Abstract
Aims
Heart failure with preserved ejection fraction (HFPEF) is common but not well understood. Electrical dyssynchrony in systolic heart failure is harmful. Little is known about the prevalence and the prognostic impact of dyssynchrony in HFPEF.
Methods and results
We have designed a prospective, multicenter, international, observational study to characterize HFPEF and to determine whether electrical or mechanical dyssynchrony affects prognosis. Patients presenting with acute heart failure (HF) will be screened so as to identify 400 patients with HFPEF. Inclusion criteria will be: acute presentation with Framingham criteria for HF, left ventricular ejection fraction ≥ 45%, brain natriuretic peptide (BNP) > 100 pg/mL or NT-proBNP > 300 pg/mL. Once stabilized, 4–8 weeks after the index presentation, patients will return and undergo questionnaires, serology, ECG, and Doppler echocardiography. Thereafter, patients will be followed for mortality and HF hospitalization every 6 months for at least 18 months. Sub-studies will focus on echocardiographic changes from the acute presentation to the stable condition and on exercise echocardiography, cardiopulmonary exercise testing, and serological markers.
Conclusion
KaRen aims to characterize electrical and mechanical dyssynchrony and to assess its prognostic impact in HFPEF. The results might improve our understanding of HFPEF and generate answers to the question whether dyssynchrony could be a target for therapy in HFPEF.
Keywords: Heart failure, Preserved ejection fraction, Diastolic dysfunction, Dyssynchrony, Echocardiography
Introduction
Congestive heart failure (CHF) affects about 2% of the western population, with prevalence increasing sharply from 1% in 40-year-olds to 10% above age 75, and it is the most common cause of hospitalization in patients over 65 years of age. CHF is defined as a syndrome characterized by impaired ability of the heart to fill with and/or to eject blood, resulting in a classical constellation of signs and symptoms.1
Heart failure with preserved ejection fraction (HFPEF) is increasingly being recognized as a pathophysiological entity.2 The proportion of patients with HFPEF is about 50% in the general heart failure (HF) population.3,4 These patients were previously classified as having diastolic heart failure (DHF) or HFPEF. However, DHF has its own definition1–3 and may not be strictly identical to HFPEF. HFPEF can be defined as an ejection fraction (EF) ≥ 45%, ≥ 50%, or ≥ 55%.2 The prognosis of HPPEF in epidemiological surveys is nearly as poor as for systolic heart failure (SHF), but in clinical trials of HFPEF (PEP-CHF, CHARM-preserved), the prognosis is much better than in clinical trials of SHF.5,6
Ventricular dyssynchrony in SHF is frequent and portends a worse outcome.7 Electrical dyssynchrony as indicated by prolonged QRS duration (≥120 ms) and/or left bundle branch block (LBBB) is present in approximately 30% of patients.7 In HFPEF, the prevalence of electrical and/or mechanical dyssynchrony during systole and/or diastole ranges from 10% to 60%.7–11 The prognostic significance of QRS-prolongation and of parameters of mechanical dyssynchrony has, to the best of our knowledge, not yet been evaluated in patients with HFPEF. In one of the few prospective studies including HFPEF patients, notably the CHARM-preserved population, the simple finding of a LBBB had a modest, or possibly no predictive impact on cardiovascular death or hospitalization for HF after a mean follow-up of 38 months.12
Methods
Study purpose
The main purpose is to test the independent prognostic (mortality and hospitalization for HF) value of electrical and/or mechanical dyssynchrony after a follow-up of 18 months.
