Abstract
Aims
Supervised exercise can benefit selected patients with heart failure, however the effectiveness of home-based exercise remains uncertain. We aimed to assess the effectiveness of a home-based exercise programme in addition to specialist heart failure nurse care.
Methods and results
This was a randomized controlled trial of a home-based walking and resistance exercise programme plus specialist nurse care (n = 84) compared with specialist nurse care alone (n = 85) in a heart failure population in the West Midlands, UK. Primary outcome: Minnesota Living with Heart Failure Questionnaire (MLwHFQ) at 6 and 12 months. Secondary outcomes: composite of death, hospital admission with heart failure or myocardial infarction; psychological well-being; generic quality of life (EQ-5D); exercise capacity. There was no statistically significant difference between groups in the MLwHFQ at 6 month (mean, 95% CI) (−2.53, −7.87 to 2.80) and 12 month (−0.55, −5.87 to 4.76) follow-up or secondary outcomes with the exception of a higher EQ-5D score (0.11, 0.04 to 0.18) at 6 months and lower Hospital Anxiety and Depression Scale score (−1.07, −2.00 to −0.14) at 12 months, in favour of the exercise group. At 6 months, the control group showed deterioration in physical activity, exercise capacity, and generic quality of life.
Conclusion
Home-based exercise training programmes may not be appropriate for community-based heart failure patients.
Keywords: Heart failure, Exercise therapy, Randomized controlled trial
Introduction
Meta-analyses of randomized controlled trials (RCTs) have demonstrated that exercise training programmes for selected patients with heart failure reduce mortality1 and improve exercise capacity and health-related quality of life.2,3 However, many of these trials were supervised exercise programmes undertaken in a hospital setting, were of short duration, and recruited a relatively small selective population of younger and predominantly male patients without co-morbidities.4
In addition, a number of trials were undertaken prior to the routine use of beta-blockers in patients with heart failure and therefore had control groups that received a level of care that now would be considered suboptimal. The effectiveness of specialist heart failure nurse and other multidisciplinary team interventions in reducing hospital admissions5 and reducing mortality6 mean that this should now be the standard care received by the comparison group in a trial of exercise therapy.
In the UK, the National Institute for Health and Clinical Excellence (NICE) has recommended that patients with heart failure should be encouraged to adopt regular aerobic and/or resistive exercise, possibly as part of an organized programme.7 Given the poor uptake and adherence to centre-based cardiac rehabilitation programmes by patients post-myocardial infarction,8 home-based exercise intervention could be more accessible and acceptable for this patient group. However, the greater age and amount of co-morbidity in patients with heart failure may argue for exercise rehabilitation for this population to be undertaken in a supervised centre-based setting.
We conducted a clinical RCT to determine the effectiveness of a home-based exercise programme. We hypothesized that the addition of a home-based exercise programme to specialist nurse care would improve the outcomes of a community-based heart failure population.
Methods
The trial protocol for BRUM-CHF has been reported in detail elsewhere (ISRCTN68886157).9 The investigation conforms with the principles outlined in the Declaration of Helsinki. Ethical approval was granted by the local research Ethics Committees.
Patients and recruitment
Recruitment took place between June 2004 and December 2005. Patients were eligible if they had a left ventricular ejection fraction of ≤40% on echocardiogram and had had a severity of at least New York Heart Association (NYHA) group II in the previous 24 months. They had to have been clinically stable for 4 weeks and in receipt of optimal medical treatment and in the care of a specialist heart failure nurse team from two acute hospital trusts and one primary care trust in the West-Midlands health region, UK, and not considered high-risk for a home-based exercise programme. Exclusion criteria included NYHA IV, MI, or revascularization within the past 4 months, hypotension, unstable angina, ventricular or symptomatic arrhythmias, obstructive aortic valvular disease, chronic obstructive pulmonary disease, hypertrophic obstructive cardiomyopathy, severe musculoskeletal problems preventing exercise, and case-note reported dementia or current severe psychiatric disorder.
Patients were identified from the specialist heart failure services of the participating centres. Clinical records were reviewed to ascertain potentially suitable patients who were then invited to participate. Those who agreed to take part and provided written informed consent undertook a shuttle walking test supervised by a trained medical professional to identify any clear contraindications (cardiovascular or skeletomuscular) that prevented their undertaking an exercise programme. Such patients were excluded.
Randomization
Patients were randomized in a 1:1 ratio to specialist heart failure nurse care with an exercise programme (exercise group) or specialist heart failure nurse care alone (control group). An independent clinical trials unit using a computerized programme undertook randomization after each patient had consented and undergone the baseline tests and questionnaires. To ensure between-group balance, minimization10 (based on NYHA group, presence or absence of atrial fibrillation, and hospital of recruitment) was used during the recruitment period.
Interventions
Both groups received specialist heart failure nurse input in primary and secondary care through clinic and home visits that included the provision of information about heart failure, advice about self-management and monitoring of their condition, and titration of beta-blocker therapy.11
In addition to specialist heart failure nurse care, the exercise group received a programme that commenced with three supervised exercise sessions to plan an individualized exercise programme. These were followed by a home-based programme, with home visits at 4, 10, and 20 weeks, telephone support at 6, 15, and 24 weeks, and a manual with details about safe progressive exercise and self-monitoring of frequency, duration, and intensity.
