofuryl)- 5-fluorouraciland level of 5-fluorouracil in rodents. Gann (Jpn J Cancer Res) 1979;70:209–14. [PubMed] [Google Scholar]
- 17.Tatsumi K, Fukushima M, Shirasaka T, Fujii S. Inhibitory effects of pyrimidine, barbituric acid and pyridine derivatives on 5-fluorouracil degradation in rats liver extracts. Jpn J Cancer Res. 1987;78:748–55. [PubMed] [Google Scholar]
- 18.Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–9. [PubMed] [Google Scholar]
- 19.Shirasaka T, Shimamoto Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anti-cancer Drugs. 1996;7:548–57. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late Phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1M tegafur-0.4 M gimestat-1M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–20. doi: 10.1016/s0959-8049(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. Oncology. 2000;58:191–7. doi: 10.1159/000012099. [DOI] [PubMed] [Google Scholar]
- 22.Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B. S-1 Cooperative Study Group (Head and Neck Cancer Working Group) Late phase II study of S-1 in patients with advanced head and neck cancer. Jpn J Cancer Chemother. 2001;28:1381–90. [PubMed] [Google Scholar]
- 23.Ohtsu A, Baba H, Sakata Y, Mitachi Y, Horikoshi N, Sugimachi K, et al. Phase II Study of S-1, a novel oral fluoropyrimidine derivative, in patients with metastatic colorectal carcinoma. Br J Cancer. 2000;83:141–5. doi: 10.1054/bjoc.2000.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirao K, Ohtsu A, Takada H, Mitachi Y, Hirakawa K, Horikoshi N, et al. Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer. 2004;100:2355–61. doi: 10.1002/cncr.20277. [DOI] [PubMed] [Google Scholar]
- 25.Ichinose Y, Yoshimori K, Sakai H, Nakai Y, Sugiura T, Kawahara M, et al. S-1 plus cisplatin combination chemotherapy in patients with advanced non-small cell lung cancer a multi-institutional phase II trial. Clin Cancer Res. 2004;10:7860–4. doi: 10.1158/1078-0432.CCR-04-1200. [DOI] [PubMed] [Google Scholar]
- 26.Hino M, Saeki T, Sano M. Late phase II of S-1 in patients with taxane resistant breast cancer. ASCO Annu Meeting. 2004 Abstract No 745. [Google Scholar]
- 27.Okusaka T, Funakoshi A, Furuse J, Boku N, Yamao K, Ohkawa S, et al. A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2008;61:615–21. doi: 10.1007/s00280-007-0514-8. [DOI] [PubMed] [Google Scholar]
- 28.Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T, et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol. 2008;62:849–55. doi: 10.1007/s00280-007-0673-7. [DOI] [PubMed] [Google Scholar]
- 29.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fijii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 30.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 31.Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine and bile. Cancer Res. 1987;47:2203–6. [PubMed] [Google Scholar]
- 32.Houghton JA, Houghton PJ, Wooten RS. Mechanism of gastrointestinal toxicity in the mouse by 5-fluorouracil, 5-fluorouridine, and 5-fluoro-2′-deoxyuridine. Cancer Res. 1979;39:2406–13. [PubMed] [Google Scholar]
- 33.Schuetz JD, Wallace HJ, Diasio RB. 5-Fluorouracil incorporation into DNA of CF-1 mouse bone marrow cells as a possible mechanism of toxicity. Cancer Res. 1984;44:1358–63. [PubMed] [Google Scholar]
- 34.Matsubara I, Kamiya J, Imai S. Cardiotoxic activity of 5-fluorouracil in the guinea pig. Jpn J Pharmacol. 1980;30:871–9. doi: 10.1254/jjp.30.871. [DOI] [PubMed] [Google Scholar]
- 35.Koenig H, Patel A. Biochemical basis for fluorouracil neurotoxicity. The role of Krebs cycle inhibition by fluoroacetate. Arch Neurol. 1970;23:155–60. doi: 10.1001/archneur.1970.00480260061008. [DOI] [PubMed] [Google Scholar]