Study design
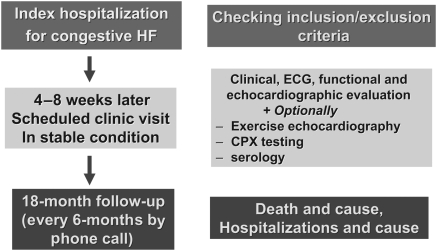
KaRen is a prospective, European, multicenter study of consecutive patients presenting with acute signs and symptoms of HF and a preserved EF. Over a 2-year period, patients presenting acutely with HF will be screened, and patients will be included based on symptoms, brain natriuretic peptide (BNP) or NT-proBNP, and EF in the acute state. Patients will return to a stable state 4–8 weeks later for detailed echocardiography and assessment of dyssynchrony, clinical examination, ECG, quality-of-life questionnaires, and serology. Sub-studies will include a detailed echocardiography also during the acute presentation, cardiopulmonary exercise (CPX) testing, as well as serology markers for myocardial fibrosis. Follow-up will be performed through phone call every 6 months until the end of the study (Figure 1). KaRen involves no intervention. Patients will be managed routinely by their own physician. The study conforms to the Declaration of Helsinki and has been approved by French and Swedish ethics committees and by the CNIL (Comité National Informatique et Libertés) in France.
Figure 1.
Karolinska-Rennes (KaRen) study design.
Inclusion/exclusion criteria
The study protocol was designed to enrol a patient population with HF symptoms similar to those observed in epidemiological and community-based studies. In practice, inclusion criteria are similar to those used in major recent or ongoing studies in HFPEF, principally the I-PRESERVE study13,14 with NT-proBNP (BNP) threshold values being defined from the CHARM data.5,6,13,15–18
Detailed exclusion criteria are given in Table 1.
Table 1.
Key exclusion criteria for patients in the Karolinska-Rennes (KaRen) study
| (1) Evidence of primary hypertrophic or restrictive cardiomyopathy or systemic illness known to be associated with infiltrative heart disease |
| (2) Known cause of right heart failure (HF) not related to left ventricular (LV) dysfunction |
| (3) Pericardial constriction |
| (4) Clinically significant pulmonary disease as evidenced by requirement of current home oxygen |
| (5) End-stage renal disease currently requiring dialysis |
| (6) Bi-ventricular pacemaker (cardiac resynchronization therapy) (patients who have a conventional pacemaker may be included) |
| (7) Anticipated or indication for cardiac surgery (patients who have indication for surgery but may not undergo surgery because of some contraindication, for example age, may not be included) |
| (8) Anticipated percutaneous intervention on aortic stenosis (patients who undergo other percutaneous intervention, for example percutaneous coronary intervention, may be included) |
The inclusion criteria are as follows:
Acute presentation to the hospital with clinical signs and symptoms of HF, according to the Framingham criteria.19
BNP > 100 pg/mL or NT-proBNP > 300 pg/mL.
LVEF ≥ 45% by echocardiography within the first 72 h. The measurement will be carried out according to guidelines.20
All inclusion criteria (clinical HF, EF, and peptide criteria) must be established within 72 h of presentation. Enrolment will occur during this time or shortly thereafter, after the presence of any of the exclusion criteria has been ruled out.
Key assessments performed 4–8 weeks after index event
All events including length of hospitalization and potential re-hospitalization will be recorded. Any re-admission prior to the 4–8-week follow-up will delay the follow-up to 4–8 weeks from that admission.
Clinical parameters
Physical exam, history since the index presentation, and serology including peptides will be collected.
Functional assessment
Patients will be evaluated by the ‘Minnesota Living with Heart Failure questionnaire’, a 21-item disease-specific instrument with scores ranging from 0 to 5 and with a summary score ranging from 0 to 105, the highest score representing the worst health-related quality-of-life. There are two specific health-related quality-of-life domains: physical limitations (maximal score = 40) and emotional limitations (maximal score = 25). Validity, reliability, and reproducibility of the Minnesota questionnaire have been established and both a French and a Swedish version exists.21 The questionnaire will be filled in by each subject at the inclusion visit prior to other assessments, the subject being alone in a quiet environment.
This functional assessment will be complemented using the EuroQOL.22
Electrocardiogram
A 12-lead surface ECG will be recorded at 50 mm/s speed. The following parameters will be analysed centrally (Karolinska University Hospital, Stockholm, Sweden): rate, rhythm, intervals (P-wave duration, PR, QRS, QT, QTc), axis (QRS), and morphology [P-wave, QRS: (LBBB, right bundle branch block, intraventricular conduction delay), abnormal Q wave, ST-T changes]. QRS duration is defined as the duration of the widest QRS complex.