The home exercise programme was based on current recommendations for exercise training in heart failure,12 consisting of both aerobic and resistance elements. The aerobic component was based on progressive walking with self-completion exercise logs. Performance on the incremental shuttle walking test (ISWT) was used to individually define walking intensity (speed) of the exercise programme. Intensity was set at 70% of peak performance, assuming the relationship with peak VO2 and distance walked on the ISWT as described by Keell et al.13 Walking time was measured and progressed during the supervised sessions in the initial 2 weeks and progressed gradually thereafter using the Borg breathlessness scale and aiming for a score of 3 (moderately breathless). The aim of the programme was to ultimately achieve continuous bouts of exercise (20–30 min) five times a week after 6 months of the home programme. Strength training was of low intensity, focusing on both upper and lower limbs, with patients completing sets of up to 10 repetitions of eight exercises using containers filled with gradually increasing volumes (thus weights) of water which were individually prescribed. Individual targets were set and patients assessed the level of difficulty using the Borg exertion scale and aimed to achieve a rating of 12–13 (somewhat hard). Individual exercises were omitted in a minority of patients with particular needs or difficulties. After 24 weeks, patients were encouraged to continue to maintain their levels of aerobic and strength exercises.
Outcome measures and data collection
The primary outcome was disease-specific quality of life measured by the Minnesota Living with Heart Failure Questionnaire (MLwHFQ), with higher scores representing a lower quality of life,14 and a change of 5 points considered to be clinically significant.15 Secondary outcomes were a composite outcome of death or admission with heart failure or myocardial infarction; ISWT,13 psychological wellbeing (Hospital Anxiety and Depression Scale—HADS),16 self-reported physical activity (exercise component of the Health Behaviours Profile,17 a modified Godin questionnaire18); blood pressure (assessed according to British Hypertension Society Guidelines); a generic measure of health-related quality of life (EQ-5D)19; and health care utilization. The ISWT has been validated as an objective measure of exercise capacity and peak oxygen uptake in the heart failure population13,20. All outcomes were assessed at baseline (prior to randomization) and at 6 and 12 month follow-ups with the exception of ISWT and blood pressure, which were not collected at 12 months.9
The nurses supporting the exercise intervention recorded the number of patient contacts. Adherence to the exercise intervention was ascertained at the home visits at 4, 10, and 20 weeks by a questionnaire detailing frequency, duration, and intensity of exercise undertaken in the previous week.
Outcomes were assessed by questionnaire completed by the patient and clinical assessment by a nurse at a hospital site at baseline and 6 months. Where possible, the nurse undertaking the assessment was blinded to the treatment allocation of the patient, but owing to staffing issues, this occurred in only 62% of participants followed up at 6 months. Follow-up at 12 months was by postal questionnaire.
Statistical analysis
On the basis of a previous trial (Belardinelli et al.21) and allowing for 20% mortality and loss to follow-up, 168 patients (84 per group) were required to detect a between-group difference of 10 points on the MLwHFQ scale at the 5% significance level and 80% power.
As pre-specified, between- and within-group analyses for primary and secondary outcomes at 6 and 12 months were performed according to intention to treat (i.e. according to initial randomized allocation to intervention or control). For outcomes measured on a continuous scale (MLwHFQ questionnaire, HADS, EQ-5D, blood pressure, self-reported physical activity, distance walked on ISWT), differences between groups were investigated using least squares linear regression. Time to first clinical event (hospital admission for heart failure or mortality or myocardial infarction) was displayed as Kaplan–Meier survival curves and compared using Cox regression analysis.
Between-group analyses were expressed as both unadjusted (corrected for baseline score) and adjusted differences (corrected for minimization variables and co-variates found to be different between groups at baseline).
Sensitivity analysis was undertaken to assess for the effect of missing outcome values using an imputation assuming not missing at randomization.22 Regression-based models at 6 months were developed to assess the relationship between co-variates and outcome measures in completers.23 As no difference in between-group inference was found in these sensitivity analyses, non-imputed results are reported here.
Two-tailed P-values of <5% level are considered to indicate statistical significance, with no adjustment for multiplicity and results expressed as means and 95% confidence intervals. All analyses were undertaken using SPSS v.12 and Stata v.8.
Results
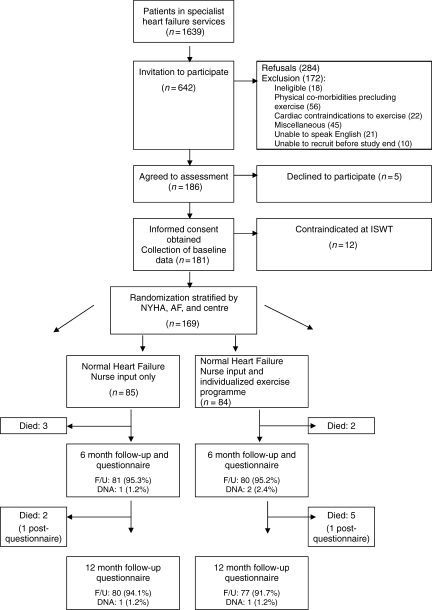
The majority of patients on the specialist heart failure nurse registers were either not eligible for the study or had an exclusion criterion (Figure 1). We randomized 169 patients, 85 assigned to the control group and 84 to the exercise group. Baseline characteristics were broadly comparable, the exception being that the exercise group was somewhat younger and had higher HADS depression scores and a lower systolic blood pressure (Table 1). Five patients died (exercise group n=2, control group n=3) and a further three patients did not complete the outcome questionnaire at 6 months. Five patients died between 6 and 12 months follow-up and two patients did not complete the outcome questionnaires at 12 months. Outcome data were therefore available for 161 (95%) patients at 6 months and 157 (92%) patients at 12 months.