- 36.Yen-Revollo JL, Goldberg RM, McLeod HL. Can inhibiting dihydropyrimidine dehydrogenase limit hand–foot syndrome caused by fluoropyrimidines? Clin Cancer Res. 2008;14:8–13. doi: 10.1158/1078-0432.CCR-07-1225. [DOI] [PubMed] [Google Scholar]
- 37.Shirasaka T, Shimamoto Y, Kato T, Fukushima M. Invention of tumor-selective 5-fluorouracil derivative named S-1 by biochemical modulation of 5-fluorouracil. Jpn J. Cancer Chemother. 1998;25:371–84. [PubMed] [Google Scholar]
- 38.Granat P, Creasey WA, Calabresi P, Handschumacher RE. Investigations with 5-azaorotic acid, an inhibitor of the biosynthesis of pyrimidine de novo. Clin Pharmacol Ther. 1965;6:436–47. doi: 10.1002/cpt196564436. [DOI] [PubMed] [Google Scholar]
- 39.Shirasaka T, Yamamitsu S, Tsuji A, Taguchi T. Conceptual change in cancer chemotherapy: From an oral fluoropyrimidine drug, UFT, to a novel oral fluoropyrimidine prodrug, S-1, and low-dose FP therapy in Japan. Investig New Drug. 2000;18:315–29. doi: 10.1023/a:1006476730671. [DOI] [PubMed] [Google Scholar]
- 40.Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, et al. Pharmacokinetic Study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res. 1999;5:2000–5. [PubMed] [Google Scholar]
- 41.Adjei AA, Reid JM, Diasio RB, Sloan JA, Smith DA, Rubin J, et al. Comparative pharmacokinetic study of continuous venous infusion fluorouracil and oral fluorouracil with eniluracil in patients with advanced solid tumors. J Clin Oncol. 2002;20:1683–91. doi: 10.1200/JCO.2002.20.6.1683. [DOI] [PubMed] [Google Scholar]
- 42.Kim WY, Nakata B, Hirakawa K. Alternative pharmacokinetics of S-1 components, 5-fluorouracil, dihydrofluorouracil and α-fluoro-β-alanine after oral administration of S-1 following total gastrectomy. Cancer Sci. 2007;98:1604–8. doi: 10.1111/j.1349-7006.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 44.Scanlon J, Newman EM, Lu Y. Biochemical basis for cisplatin and 5-fluolourasil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986;83:8923–5. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirasaka T, Shimamoto Y, Ohshimo H, Saito H, Fukushima M. Metabolic basis of the synergistic antitumor activities of 5-fluorouracil and cisplatin in rodent tumour models in vivo. Cancer Chemother Pharmacol. 1993;32:167–72. doi: 10.1007/BF00685830. [DOI] [PubMed] [Google Scholar]
- 46.Araki H, Fukushima M, Kamiyama Y, Shirasaka T. Effect consecutive lower-dose cisplatin in enhancement of 5-fluorouracil cytotoxicity in experimental tumor cells in vivo. Cancer Lett. 2000;160:185–91. doi: 10.1016/s0304-3835(00)00583-8. [DOI] [PubMed] [Google Scholar]
- 47.Kondo K, Murase M, Kodera Y, Akiyama S, Ito K, Yokoyama Y, et al. Feasibility study on protracted infusional 5-fluorouracil and consecutive low-dose cisplatin for advanced gastric cancer. Oncology. 1996;53:64–7. doi: 10.1159/000227537. [DOI] [PubMed] [Google Scholar]
- 48.Chung Y, Yamashita Y, Matsuoka T, Nakata T, Onoda N, Maeda K, et al. Continuous infusion of 5-fluorouracil and low dose cisplatin infusion for the treatment of advanced and recurrent gastric adenocarcinoma. Cancer. 1997;80:1–7. [PubMed] [Google Scholar]
- 49.Nakata B, Mitachi Y, Tsuji A, Yamamitsu S, Hirata K, Shirasaka T, et al. Combination phase I trail of a novel oral fluorouracil derivative S-1 with low-dose cisplatin for unresectable and recurrent gastric cancer (JFMC27-9902) Clin Cancer Res. 2004;10:1664–9. doi: 10.1158/1078-0432.ccr-03-0045. [DOI] [PubMed] [Google Scholar]
- 50.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 51.Hara T, Koizumi W, Narahara H, Takagane A, Akiya T, Takagi M, et al. Report of safety analysis in a randomized phase III study comparing S-1 alone with S-1 plus CDDP in advanced gastric cancer (The SPIRITS trial; TS-1 Advanced Gastric Cancer Clinical Trial Group) EJC. 2007;5:264. #3516 (abstr) [Google Scholar]