At least 10 beats will be requested in patients with atrial fibrillation and 5 in patients with sinus rhythm. Dyssynchrony will be defined as QRS ≥ 120 ms, but patients will be analysed according to QRS width as a continuous variable and in 10 ms increments.
The definition of electrical dyssynchrony is a QRS > 120 ms in accordance with the current ESC guidelines.23
Echocardiography
Technical requirements
Each echocardiographic examination will be performed according to a checklist using the same machine (ViVid Seven, GE Healthcare, Horten, Norway). The quantitative analysis will be conducted in a ‘Core Lab’ (Rennes University Hospital, France). Specific attention will be given to the quality of echocardiographic images and of the ECG traces. In patients with atrial fibrillation, at least 10 beats are required for each recording.
The recent PROSPECT trial drew attention to the difficulty of assessing mechanical dyssynchrony with sufficient reliability.24 As requested in the recent ESC guidelines for cardiac pacing and resynchronization therapy,23 all examinations for dyssynchrony assessment will be performed by experienced echocardiographers (at least 50 exams per year investigating dyssynchrony). In addition, a pre-study validation of each echocardiographer will be performed.
In addition to the study of dyssynchrony, assessment of LV diastolic function will be performed based on the variables reported in Table 2. Left ventricular mass, dimensions, volumes, and function will be assessed.23 Right ventricular (RV) function will also be explored by tricuspid annulus excursion (TAPSE), pulse Doppler tissue imaging (DTI), estimated pulmonary pressures, inferior vena cava dimensions, and pulmonary pre-ejection time delay, which are also used for the calculation of interventricular mechanical delays.25
Table 2.
Diastolic dysfunction characterization according to echo-Doppler information
| Mitral inflow | Pulmonary venous inflow | E/Ea | |
|---|---|---|---|
| Normal | 0.75 < E/A < 1.5 DT > 160 ms | S > D MV A-dur > PV A-dur | <10 |
| Mild LV diastolic dysfunction | E/A < 0.75 DT > 240 ms | S > D MV A-dur > PV A-dur | <10 |
| Moderate LV diastolic dysfunction | 0.75 < E/A < 1.5 DT > 160 ms | S < D MV A-dur + 30 < PV A-dur | ≥10 |
| Severe LV diastolic dysfunction | E/A > 5 DT < 160 ms | S < D MV A-dur + 30 < PV A-dur | ≥10 |
DT, deceleration time; MV A-dur, mitral valve A-wave duration; PV A-dur, pulmonary vein A-wave duration.
Dyssynchrony analysis
In addition to conventional echo Doppler data, two-dimensional (2D) loops (parasternal short axis and apical four-, two-, and long-axis views) of the LV and the RV will be recorded successively in colour-mode (frame rate > 140/s) and in conventional grey scale mode (frame rate about 75/s).
For the same purpose, a three-dimensional (3D) matrix for further 3D volumetric dyssynchrony analysis will be recorded so as to measure the systolic dyssynchrony index (SDI) according to Image Arena (TomTec Imaging Systems, Unterschleissheim, Germany).
The following measurements will be carried out:
Atrioventricular, interventricular (difference between aortic and pulmonary pre-ejection time delays), and intra-LV dyssynchrony will be first assessed by conventional Doppler echo. In 2D, left ventricular filling duration (mitral inflow), aortic, and pulmonary pre-ejection time delays will be measured.
Temporal dyssynchrony will be assessed by M-mode in parasternal and apical four-chamber view, so as to measure the time delay between the ECG Q-wave and the maximal excursion of the posterior and lateral part of the mitral annulus in systole.
Intra-LV spatial dyssynchrony will be assessed by: (i) M-mode in parasternal long- and short-axis (Table 3); (ii) myocardial velocity and strain curves (colour-coded DTI loops being post-processed on ECHOPAC, GE-Healthcare, Horten, Norway) in three apical views will be used to measure the time-to-peak difference within each LV wall (Table 3); (iii) a radial, circumferential, and longitudinal time to peak of deformation in the basal, and mid-segments of each LV wall will also be studied using the 2D-strain application; (iv) using 3D-volume information, significant intra-LV dyssynchrony will be defined by an SDI > 14%.