Figure 1.
Flow of patients through the BRUM-CHF study. NYHA, New York Heart Association class; AF, atrial fibrillation; ISWT, incremental shuttle walking test; DNA, did not attend; F/U, follow-up.
Table 1.
Baseline characteristics
| Exercise arm (n = 84) | Control arm (n = 85) | |
|---|---|---|
| Sex, n (%) | ||
| Males | 64 (76.2) | 62 (72.9) |
| Females | 20 (23.8) | 23 (27.1) |
| NYHA on recruitment, n (%) | ||
| I | 4 (4.8) | 6 (7.1) |
| II | 63 (75.0) | 62 (72.9) |
| III | 17 (20.0) | 17 (20.0) |
| AF present on recruitment, n (%) | 17 (20.2) | 18 (21.2) |
| Age, mean (SD) | 65.9 (12.5) | 70.0 (12.5) |
| Ethnicity, n (%) | ||
| White | 69 (83.1) | 74 (87.1) |
| South Asian | 5 (6.0) | 5 (5.9) |
| Other | 9 (10.8) | 6 (7.1) |
| Past medical history, n (%) | ||
| Diabetes | 18 (21.4) | 19 (22.4) |
| Stroke | 8 (9.5) | 7 (8.2) |
| Peripheral vascular disease | 7 (8.3) | 7 (8.2) |
| Angina | 34 (40.5) | 36 (42.4) |
| Hypertension | 30 (35.7) | 38 (44.7) |
| Valvular heart disease | 14 (16.7) | 19 (22.4) |
| Myocardial infarction | 40 (47.6) | 33 (38.8) |
| CABG | 16 (19.0) | 10 (11.8) |
| Angioplasty | 15 (17.9) | 14 (16.5) |
| Attended cardiac rehabilitation | 14 (16.7) | 10 (11.8) |
| In paid employment, n (%) | 7 (8.3) | 4 (4.7) |
| Years in full-time education, mean (SD) | 10.9 (3.7) | 10.6 (2.2) |
| Current smoker, n (%) | 7 (8.3) | 8 (9.4) |
| Units of alcohol/week, mean (SD) | 5.85 (8.0) | 5.23 (8.7) |
| MLwHFQ score, mean (SD) | 33.35 (21.3) | 31.82 (22.5) |
| ISWT distance (m), mean (SD) | 234.0 (132.6) | 220.5 (130.7) |
| Self-reported physical activity score, mean (SD) | 4.55 (2.5) | 4.67 (2.6) |
| HADS anxiety score, mean (SD) | 6.05 (4.36) | 5.48 (4.07) |
| HADS depression score, mean (SD) | 6.27 (3.58) | 5.01 (3.07) |
| EQ5D, mean (SD) | 0.675 (0.25) | 0.696 (0.26) |
| SBP (mmHg), mean (SD) | 116.63 (15.6) | 124.0 (19.7) |
| DBP (mmHg), mean (SD) | 67.85 (10.8) | 68.29 (12.4) |
| Drugs, n (%) | ||
| Diuretic | 77 (92.8) | 78 (91.8) |
| ACE-inhibitor or A-II receptor antagonists | 82 (98.8) | 80 (94.1) |
| Beta-blockers | 61 (73.5) | 52 (61.2) |
NYHA, New York Heart Association class; AF, atrial fibrillation; CABG, coronary artery bypass graft; MLwHFQ, Minnesota Living with Heart Failure Questionnaire; ISWT, incremental shuttle walking test; HADS, Hospital Anxiety and Depression Scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE, angiotensin-converting enzyme; A-II, angiotensin II receptor blocker.
Primary and secondary outcomes
Between-group analysis
At 6 months, there was no between-group difference in the primary outcome (MLwHFQ) or secondary outcomes, with the exception of a higher EQ-5D score (mean 0.11, 95% CI 0.04 to 0.18) in the exercise group (Table 2).
Table 2.
