Abstract
Aims
Attenuation of the effects of natriuretic peptides has been demonstrated in animal models but studies in humans are scarce, particularly concerning renal attenuation. We investigated the attenuation of B-type natriuretic peptide (BNP) in chronic advanced heart failure (HF).
Methods and results
We included 62 outpatients with HF and severe left ventricular systolic dysfunction. Cases had at least one hospital admission or emergency department visit for acute HF in the previous year and were in NYHA class III/IV despite optimized therapy. The individual age- and sex-matched controls were symptomatically controlled (NYHA I and II). We collected 24 h urine and a blood sample from all patients. Plasma BNP and plasma (pcGMP) and urine cyclic guanosine monophosphate (ucGMP) were measured. Patients were followed for 3 months for hospital admission or all-cause death. ucGMP to plasma BNP (ucGMP/BNP) ratio was attenuated in cases vs. controls [median (IQR): 8354 (4293–16 456) vs. 12 693 (6896–22 851)]. There were no differences in pcGMP to BNP (pcGMP/BNP) ratio or urine cGMP excretion. Patients with worse outcome had lower pcGMP/BNP [260 (86–344) vs. 381 (244–728) in patients without adverse outcome events] and lower ucGMP/BNP [4146 (2207–9363) vs. 10 922 (7495–19 971)].
Conclusion
Renal NP’s second messenger production is attenuated in advanced HF. Patients with worse outcome have lower ucGMP/BNP and pcGMP/BNP ratios.
Keywords: Heart failure, Attenuation, B-type natriurtic peptide, Second messenger systems
Introduction
The natriuretic peptide (NP) system is activated in heart failure (HF). Elevated NP levels are a hallmark in both acute and chronic HF, and they have well-known beneficial effects—diuretic, natriuretic, vasodilating, anti-fibrotic, and anti-hypertrophic.
The compensatory actions of NPs are attenuated in HF.1–4 This attenuation is probably multifactorial,5 and several mechanisms may contribute, including increased NP degradation whether clearance receptor-mediated (natriuretic peptide receptor C)6 or enzyme-mediated (neutral endopeptidase);2,7 and down-regulation of the natriuretic-peptide-receptor A8–10 — the main atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) biological receptor. Altered guanylate cyclase-coupled receptor function, altered intracellular cyclic guanosine monophosphate (cGMP) processing, or increased cGMP intracellular degradation are also possible contributors. The former two hypotheses are speculative,11 but increased intracellular degradation is well documented in animal models of clinical conditions associated with sodium retention and oedema formation.12–14 NP receptor down-regulation is still controversial. In humans, such a phenomenon has not been specifically investigated, particularly at the kidney level. However, ANP binding sites on platelets are diminished in severe HF.15 Counter-regulatory mechanisms, such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, endothelin, and arginine–vasopressin might also play a role.5
Another issue to take into account when discussing the relative deficit of natriuretic effect is the functional heterogeneity of circulating BNP suggested by various recent reports.16–21 Indeed, raised circulating levels of immunoreactive BNP are a sensitive biomarker of HF and correlate positively with severity. There is increasing evidence that immunoreactive BNP consists of both high-molecular-weight BNP forms—presumably proBNP forms—and the biologically active low-molecular-weight BNP forms—corresponding to more mature BNP forms. Hawkridge et al.16 even suggested that in patients with severe HF, the biologically active mature BNP1-32 peptide is either very decreased or non-existent. ProBNP, which is six to eight times less active than BNP, is the predominant form in this context.17 Besides these two major groups, growing evidence supports the existence of diverse circulating proBNP and BNP products—aminopeptidase cleavage by dipeptidyl peptidase 4 may be responsible for the generation of proBNP3-108 from proBNP1-108 and BNP3-32 from BNP1-32.18 The available commercial assays are known to be non-specific for the BNP1-32 form and cross-reactivity exists between the proBNP and the aminopeptidase proteolyzed fragments.17–19 This might be another circumstance contributing to the high plasma levels of immunoreactive BNP detected by the commercial assays in HF patients, particularly in patients with the most severe HF forms, and the paradoxical lack of BNP activity.
Cyclic GMP is an intracellular second messenger of ANP, BNP, and nitric oxide (NO).22,23 Tsutamoto et al.24 considered the peripheral blood concentration of cGMP as an index of NP biological activity and reported that plasma cGMP (pcGMP) concentration correlated with concentrations of ANP and BNP and increased with the severity of chronic HF.