Table 3.
Review of the criteria currently used to characterize and quantify mechanical dyssynchrony by Doppler-echocardiography25
| Methods | Measures | Cut-off values for dyssynchrony |
|---|---|---|
| Atrioventricular dyssynchrony | ||
| Conventional Doppler | Mitral inflow duration | <40% of the cycle length |
| Interventricular dyssynchrony | ||
| Conventional Doppler | Difference between aortic and pulmonary pre-ejection times | ≥40 ms |
| Intra-LV dyssynchrony | ||
| Conventional Doppler | Aortic pre-ejection interval during spontaneous rhythm | ≥140 ms |
| M-Mode | Time to peak LV posterior and/or lateral wall maximal excursion > time to mitral valve opening | Overlap systole–diastole |
| TVI | Maximal delay between peak systolic velocities of any two of 12 LV segments | >100 ms |
| TVI | Maximal delay between peak systolic velocities in four LV segments | ≥65 ms |
| TVI | Standard deviation of time to peak systolic velocity of 12 LV segments | ≥33 ms |
| TSI | Time to peak velocities of opposing ventricular walls | ≥65 ms |
| Longitudinal strain | Temporal difference in septal-lateral peak systolic strain | ≥50 ms |
| Radial strain | Time difference of peak radial strain in the septum vs. the posterior wall | ≥130 ms |
| Real-time 3D | SDI | ≥14.7% |
LV, left ventricular; SDI, systolic dyssynchrony index; TSI, tissue synchronization imaging analysis; DT, deceleration time.
Systolic and diastolic intraventricular dyssynchrony will also be measured by tissue synchronization imaging analysis (TSI): TSI is a parametric imaging tool derived from the 2D tissue Doppler images. It will automatically calculate Ts (time from the beginning of the QRS complex to peak systolic velocity) in every position in the image with reference to the QRS. The operator can also manually adjust the start and end times of the TSI in order to analyse the ejection and/or diastolic phase. We will extend into early diastole (MVO + 250 ms) in order to detect dyssynchrony during this phase as well as during the ejection phase (AVO-AVC).
For the purpose of this study, mechanical dyssynchrony will be classified according to whether one, two, or three levels of dyssynchrony (inter- and/or atrioventricular and/or intra-LV) are found.
We do not have any predetermined idea of the parameters that are the most relevant in HFPEF. Different combinations of intra-LV parameters of mechanical dyssynchrony will be tested to find the best way to assess their prognostic value. We will distinguish parameters describing: radial dyssynchrony, longitudinal dyssynchrony, and 3D volumic assessment of LV dyssynchrony.
Data collection
Each investigator will enter clinical data into a web-based electronic case report form (Clinsource, Brussels, Belgium). The echocardiographic analysis will be performed by the Core Laboratory. The follow-up data will be obtained and entered onto the web-based electronic case reports by dedicated research staff.
Long-term follow-up
Patients will be followed by phone call and/or chart and death registry review every 6-months for 18-months after closure of enrolment, such that all patients will have a minimal of 18-month follow-up period.
Study endpoints
The primary outcome will be all-cause death and/or hospitalization for HF. Hospitalization for HF is defined as admission to hospital for any length of time, including day-care, with either treatment of HF or for HF being the main component of the admission. Acute presentation to an outpatient clinical or emergency room that does not result in admission will be registered but not counted as a primary endpoint. Evidence of worsening HF must include at least one of the following items: increasing dyspnoea on exertion, orthopnoea, nocturnal dyspnoea, pulmonary oedema, increasing peripheral oedema, increasing fatigue or decreasing exercise tolerance, renal hypoperfusion (i.e. worsening renal function), raised jugular venous pressure, and radiological signs of CHF.
The secondary outcome will be overall mortality. This is of particular interest as one possible explanation for the high mortality observed in epidemiological surveys15,16 is mortality from non-cardiovascular causes.