Primary and secondary outcomes at 6 and 12 months and between-group differences
| Exercise |
Usual care |
Mean differencea | 95% CI of mean difference | Adjusted mean differenceb | 95% CI of adjusted mean difference | P-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||||
| Outcomes at 6 months | |||||||||
| MLwHFQc | 36.26 | 24.08 | 34.49 | 23.98 | 0.25 | −5.03 to 5.53 | −2.53 | −7.87 to 2.80 | 0.3 |
| Distance on ISWT/m | 226.95 | 120.3 | 206.18 | 135.2 | 21.18 | −5.79 to 48.12 | 14.98 | −11.89 to 41.86 | 0.1 |
| Self-reported physical activityd | 4.63 | 3.54 | 3.54 | 3.81 | 1.14* | 0.05 to 2.23 | 1.05 | −0.05 to 2.14 | 0.06 |
| HADS anxiety score | 6.39 | 4.34 | 5.45 | 4.23 | 0.57 | −0.42 to 1.56 | 0.45 | −0.55 to 1.45 | 0.4 |
| HADS depression score | 6.51 | 4.07 | 5.90 | 3.78 | −0.57 | −1.52 to 0.39 | −0.68 | −1.64 to 0.28 | 0.2 |
| EQ-5Dd | 0.663 | 0.242 | 0.617 | 0.319 | 0.064 | −0.01 to 0.14 | 0.11* | 0.04 to 0.18 | 0.004 |
| Systolic BP | 122.66 | 16.62 | 126.91 | 21.03 | 0.30 | −4.92 to 5.52 | −0.30 | −5.52 to 4.94 | 0.9 |
| Diastolic BP | 70.86 | 11.50 | 69.62 | 14.11 | 2.07 | −1.06 to 5.19 | 1.94 | −1.17 to 5.06 | 0.2 |
| Outcomes at 12 months | |||||||||
| MLwHFQc | 37.61 | 20.97 | 34.91 | 24.80 | 2.23 | 5.99 to 16.81 | −0.55 | −5.87 to 4.76 | 0.8 |
| Self-reported physical activityd | 3.60 | 2.64 | 3.78 | 4.09 | −0.12 | −1.11 to 0.86 | 0.05 | −1.1 to 0.96 | 0.9 |
| HADS anxiety score | 6.68 | 4.31 | 5.62 | 4.50 | 0.75 | −0.28 to 1.79 | 0.23 | −0.81 to 1.27 | 0.7 |
| HADS depression score | 6.26 | 3.73 | 5.50 | 3.91 | −0.98* | −1.90 to −0.06 | −1.07* | −2.00 to −0.14 | 0.02 |
| EQ-5Dd | 0.679 | 0.21 | 0.691 | 0.28 | 0.059 | −0.003 to 0.121 | 0.058 | −0.01 to 0.12 | 0.07 |
BP, blood pressure; HADS, Hospital Anxiety and Depression Scale; ISWT, incremental shuttle walking test; MLwHFQ, Minnesota Living with Heart Failure Questionnaire.
aAdjusted for baseline value.
bAdjusted for baseline value, age, sex, NYHA class, centre of recruitment, presence or absence of AF, HADS, and blood pressure at baseline.
cScores range from 0 to 121, with higher scores indicating worse quality of life.
dSelf-reported physical activity using score on 0–18 scale.
eScores range from 1.00 (indicating perfect health) to –0.59 (worse imaginable health state).
*P < 0.05.
At 12 months, there was no significant between-group difference in the primary outcome (MLwHFQ) at 12 months follow-up (mean −0.55, 95% CI −5.87 to 4.76) or secondary outcomes, with the exception of a lower HADS depression score (mean −1.07, 95% CI −2.00 to −0.14) at 12 months in the exercise group (Table 2).
During the year from recruitment (pre-defined as 12 months + 30 days), 12 participants died, 7 in the exercise arm, and there were 6 admissions for heart failure, 4 in the exercise arm. No participant had a myocardial infarction. Mean time to first event (admission for heart failure, a myocardial infarction, or death) was 425 days (95% CI 407–443 days) in the exercise arm and 531 days (95% CI 513–550 days) in the control arm. In a Cox regression analysis, the adjusted hazard ratio for an event in the exercise compared with control group was 1.45 (95% CI 0.43 to 4.86).
Within-group analysis
In the exercise group, there was no change in primary or secondary outcomes from baseline to 6 month follow-up, with the exception of an increase in mean systolic (mean 5.8, 95% CI 1.9 to 9.8 mmHg) and diastolic (mean 3.3, 95% CI 1.2 to 5.4 mmHg) blood pressure (Table 3). Compared with baseline, the control group showed a significant reduction in exercise capacity (mean −24.8, 95% CI −45.70 to −4.01 m), self-reported physical activity (−1.1, 95% CI −2.0 to −0.2), HADS depression score (mean 1.0, 95% CI 0.5 to 1.56), and EQ-5D score (mean −0.08, 95% CI −0.14 to −0.02) at 6 months.
Table 3.