A decrease in the pcGMP/BNP ratio with ageing has been described even in patients with normal LV systolic function.25 The cGMP to NP plasma ratio is also decreased in the HF context, highlighting the insufficient response and resistance to increasing levels of NPs.8,24,26 NP levels increase with the severity of HF but cGMP levels eventually reach a plateau.
Renal ANP responsiveness is also decreased in chronic HF. This is expressed by an absence of increase in renal cGMP generation1,2,26 and a reduced urinary cGMP (ucGMP) to plasma BNP (ucGMP/BNP) ratio.26 Renal NP desensitization is thought to contribute to decreased natriuresis in chronic HF.1
There is limited information about the potential alterations in NP intracellular signalling pathways in HF, and most studies have focused on ANP-induced cGMP production. Most data on this issue have been obtained from animal models of HF.
The aims of this study were (i) to study the attenuation of BNP in chronic advanced HF by comparing cases of severely symptomatic chronic HF with age- and gender-matched controls and (ii) to assess the association of this attenuation with the incidence of adverse outcomes at 3 months.
Methods
Study design
Outpatients were recruited from our HF clinic and were included in a matched case–control study. Cases (n = 31) were patients with an established HF diagnosis for at least 2 years, severe LVSD assessed by echocardiography during the previous year, hospital admissions or emergency department visits for worsening HF in the previous year and persistently in NYHA class III or IV despite optimized medical therapy—adaptation of chronic advanced HF definition by Metra et al.27 Controls (n = 31) had severe LVSD (echocardiogram in the previous year), and were individually matched with the cases by age (±5 years) and sex, but were symptomatically controlled by optimized medical therapy (NYHA class I or II). We excluded patients with acute coronary syndrome in the previous 3 months as well as those with hospital admission, therapeutic adjustment, or any infection in the previous month.
All eligible patients were included in this prospective cohort study and followed for 3 months. Adverse outcome was defined as admission for worsening HF or all-cause death. Follow-up data were obtained by consulting the hospital records for each patient and by telephone contact. No patient was lost during follow-up.
All patients provided written informed consent to participate in the study. The study protocol conforms to the ethical guidelines of the declaration of Helsinki. The study was approved by the institution’s ethics committee.
Laboratory procedures and data collection
All patients collected a 24 h urine sample (8 a.m. to 8 a.m. the next morning) in a non-acidic medium for creatinine and cGMP determination. Urine collections were made according to the routine hospital protocol—patients were instructed to keep the receptacle in the refrigerator between micturitions and to deliver it to the clinic as soon as possible after the final collection. On the second day, when the urine sample was returned to the clinic, fasting blood samples were obtained from all the study patients between 8 a.m. and 9 a.m. A physical examination was also performed and a record of demographic data, co-morbidities, and medication in use was also completed. We based the assessment of hypervolaemia on physical examination findings (rales, peripheral oedema, hepatomegaly, S3 gallop, increased jugular venous pressure, and weight change) and determined the sodium retention score as previously described.28
BNP determination is a routine laboratory procedure in our hospital; an Abbott chemiluminescent microparticle immunoassay (two-step immunoassay) is used. The measurement range of this assay is 10–5000 pg/mL and the assay is designed to have an upper 95% CI of ≤12% total coefficient of variation (CV). Within-run and total % CV according to BNP measurement are as follows: ±180 pg/mL: <6 and <7%; ±1000 pg/mL: <5 and<6%; and ±3800 pg/mL: <4 and <5%. All patient samples were measured on separate assays.
Cyclic guanosine monophosphate measurement
Urine samples were stored at −20°C or lower prior to analysis. Blood for cGMP measurement was collected in heparin-lithium tubes; samples were immediately centrifuged (4500 r.p.m. for 15 min) and then stored at −70°C or below within 2 h. Plasma and urine cGMP was measured using a commercially available ELISA test—cyclic GMP EIA kits (BT-740) purchased from Biomedical Technologies Inc. The BTI cyclic GMP EIA system provides a simple and sensitive method for measuring cGMP in biological samples, which can detect a minimum of 0.05 pmol/mL.
Urine cyclic GMP excretion (pmol/24 h) was calculated as ucGMP (pmol/mL) × 24 h urine volume (mL); the filtered load of cGMP by the kidney (pmol/min) was calculated as pcGMP (pmol/mL) × creatinine clearance (mL/min), used as an estimate of the glomerular filtration rate.