- 52.Tsuji A, Shima Y, Morita S, Uchida M, Okamoto K, Morita M, et al. Combination chemotherapy of S-1 and low-dose twice-weekly cisplatin for advanced and recurrent gastric cancer in an outpatient setting: a retrospective study. Anticancer Res. 2008;28:1433–8. [PubMed] [Google Scholar]
- 53.Lipkin M, Sherlock P, Bell B. Cell proliferation kinetics in the gastrointestinal tract of man. II. Cell renewal in stomach, ileum, colon, and rectum. Gastroenterogy. 1963;45:721–9. [PubMed] [Google Scholar]
- 54.Clarkson B, Ota K, Ohkita T, O’Connor A. Kinetics of proliferation of cancer cells in neoplastic effusions in man. Cancer. 1965;18:1189–213. doi: 10.1002/1097-0142(196510)18:10<1189::aid-cncr2820181002>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 55.Cronkite EP, Bond VP, Fliedner YM, Killmann SA. The use of tritiated thymidine in the study of haemopoietic cell proliferation. In: Wolsteinholme GEW, O'Connor M, editors. Ciba Foundation Symposium on Haemopoiesis. Boston: Little Brown &Co; 1960. pp. 56, 70–98. [Google Scholar]
- 56.Saga Y, Suzuki M, Sato I, Shirasaka T. An in vitro examination of a 5-fluorouracil regimen involving continuous venous infusion using cultured cell lines derived from ovarian cancers. Oncol Rep. 2000;7:625–8. doi: 10.3892/or.7.3.625. [DOI] [PubMed] [Google Scholar]
- 57.Arai W, Hosoya Y, Hyodo M, Haruta H, Kurashima K, Saito S, et al. Comparison of alternate-day versus consecutive-day treatment with S-1: assessment of tumor growth inhibition and toxicity reduction in gastric cancer cell line in vitro and in vivo. Int Clin Oncol. 2008;9 doi: 10.1007/s10147-008-0780-4. in press. [DOI] [PubMed] [Google Scholar]
- 58.Arai W, Hosoya Y, Hyodo M, Yokoyama T, Hirashima Y, Yasuda Y, et al. Alternate-day oral therapy with TS-1 for advanced gastric cancer. Int Clin Oncol. 2004;9:143–8. doi: 10.1007/s10147-004-0381-9. [DOI] [PubMed] [Google Scholar]
- 59.Yamamitsu S, Kimura K, Yamada Y, Inui N, Hiyama S, Hirata K, et al. The first report from Sapporo Tsukisamu Hospital―Chemotherapy for patients with advanced pancreatic cancer. Jpn J Cancer Chemother. 2007;34:1059–66. [PubMed] [Google Scholar]
- 60.Yamamitsu S, Kimura K, Yamada Y, Inui N, Hiyama S, Hirata K, et al. The second report from Sapporo Tsukisamu Hospital―Chemotherapy for patients with advanced colorectal cancer. Jpn J Cancer Chemother. 2007;34:1241–7. [PubMed] [Google Scholar]
- 61.Yamamitsu S, Kimura K, Yamada Y, Inui N, Hiyama S, Hirata K, et al. The third report from Sapporo Tsukisamu Hospital―Chemotherapy for patients with advanced gastric cancer (peritoneal dissemination, peritonitis carcinomatosa) Jpn J Cancer Chemother. 2007;34:1241–7. [PubMed] [Google Scholar]
- 62.Yamamitsu S, Kimura K, Yamada Y, Inui N, Hiyama S, Hirata K, et al. The fourth report from Sapporo Tsukisamu Hospital―Chemotherapy and its regimen as second choice for patients with earky gastric cancer (peritoneal dissemination, peritonitis carcinomatosa) Jpn J Cancer Chemother. 2007;34:1589–94. [PubMed] [Google Scholar]
- 63.Janu P, Li J, Renegar KB, Kudsk KA. Recovery of gut-associated lymphoid tissue and upper Respiratory tract immunity after parenteral nutrition. Ann Surg. 1997;225:707–17. doi: 10.1097/00000658-199706000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.King BK, Li J, Kudsk KA. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;123:1303–9. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 65.Yamashita T, Ueda Y, Fuji N, Itoh T, Kurioka H, Shirasaka T, et al. Potassium oxonate, an enzyme inhibitor compounded in S-1, reduces the suppression immunity induced by 5-fluorouracil. Cancer Chemother Pharmacol. 2005;30:1–6. doi: 10.1007/s00280-005-0150-0. [DOI] [PubMed] [Google Scholar]