Within-group differences from baseline to 6 months and 6–12 months
| Exercise intervention |
Usual care |
|||
|---|---|---|---|---|
| Mean difference | 95% CI | Mean difference | 95% CI | |
| 0–6 months | ||||
| MLwHFQ | 2.18 | −2.10 to 6.45 | 2.37 | −1.15 to 5.89 |
| Distance on ISWT/m | −2.20 | −23.23 to 16.11 | −24.80* | −45.70 to −4.01 |
| Self-reported physical activity | 0.10 | −0.67 to 0.87 | −1.10* | −1.98 to −0.22 |
| HADS anxiety score | 0.31 | −0.48 to 1.10 | −0.09 | −0.82 to 0.64 |
| HADS depression score | 0.08 | −0.68 to 0.86 | 1.00** | 0.42 to 1.58 |
| EQ-5D | −0.005 | −0.06 to 0.05 | −0.08* | −0.14 to −0.02 |
| Systolic BP | 5.85* | 1.92 to 9.79 | 0.80 | 2.16 to 6.05 |
| Diastolic BP | 3.26* | 1.16 to 5.36 | −0.08 | 1.69 to 3.42 |
| 6–12 months | ||||
| MLwHFQ | 1.97 | −1.50 to 5.44 | 0.63 | −2.73 to 4.00 |
| Self-reported physical activity | −1.08* | −1.98 to −0.18 | 0.19 | −0.74 to 1.11 |
| HADS anxiety score | 0.36 | −0.36 to 1.07 | 0.13 | −0.52 to 0.78 |
| HADS depression score | −0.11 | −0.76 to 0.54 | 0.30 | −0.27 to 0.86 |
| EQ-5D | 0.007 | −0.04 to 0.06 | 0.009 | −0.04 to 0.06 |
BP, blood pressure; HADS, Hospital Anxiety and Depression Scale; ISWT, incremental shuttle walking test; MLwHFQ, Minnesota Living with Heart Failure Questionnaire.
*P < 0.05.
**P = 0.001.
From 6 to 12 month follow-up in the exercise arm, there was no within-group change apart from a significant decrease in self-reported physical activity (Table 3). In the control arm, there were no significant differences over time.
Adherence to the exercise intervention
Of the 84 patients randomized to the exercise group, 68 (81%) completed the initial assessment and attended the three hospital-based classes. At the 4 week home visit, 62 (74%) patients in the intervention arm were participating in the exercise programme, by 10 weeks, this had fallen to 53 (63%) and by 20 weeks, 45 (54%). At the home visits, participants who were still exercising reported that they were doing a mean of five sessions of aerobic exercise (walks) per week, as prescribed, with a median duration (IQR) increasing from 12 (8–20) min at 4 weeks to 15 (11–27.5) min at 20 weeks. Participants also reported to be undertaking the two strength training sessions per week. The mean (SD) Borg breathlessness scores achieved during walking at 4, 10, and 20 weeks were 3.13 (0.87), 2.94 (1.13), and 3.18 (0.86), respectively, and the Borg perceived exertion scores were 11.0 (1.83), 11.0 (1.70), and 11.56 (1.48), respectively.
In a post hoc analysis comparing those adhering to the exercise at 20 weeks (reporting at least two exercise sessions per week) with the controls, there were no significant differences at 6 months, but all the outcomes were in a direction favouring the exercise group. At 6 months, the adjusted mean difference for the MLwHFQ was –4.97 (95% CI –7.87 to 2.80) and the mean distance walked on the ISWT 17.3 (95% CI –11.89 to 41.86). At 12 months, the adherent exercise participants had a significantly higher EQ-5D (adjusted mean difference 0.14, 95% CI 0.05 to 0.22, P = 0.001) than controls.
Health service utilization
Over the 12 months of follow-up, a similar number of patients in the two groups were admitted to hospital for any cause (exercise16/84 vs. control 20/85) or for a cardiac condition (11/85 vs. 11/84) and there was no difference in mean number of nights of admissions for any cause or a cardiac condition [mean nights for a cardiac condition: exercise group 1.2 (standard deviation 6.3) vs. control group 1.4 (standard deviation 5.8)] (Table 4).
Table 4.
Health service resource use 0–12 months
| Exercise intervention |
Control |
P-valuea | |||||
|---|---|---|---|---|---|---|---|
| Number of patients | Mean | SD | Number of patients | Mean | SD | ||
| Hospital admissions all causes (nights) | 16b | 3.07 | 11.72 | 20 | 2.38 | 7.75 | 0.2 |
| Hospital admissions cardiac causes (nights) | 11 | 1.15 | 6.28 | 11 | 1.38 | 5.84 | 0.1 |
| Day case admissions | 13 | 0.27 | 0.70 | 16 | 0.41 | 1.09 | 0.2 |
| Out-patient visits | 54 | 1.89 | 2.02 | 38 | 1.33 | 1.85 | 0.3 |
| Specialist heart failure nurse consultations | 61 | 2.24 | 1.98 | 57 | 2.63 | 2.60 | 0.4 |
| Visits to general practitioner | 31 | 1.89 | 3.04 | 30 | 1.65 | 2.49 | 0.7 |
| Home visits from general practitioner | 3 | 0.16 | 0.99 | 4 | 0.22 | 0.98 | 0.8 |
| Practice nurse consultations | 24 | 1.12 | 2.58 | 20 | 0.99 | 1.92 | 0.6 |
Overall, only 19% of participants were admitted for any cause, and 12% for a cardiac cause.
aAdjusted for age, sex, NYHA class, centre of recruitment, presence or absence of AF, with HADS, and blood pressure at baseline added stepwise.
bSeventy-three nights from 1 patient admitted with a CVA.