Analysis will be performed according to pre-specified subgroups as defined by parameters measured at inclusion (see Supplementary material).
Sample sizes
In unselected populations with HF, approximately 50% have HFPEF. Prognosis ranges from 8–9% annual cardiovascular mortality or hospitalization for HF over 3 years as observed in the CHARM-Preserved trial, to 22–29% overall mortality over 1 year in unselected HF populations. The prevalence of dyssynchrony in HFPEF is not well known. In the Swedish Heart Failure Registry (S-HFR), approximately 20% of patients had a QRS > 120 ms (Uppsala Clinical Research Centre) while another study reported a figure of 40%. The prognosis of dyssynchrony in HFPEF is not well-determined. In the aforementioned study, 6-month mortality or hospitalization for HF amounted to 66% in patients with dyssynchrony and to 40% in patients without dyssynchrony.6
Based on the above data, we estimate that in our unselected population, 50% of HF patients will have HFPEF, 20–40% of those will have dyssynchrony; we further estimate that the combined endpoint of all-cause mortality or HF hospitalization over 18 months will occur in 35–40% of patients without dyssynchrony and in 50–55% of patients with dyssynchrony. Taking these estimations as a base along with an 80% statistical power and a two-sided significance level of 5%, to detect a significant difference in the endpoint at 18 months, we would need to screen approximately 800 patients with HF so as to enrol 400 patients with HFPEF.
Statistical analysis
Patients will be analysed according to the study endpoints. Kaplan-Meier curves of event-free survival for the predefined subpopulation (no dyssynchrony, electrical dyssynchrony, mechanical dyssynchrony) will be plotted and they will be compared using the log-rank test. Any patient who is lost to follow-up will be censored from the Kaplan-Meier analysis, that is, removed from the population ‘at risk’ without being counted as an event. Baseline characteristics will be presented as median and quartiles for continuous variables and as number and percentage for categorical variables. Comparisons between groups for baseline variables will be made using independent sample t-tests and analysis of variance (ANOVA) for normally distributed continuous variables and Mann–Whitney U test and Kruskal-Wallis ANOVA for non-normal distributions. Partial correlation coefficients will be calculated to assess relationships between markers and other parameters, such as echocardiography parameters. Non-normally distributed variables will be log-transformed. To determine if dyssynchrony is a significant predictor of mortality or cardiovascular hospitalization, we will first screen for univariate predictors. Secondly, a multiple Cox proportional hazards regression will be used for the best subset selection with Mallow's Cox proportional being performed, adjusting for age, gender, and other known risk factors for mortality or hospitalization. In parallel to this selection, we will focus on dyssynchrony and assess the minimal predicting combination of predictors. Age, BNP, and/or NT-proBNP (values obtained at the time of decompensation and at the scheduled hospitalization will be considered separately in the analysis) will be used in the prognostic models as important control variables. The results of the logistic regression models will be reported as odds ratios (OR) and 95% confidence intervals (CI). The discriminatory ability of the models will be compared using computed areas under the receiver-operating curve (ROC). The relationship between continuous predictors will be examined using Spearman correlation coefficients as appropriate, whereas the relationship between a categorical and a continuous predictor will be assessed using t-test analysis. Significant differences will be defined as a P-value < 0.05.
The reproducibility of echocardiographic and ECG measurements will be analysed using the intra-class correlations searching for indices ranging from 0.8 to 0.9 for the intra-observer variability, and from 0.7 to 0.9 for the inter-observer variability. The agreement between measurements performed by different observers will be analysed using the Bland–Altman method. The bias and 95% CIs will be calculated as described by Bland–Altman.
Substudies
Patients will be entered into the main trial and subsequently, after giving separate informed consent, will be entered into substudies depending on their own free choice and also on local participation in the substudies.
Extended Doppler echocardiography performed at the index event
To assess whether cardiac functions and mechanical dyssynchrony alter from the acute to the stable phase, an extended echocardiography protocol similar to that requested at the 4–8-week visit will be performed in the acute setting (first 72 h following admission or visit).