Statistical analysis
Numerical variables are presented as mean (standard deviation) or median (interquartile range) if non-normally distributed. Categorical variables are presented as count (percent). In the case–control study, continuous variables were compared between cases and matched controls using paired-samples t-test for normally distributed variables and Wilcoxon signed ranks test for two related samples for non-normally distributed variables. Categorical variables were compared using the McNemar test.
In the cohort study, the ucGMP/BNP and pcGMP to plasma BNP (pcGMP/BNP) ratios were compared between patients with and without adverse outcome events during follow-up by the Mann–Whitney U-test. We used a box plot to present the distribution of ratios between the two groups.
All the analyses were conducted using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
Clinical characteristics as well as laboratory data are displayed in Table 1. Baseline characteristics of cases and controls are shown and compared. Plasma BNP and pcGMP were significantly higher in cases than in controls. Beta-blocker use was more prevalent in the control group. Creatinine clearance was significantly lower in cases than in controls. Cases were more fluid overloaded than controls, as suggested by a borderline significantly higher sodium retention score; and they were also receiving higher diuretic doses [median (IQR) 120 (80–160) mg in cases against 80 (60–160) mg in controls, P = 0.06]. There was no difference in 24 h urine volumes [mean (SD) 1792 (617) mL in cases vs. 1689 (584) mL in controls; P = 0.41].
Table 1.
Baseline characteristics of the study sample and comparison between cases and controls
| All patients | Cases (N = 31) | Controls (N = 31) | P-value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years), mean (SD) | 74 (10) | |||
| Male, n(%) | 42 (67.7) | |||
| Hypertension, n(%) | 36 (58.1) | 18 (58.1) | 18 (58.1) | 1.00 |
| Diabetes mellitus, n(%) | 24 (38.7) | 15 (48.4) | 9 (29.0) | 0.15 |
| Coronary artery disease, n(%) | 32 (51.6) | 19 (63.3) | 13 (44.8) | 0.39 |
| Atrial fibrillation, n(%) | 34 (38.7) | 15 (48.4) | 9 (32.1) | 0.29 |
| COPD, n(%) | 15 (24.2) | 7 (22.6) | 8 (26.7) | 1.00 |
| Systolic blood pressure (mmHg), mean (SD) | 118 (20) | 114 (20) | 121 (19) | 0.24 |
| Heart rate (/min), mean (SD) | 76 (15) | 79 (16) | 74 (4) | 0.27 |
| Sodium retention score (−1 to 11), median (IQR) | 1 (0–3) | 2 (1–3) | 1 (0–2) | 0.04 |
| NYHA, n(%) | ||||
| I | 5 (8.1) | 0 (0) | 5 (16.1) | |
| II | 26 (41.9) | 0 (0) | 26 (83.9) | |
| III | 27 (43.5) | 27 (87.1) | 0 (0) | |
| IV | 4 (6.5) | 4 (12.9) | 0 (0) | |
| Medication | ||||
| Aspirin, n(%) | 30 (48.4) | 13 (41.9) | 17 (54.8) | 0.45 |
| Nitrates, n(%) | 17 (27.4) | 12 (38.7) | 5 (16.1) | 0.12 |
| Furosemide, n(%) | 62 (100) | 31 (100) | 31 (100) | |
| Furosemide dose (mg), median (IQR) | 90 (80–160) | 120 (80–160) | 80 (60–160) | 0.06 |
| ACEi or ARB, n(%) | 55 (88.7) | 25 (80.6) | 30 (96.8) | 0.12 |
| Beta-blockers, n(%) | 48 (77.4) | 20 (64.5) | 28 (90.3) | 0.02 |
| Spironolactone, n(%) | 27 (43.5) | 15 (48.4) | 12 (3.7) | 0.61 |
| Statins, n(%) | 41 (66.1) | 22 (71) | 19 (61.3) | 0.58 |
| Digoxin, n(%) | 17 (27.4) | 12 (38.7) | 5 (16.1) | 0.12 |
| Laboratory parameters | ||||
| Haemoglobin (g/dL), mean (SD) | 12.5 (1.9) | 12.6 (2.1) | 12.4 (1.7) | 0.64 |