Discussion
The findings of this study failed to confirm the hypothesis that the addition of a home-based exercise programme to specialist nurse care would improve the outcomes of a community-based heart failure population. However, there was some evidence of improvement in the exercise group compared with control, i.e. higher quality of life (EQ-5D) at 6 months and reduced psychological distress (HAD scale) at 12 months.
Comparison with previous studies
Our results differ from the one other UK-based trial of the addition of exercise to specialist heart failure nurse care to date. Austin et al.24 reported a greater improvement in health-related quality of life, NYHA class, and exercise capacity in exercisers compared with controls at 6 month follow-up. However, our study differs from this previous trial in a number of important ways. First, the exercise intervention was centre-based and supervised by a nurse specialist and had group-based education sessions as part of the intervention. Therefore, there was more opportunity for the monitoring and maintenance of an optimal exercise dosage, particularly training intensity. Second, patients in the previous trial were not on optimized therapy at entry to the study, so there were major changes in patients’ pharmacological management and lifestyle that took place in the first few weeks of the trial. Although these changes would have been experienced by both groups, there were differences between groups and it is plausible that they may have facilitated the impact of exercise training.
Our findings are consistent with previous trials of home-based exercise programmes in heart failure. Only three trials of entirely home-based exercise have been published.25–27 In accord with the present study, none of these trials achieved an improvement in exercise capacity in the exercise group, but one trial with a high loss to follow-up (40%) did report a significant improvement in quality of life,25 and Dracup et al.26 reported a reduction in hospital admissions.
The Cochrane review of exercise training in heart failure showed that the largest effect sizes were seen in those studies with the most intensive (i.e. most frequent and longest duration of exercise sessions) and monitored programmes.2 In addition, many of these trials were undertaken before the widespread use of beta-blockers and multidisciplinary care. We believe these two factors are the likely explanation of our findings. As patients in the BRUM-CHF trial were on optimized therapy, this may have reduced the potential for improvement with an exercise programme. Furthermore, given the real-world nature of this trial, the exercise programme, although meeting current guidance for exercise provision for patients with heart failure,12,28 was designed to be inexpensive and to be able to be implemented widely, avoiding the need for patients to attend more than a small number of centre-based sessions.
Of interest is the significantly higher quality of life as measured using a generic measure (EQ-5D) at 6 month follow-up, which is of a similar order to the difference seen in Austin et al.24 However, this change in generic quality of life was not directly associated with the change in disease-specific quality of life as assessed by the MLwHFQ. The population in our study found the MLwHFQ difficult to complete, being uncertain about whether their symptoms were due to their heart condition, old age, or another chronic disease. This finding is concordant with the results of a qualitative validation of the MLwHFQ29 and our parallel qualitative study, in which many participants did not appear to know that they had heart failure, or in which way their heart failure affected them. The quality of life of a large sample of patients enrolled in the CARE-HF study was evaluated using the EQ-5D and MLwHFQ and compared with the NYHA class and population norms.30 This concluded that the EQ-5D may be an acceptable, valid measure for use in heart failure patients owing to its high response rates, sensitivity to NYHA status, and statistically significant relationship with the MLwHFQ. Many (33.7%) participants in the CARE-HF study did not fully complete the MLwHFQ.
Approximately, a quarter of participants scored as having possible or probable depression or anxiety on the HADS at baseline, so there was the potential to improve with the exercise programme. Indeed, at 12 month follow-up, the exercise group reported a significantly lower mean HADS depression score. Given that by the 12 month follow-up the exercise group did not report a higher level of physical activity, this cannot explain this lower level of depression.31,32
Study strengths and limitations
The BRUM-CHF trial has several strengths. First, it is large in comparison with many studies of home-based exercise for patients with heart failure. Second, it had very low rates of loss to follow-up and therefore risk of attrition bias. Third, the control group was chosen to reflect current optimal management of heart failure, i.e. specialist nurse care. This mirrors current clinical practice, enabling greater generalizability of the findings, although controlling for confounding and selection bias through randomization. Fourth, we recruited via a community-based population of heart failure patients. Recruitment to the trial has highlighted that a home-based exercise programme will be appropriate for a minority of patients with heart failure. The majority of patients seen by the specialist heart failure service were not eligible for the trial, owing to factors that included insufficient or too severe disease or preclusion of safe home exercise. In addition, co-morbidities accounted for large numbers of patients not being suitable for exercise. The older age of patients with heart failure means that there will be a high level of co-morbidity, which was not an issue in previous trials of highly selected, atypical patients.33 Poor recruitment to trials of patients with heart failure has been previously reported, with a high proportion declining to participate when contacted, with reasons given including a perception of being too old, too unwell, or too busy.33 This is an issue that will need to be considered when planning rehabilitation services for patients with heart failure.