Exercise echocardiography
Patients with HFPEF may be symptomatic only during exercise. Although they may have normal diastolic parameters on echocardiography at rest, exercise can unmask signs of diastolic dysfunction and increased filling pressures.8,26 Relaxation and/or compliance abnormalities associated with a rapid increase in LV end-diastolic pressure when exercising may explain symptoms. Furthermore, exercise echocardiography will provide a complementary assessment of electrical and mechanical dyssynchrony as evaluated in systolic HF.27 Except for the real time 3D assessment of dyssynchrony, we intend to perform the same measurements during exercise as we do at rest. Women will not exceed 45 W and men 60 W.
Cardiopulmonary exercise testing
CPX testing with calculation of peak VO2 consumption (peak VO2) closely correlates with severity of HF and is the best single prognostic predictor in SHF. The HF survival score (HFSS) and the Seattle Heart Failure Model (SHFM) are the best multivariable prognostic predictors in SHF.28 In SHF, exercise intolerance is thought to reflect both increased filling pressures (certainly a feature of HFPEF as well) and reduced cardiac output, resulting in impaired skeletal muscle function and metabolic disturbances. Little is known about these aspects in HFPEF. CPX parameters and their prognostic significance have not been studied thoroughly in HFPEF and results are conflicting.29 Therefore, in this substudy we will look for the prognostic value of conventional parameters currently used for patients with systolic heart failure (peak VO2, slope VE/VCO2 slope).30
Serology
This substudy aims to characterize serum markers in order to assess HF severity and its pathophysiology. SHF is known to be associated with neuroendocrine activation, inflammation, and anabolic–catabolic imbalance.31–33 We intend to assess the prevalence and importance of these factors in HFPEF. We will also examine collagen turn-over and determine whether an increase in collagen turn-over is correlated with markers of dyssynchrony, systolic, and diastolic function, neurohormonal activation, inflammation and anabolic–catabolic imbalance. These substudies will further improve our understanding of HFPEF, its determinants, and its prognosis.
Discussion
To our knowledge, this is the first study to describe the prevalence and prognostic impact of electrical and mechanical dyssynchrony in HFPEF. The prospective multicenter design will allow detailed assessment of many potentially important parameters in a broad population.
How to define HFPEF is still a matter of debate.2,26 Since patients with acute decompensated HF along with HFPEF and patients with SHF share clinical similarities,34 the clinical syndrome of acute HF will be defined by the Framingham criteria and by BNP or NT-proBNP measurement. To date, no clear BNP cut-off value has been proposed for patients with HFPEF. Among patients in the I-PRESERVE study, the median value of NT-proBNP at baseline was 339 pg/mL.14 We have chosen relatively low cut-offs of 100 pg/mL for BNP and of 300 pg/mL for NT-proBNP so as to achieve a high sensitivity. However, this cut-off value may be adjusted depending on an intermediate analysis after 6 months.
ACC/AHA and ESC guidelines state that echocardiography is the single most useful test in the diagnosis of HF.30 The definition of HFPEF varies and the knowledge of patient characteristics in this population is evolving. In the EuroHeart Survey, which included 6806 patients, a LVEF > 40% was considered to define HFPEF.4 Patients with HFPEF were 4 years older on average, were more often female, had more hypertension or atrial fibrillation, and received less cardiovascular medication when compared to those with SHF. In the European Study Group on Diastolic Heart Failure,2 a normal LVEF was defined as LVEF > 50%. The cut-off value in defining systolic and non-systolic HF was 40% in the CHARM-preserved trial,6 and 45% in I-PRESERVE trial.13 In the recent OPTIMIZE-HF registry on 20,118 patients, no real difference in characteristics of HFPEF patients was observed regardless of whether the cut-off value for LVEF was >40% or >50%.34 For these reasons, in KaRen, HFPEF will be defined as LVEF > 45%, the same as in the I-PRESERVE trial.13
The prognostic importance and prevalence of HFPEF has recently been recognized. The prevalence of HFPEF increased between 1987 and 2001 and mortality was similar in patients with either SHF or HFPEF. In subsequent large studies, patients with preserved/reduced EF had a 1-year mortality ratio ranging from 22/26% to 27/36%.15,16 The 5-year survival was also similar regardless of whether the EF was reduced or not: 43/46%.18 None of these studies provided the precise cause of death. In contrast, clinical trials of HFPEF suggest a much better prognosis. In CHARM-preserved,6 3-year cardiovascular mortality of HF hospitalization was only 8–9%; in PEP-CHF, 1 year all-cause mortality, or HF hospitalization was 11–15%.5 The cause of death was unknown in most recently published registries.15,16 Thus, it remains unclear whether HFPEF has an equally poor cardiovascular prognosis compared with SHF. Since KaRen will collect detailed data on prognosis, including causes of death and hospitalization, the principal aim of KaRen is to settle this issue.