The principal limitation of this trial was the self-monitored nature of exercise training so that it was not possible to ensure that patients complied with their individualized exercise prescription. However, unlike most previous trials of exercise in patients with heart failure, we assessed adherence. Adherence to the exercise programme is similar to the adherence rates for cardiac rehabilitation in patients post-myocardial infarction.8 Trials that have reported adherence, report very high adherence rates (75–100%),21,34–36 which are very unlikely outside supervised exercise training programmes in highly selected patient populations. The adherence to exercise may be an important factor in our findings since a per protocol analysis, including only participants in the exercise arm who were still adhering at 20 weeks, found increased effect sizes. It is possible that the participants undertaking the home-based exercise programme did not achieve an adequate dose of aerobic exercise, as they did not achieve our recommendations for duration of walking or intensity of exercise. Additional potential limitations were the lack of an attention control (i.e. controls receiving similar numbers of contacts as the intervention) and difficulties in blinding. However, although this could have had an effect at the 6 month follow-up, by 12 months both groups had been receiving ‘usual care’ for the previous 6 months. Given the nature of the exercise intervention, it was not possible to blind patients and caregivers. Although we aimed to blind outcome assessment, this was not always logistically possible, as patients may have discussed their allocation with the research nurses. To check the potential impact of this loss of observer blinding, we undertook a post hoc sensitivity analysis comparing the between-group primary outcome analysis in only those patients who had blinded follow-up at 6 months. There was no change in the findings. Baseline differences between intervention and control groups were controlled for in the analyses and thus should not have affected the findings.
Conclusions
This study failed to demonstrate a benefit from the addition of a home-based exercise programme in a community-based heart failure population. Further evidence is needed to assess the suitability of home-based exercise programmes in this population.
Contributors
K.J., R.S.T., G.Y.H.L., M.D., R.D., A.S., J.M., S.G., S.S., and J.S. conceived the study, wrote the original grant proposal, and were members of the trial management group. Trial implementation was undertaken by K.J. with support from J.I.; analysis was largely undertaken by K.J. with support from R.T. The paper was drafted by K.J.; all authors contributed to the final text. K.J. is the guarantor.
Conflict of interest: none declared.
Funding
This study was funded by the Department of Health’s Policy Research Programme, as part of a joint DH/British Heart Foundation Heart Failure research initiative. The views and opinions expressed in this paper do not necessarily reflect those of the Department of Health.
Copyright
HADS copyright © R.P. Snaith and A.S. Zigmond, 1983, 1992, 1994. Record forms originally published in Acta Psychiatrica Scandinavica, 67, 361–70, copyright © Munksgaard International Publishers Ltd, Copenhagen, 1983. Reproduced by permission of the publisher, nferNelson Publishing Company Ltd, The Chiswick Centre, 414 Chiswick High Road, London W4 5TF, UK. All rights reserved, including translation. nferNelson is a division of Granada Learning Limited, part of ITV plc.
Acknowledgements
We thank the specialist heart failure nurses, cardiac rehabilitation teams, cardiologists, and participants at the hospitals (City, Sandwell, University Hospital Birmingham) and South Birmingham PCT that took part. We thank Dr Jo Dodwell, who provided medical support for the recruitment phase of the study, and the research nurses and Dr Lynne Williams, who undertook some recruitment and follow-up. Robert Lancashire provided data support and Deb Bird and Leti Perez-Martin provided administrative support.
References
- 1.ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189–192. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees K, Taylor RS, Singh S, Coats A, Ebrahim S. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews 2004. doi: 10.1002/14651858.CD003331.pub2. Issue 3. Art. No.: CD003331. doi:10.1002/14651858.CD003331.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Tol BAF, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkal G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8:841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Williams F, Mair F, Leitner M. Exercise training and heart failure: a systematic review of current evidence. Br J Gen Pract. 2002;52:47–55. [PMC free article] [PubMed] [Google Scholar]
- 5.Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005;91:899–906. doi: 10.1136/hrt.2004.048389. doi:10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stromberg A, Martensson J, Fridlund B, Levin L-A, Karlsson J-E, Dahlstrom U. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failure. Eur Heart J. 2003;24:1014–1023. doi: 10.1016/s0195-668x(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 7.The National Collaborating Centre for Chronic Conditions. NICE Guideline No. 5. Chronic Heart Failure. National clinical guideline for diagnosis and management in primary and secondary care. http://guidance.nice.org.uk/CG5/guidance/pdf/English . [Google Scholar]
- 8.Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, Victory J, Brown J, Taylor RS, Ebrahim S. Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups. Health Technol Assess. 2004;41:2–5. doi: 10.3310/hta8410. [DOI] [PubMed] [Google Scholar]
- 9.Jolly K, Taylor RS, Lip GYH, Davies M, Davis R, Mant J, Singh S, Greenfield G, Ingram J, Stubley J, Bryan S, Stevens A. Home-based exercise rehabilitation in addition to specialist heart failure nurse care: design, rationale and recruitment to the Birmingham Rehabilitation Uptake Maximisation study for patients with congestive heart failure (BRUM-CHF): a randomised controlled trial. BMC Cardiovasc Disord. 2007;7:9. doi: 10.1186/1471-2261-7-9. doi:10.1186/1471-2261-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacRae KD. Minimisation: the platinum standard for trials? BMJ. 1998;317:362–363. doi: 10.1136/bmj.317.7155.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis R, Lip GYH, Davies MK. 2nd ed. Blackwell; 2007. ABC of Heart Failure. [Google Scholar]
- 12.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Meyers JN, Sullivan MJ. Exercise and heart failure: a statement from the American Heart Association Committee on Exercise, Rehabilitation and Prevention. Circulation. 2003;107:1210–1225. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 13.Keell S, Chambers J, Francis D, Edwards D, Stables R. Shuttle-walk test to assess chronic heart failure. Lancet. 1998;352:705. doi: 10.1016/s0140-6736(05)60821-5. [DOI] [PubMed] [Google Scholar]
- 14.Rector T, Kubo S, Cohn J. Patient self-assessment of their heart failure. Part 2. Content, reliability and validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart Fail. 1987;Oct/Nov:198–209. [Google Scholar]
- 15.Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, Keeler CA. Use of the living with heart failure questionnaire to ascertain patients’ perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail. 1995;1:201–206. doi: 10.1016/1071-9164(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond A, Snaith R. The Hospital Anxiety and Depression Scale. Acta Psych Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 17.Marmot M, Davey Smith G, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: The Whitehall II Study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 18.Godin G, Shepherd R. A simple method to assess exercise behaviour in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 19.EuroQol Group. EuroQol: a new facility for the measurement of health related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 20.Morales FJ, Martinez A, Mendez M, Agarrado A, Ortega F, Fernandez-Guerra J, Montemayor T, Burgos J. A shuttle walk test for assessment of functional capacity in chronic heart failure. Am Heart J. 1999;138:291–298. doi: 10.1016/s0002-8703(99)70114-6. [DOI] [PubMed] [Google Scholar]
- 21.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomised, controlled trial of long-term moderate exercise training in chronic heart failure. Effects on functional capacity, quality of life and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 22.Choi SC, Lu IL. Effect of non-random missing data mechanisms in clinical trials. Stat Med. 1995;14:2675–2684. doi: 10.1002/sim.4780142407. [DOI] [PubMed] [Google Scholar]
- 23.Schafer J. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 24.Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail. 2005;7:411–417. doi: 10.1016/j.ejheart.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Oka R, De Marco T, Haskell W, Botvinick E, Dae M, Bolen K, Chatterjee K. Impact of a home-based walking and resistance training programme on quality of life in patients with heart failure. Am J Cardiol. 2000;85:365–369. doi: 10.1016/s0002-9149(99)00748-1. [DOI] [PubMed] [Google Scholar]
- 26.Dracup K, Evangelista LS, Hamilton MA, Erickson V, Hage A, Moriguchi J, Canary C, MacLellen WR, Fonarow GC. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J. 2007;154:877–883. doi: 10.1016/j.ahj.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Corvera-Tindel K, Doering LV, Woo MA, Khan S, Dracup K. Effects of a home walking exercise program on functional status and symptoms in heart failure. Am Heart J. 2004;147:339–346. doi: 10.1016/j.ahj.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Meyer K, Hajric R, Westbrook S, Haag-Wildi S, Holtkamp R, Leyk D, Schnellbacher K. Hemodynamic responses during leg press exercise in patients with chronic congestive heart failure. Am J Cardiol. 1999;83:1537–1543. doi: 10.1016/s0002-9149(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 29.Hak T, Willems D, van der Wal G, Visser F. A qualitative validation of the Minnesota Living with Heart Failure Questionnaire. Quality of Life. 2004;13:417–426. doi: 10.1023/B:QURE.0000018487.35591.6e. [DOI] [PubMed] [Google Scholar]
- 30.Calvert MJ, Freemantle N, Cleland JGF. The impact of chronic heart failure on health-releted quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251. doi: 10.1016/j.ejheart.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Dorn J, Naughton J, Imamura D, Trevisan M. Correlates of compliance in a randomised exercise trial in myocardial infarction patients. Med Sci Sports Exerc. 2001;33:1081–1089. doi: 10.1097/00005768-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Turner SC, Bethell HJN, Evans JA, Goddard JR, Mullee MA. Patient characteristics and outcomes of rehabilitation. J Cardiopulm Rehabil. 2002;22:253–260. doi: 10.1097/00008483-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd-Williams F, Mair F, Sheils C, Hanratty B, Goldstein P, Beaton S, Capewell S, Lye M, Mcdonald R, Roberts C, Connelly D. Why are patients in clinical trials of heart failure not like those we see in everyday practice? J Clin Epidemiol. 2003;56:1157–1162. doi: 10.1016/s0895-4356(03)00205-1. [DOI] [PubMed] [Google Scholar]
- 34.Cider A, Tygesson H, Hedberg M, Seligman L, Wennerblom B, Sunnerhagen KS. Peripheral muscle training in patients with clinical signs of heart failure. Scand J Rehabil Med. 1997;29:121–127. [PubMed] [Google Scholar]
- 35.Tyni-Lenne R, Dencker K, Gordon A, Jansson E, Sylven C. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail. 2001;3:47–52. doi: 10.1016/s1388-9842(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 36.Gordon A, Tyni-Lenne R, Persson H, Kaijser L, Hultman E, Sylven C. Markedly improved skeletal muscle function with local muscle training in patients with chronic heart failure. Clin Cardiol. 1996;19:568–574. doi: 10.1002/clc.4960190709. [DOI] [PubMed] [Google Scholar]