In SHF, an inverse correlation exists between QRS duration and LVEF. As QRS duration increases, LV systolic function decreases, thereby worsening prognosis.3,34 Large multicenter trials including patients with moderate to severe SHF have established the value of electrical dyssynchrony (QRS ≥ 120 ms) as an outcome marker and in selecting candidates for cardiac resynchronization therapy (CRT).23 The presence and prognostic implications of electrical dyssynchrony in HFPEF are less clear. The baseline characteristics of the I-PRESERVE study showed that LBBB (QRS > 120 ms) was present in 9.1% of the 4133 patients.14 Our study aims to prospectively investigate the prognosis of HFPEF patients by examining mortality and cardiovascular events. It should be possible to determine whether electrical and/or mechanical dyssynchrony have a significant and an independent impact on prognosis.
Cardiac dyssynchrony will not only be defined on the basis of QRS width. Mechanical dyssynchrony will also be analysed by means of echocardiography. We have designed the protocol by taking into account the results of recent multicentre studies using echocardiography.24 Several Doppler echocardiography techniques have been used to explore cardiac dyssynchrony.25 However, despite numerous studies, no single echocardiographic criterion has been validated and proven clinically useful for predicting individual patient response to CRT.25 Our study may be able to demonstrate that dyssynchrony can be reliably assessed by echocardiography. The HFPEF population might prove easier to explore than the SHF population, as contractility is only weakly depressed and LV non-enlarged. Consequently, Doppler echocardiography criteria could prove easier to collect. Furthermore, mechanical dyssynchrony will be defined by a multi-parametric approach. Patients will be classified according to the presence of interventricular and/or atrioventricular and/or intra-LV criteria of mechanical dyssynchrony and correlations with outcome will be examined according to the degree of dyssynchrony.25
Limitations of the study
The inclusion of patients with many co-morbidities along with HFPEF in a prospective registry is a difficult challenge that we will have to take up.5,6,13
Characterizing mechanical dyssynchrony using Doppler echocardiography has been very challenging in most multicenter trials. This has been a learning experience. Therefore, the reproducibility and feasibility of the assessment of mechanical dyssynchrony will obviously be a major task. To minimize these difficulties, each echocardiographic examination will be recorded digitally by trained investigators and analysed in a Core Laboratory.
Conclusion
This study is being conducted to provide answers to the following questions:
What is the prevalence of electrical and/or mechanical dyssynchrony in the HFPEF population?
How do electrical and mechanical dyssynchrony correlate with outcome as assessed by a combined endpoint of either all-cause death or HF hospitalization?
This prospective observational study also aims to assess the potential usefulness of conducting clinical trials on CRT in patients with HFPEF.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
We would also like to thank Medtronic Europe for their research grant.
Acknowledgements
We wish to thank the French Society of Cardiology, the French Federation of Cardiology, and the Swedish Heart and Lung Foundation for their support. Finally, we wish to express our gratitude to the following co-investigators: Jean-Noel Trochu, Pascal de Groote, Gilbert Habib, Pascal Lim, François Tournoux, Thierry LeTourneaux, Patricia Réant, Christine Selton-Suty, Cyrille Bergerot, Christian de Place, Christophe Leclercq, and Philippe Mabo.
Conflict of interest: Some of the investigators have received grants or consultancy fees from Medtronic, General Electric, Bristol Myers, Squibb, Philips, Sorin Group, Bracco and St. Jude Medical.
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007 doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 3.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 4.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25:1214–1220. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 7.Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 8.Burgess MI, Fang ZY, Marwick TH. Role of diastolic dyssynchrony in the delayed relaxation pattern of left ventricular filling. J Am Soc Echocardiogr. 2007;20:63–69. doi: 10.1016/j.echo.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.De Sutter J, Van de Veire NR, Muyldermans L, De Backer T, Hoffer E, Vaerenberg M, Paelinck B, Decoodt P, Gabriel L, Gillebert TC, Van Camp G Working Group of Echocardiography and Cardiac Doppler of the Belgian Society of Cardiology. Prevalence of mechanical dyssynchrony in patients with heart failure and preserved left ventricular function (a report from the Belgian Multicenter Registry on dyssynchrony) Am J Cardiol. 2005;96:1543–1548. doi: 10.1016/j.amjcard.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson JE. Systolic and diastolic ventricular dyssynchrony in systolic and diastolic heart failure. J Am Coll Cardiol. 2007;49:106–108. doi: 10.1016/j.jacc.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96. doi: 10.1016/j.jacc.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Hawkins NM, Wang D, McMurray JJ, Pfeffer MA, Swedberg K, Granger CB, Yusuf S, Pocock SJ, Ostergren J, Michelson EL, Dunn FG CHARM Investigators and Committees. Prevalence and prognostic implications of electrocardiographic left ventricular hypertrophy in heart failure: evidence from the CHARM programme. Heart. 2007;93:59–64. doi: 10.1136/hrt.2005.083949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson P, Massie BM, McKelvie R, McMurray J, Komajda M, Zile M, Ptaszynska A, Frangin G for the I-PRESERVE Investigators. The irbesartan in heart failure with preserved systolic function (I-PRESERVE) trial: rationale and design. J Card Fail. 2005;11:576–585. doi: 10.1016/j.cardfail.2005.06.432. [DOI] [PubMed] [Google Scholar]
- 14.McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, Staiger C, Donovan JM, Massie BM. Heart failure with preserved ejection fraction: Clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10:149–156. doi: 10.1016/j.ejheart.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 16.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 17.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS Investigators of the CHARM Echocardiographic Substudy-CHARMES. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence:results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 18.Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 19.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W American Society of Echocardiography's Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients' perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12:87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Nord E. EuroQol: health-related quality of life measurement. Valuations of health states by the general public in Norway. Health Policy. 1991;18:25–36. doi: 10.1016/0168-8510(91)90141-j. [DOI] [PubMed] [Google Scholar]
- 23.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, Gasparini M, Linde C, Morgado FB, Oto A, Sutton R, Trusz-Gluza M European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in Collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–2295. doi: 10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- 24.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 25.Gorcsan J, 3rd, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, Martin R, Steinberg JS, Sutton MS, Yu CM American Society of Echocardiography Dyssynchrony Writing Group. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting–a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–506. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Lafitte S, Bordachar P, Lafitte M, Garrigue S, Reuter S, Reant P, Serri K, Lebouffos V, Berrhouet M, Jais P, Haissaguerre M, Clementy J, Roudaut R, DeMaria AN. Dynamic ventricular dyssynchrony: an exercise-echocardiography study. J Am Coll Cardiol. 2006;47:2253–2259. doi: 10.1016/j.jacc.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 28.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 29.Lund LH, Mancini DM. Peak VO(2) in elderly patients with heart failure. Int J Cardiol. 2008;125:166–171. doi: 10.1016/j.ijcard.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazza A, Nieminen M, Priori SG, Swedberg K. ESC Guidelines for the diagnosis, treatment of acute and chronic heart failure 2008. Authors/Task Force Members. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Drexler H, Kastner S, Strobel A, Studer R, Brodde OE, Hasenfuss G. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J Am Coll Cardiol. 1998;32:955–963. doi: 10.1016/s0735-1097(98)00336-2. [DOI] [PubMed] [Google Scholar]
- 32.Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 33.Martos R, Baugh J, Ledwidge M, O'Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 34.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]