| Creatinine clearance (ml/min), mean (SD) | 57.1 (27.4) | 50.3 (26.2) | 63.9 (27.4) | 0.01 |
| Na (mEq/L), mean (SD) | 140 (3) | 140 (3) | 140 (3) | 0.61 |
| K (mEq/L), mean (SD) | 4.4 (0.5) | 4.3 (0.5) | 4.5(0.5) | 0.40 |
| Aldosterone (ng/dL), mean (SD) | 15.2 (11.4) | 16.8 (10.4) | 13.6 (12.3) | 0.30 |
| BNP (pg/mL), median (IQR) | 608.3 (298.1–858.2) | 716.4 (374.9–988.3) | 442.0 (278.2–775.0) | 0.04 |
| Plasma cGMP (pmol/mL), mean (SD) | 61.8 (28.6) | 69.5 (22.5) | 54.1 (32.1) | 0.03 |
| Urine cGMP (pmol/mL), mean (SD) | 1729.2 (727.7) | 1629.4 (596.7) | 1829.0 (836.7) | 0.30 |
| 24 h urinary volume (mL), mean (SD) | 1740 (598) | 1792 (617) | 1689 (584) | 0.41 |
| Plasma cGMP/plasma BNP, median (IQR) | 350 (200–604) | 345 (202–617) | 374 (196–600) | 0.88 |
| Urine cGMP/plasma BNP, median (IQR) | 9171 (6029–18186) | 8354 (4293–16456) | 12693 (6896–22851) | 0.02 |
| cGMP in 24 h urine (×1000 pmol/24 h), mean (SD) | 2975.4 (1568.4) | 2961.6 (1695.3) | 2989.1 (1458.6) | 0.87 |
| cGMP filtered load (×1000 pmol/min), mean (SD) | 3.53 (2.54) | 3.57 (2.36) | 3.49 (2.76) | 0.87 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor I blocker; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; K, serum potassium; Na, serum sodium; SD, standard deviation.
There was no statistically significant difference in pcGMP/BNP ratio [median (IQR)] between the chronic advanced HF patients and controls: 345 (202–617) vs. 374 (196–600), respectively, P = 0.88. Cyclic GMP excretion in 24 h urine and the filtered cGMP load at the kidney were also similar between cases and controls.
UcGMP/BNP ratio [median (IQR)] was significantly lower in cases than in controls 8354 (4293–16 456) vs. 12693 (6896–2 2851) respectively; P = 0.02.
During the 3-month follow-up period, 12 (19%) patients died or were hospitalized—in 7 of these (58%), death was the final outcome. Among the 12 patients who experienced an adverse outcome event, 10 (83.3%) were cases and 2 (16.7%) were controls (P = 0.02).
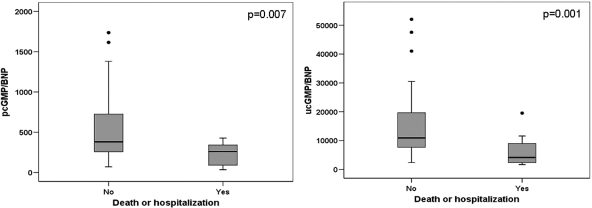
The ucGMP/BNP ratio [median (IQR)] in patients with (n = 12) and those without (n = 50) an adverse outcome occurrence was 4146 (2207–9363) vs. 10 922 (7495–19 971), respectively; P = 0.001. The pcGMP/BNP ratio between patients with and without an adverse outcome was 260 (86–344) vs. 381 (244–728), respectively; P = 0.007 (Table 2). Figure 1 shows the distribution of ucGMP/BNP and of pcGMP/BNP ratios according to outcome. Patients who developed an adverse outcome had lower pcGMP/BNP and ucGMP/BNP ratios and higher plasma BNP levels. However, there were no differences between the 12 patients with an adverse outcome and the 50 outcome-free patients for ucGMP and pcGMP, 24 h-urine cGMP excretion, and cGMP filtered load by the kidney; these data are shown in Table 2.
Table 2.
Laboratory parameters in patients with and without adverse outcome events
| Patients with outcome (n = 12) | Outcome-free patients (n = 50) | P-value | |
|---|---|---|---|
| Creatinine clearance (mL/min), mean (SD) | 51.5 (27.9) | 58.4 (27.4) | 0.44 |
| BNP (pg/mL), median (IQR) | 955.8 (790.4–2652.8) | 479.6 (276.5–753.7) | <0.001 |
| Plasma cGMP (pmol/mL), mean (SD) | 1650.0 (677.6) | 1748.2 (744.5) | 0.69 |
| Urine cGMP (pmol/mL), mean (SD) | 65.5 (22.3) | 60.9 (30.0) | 0.62 |
| Plasma cGMP/plasma BNP, median (IQR) | 260 (86–344) | 381 (244–728) | 0.007 |
| Urine cGMP/plasma BNP, median (IQR) | 4146 (2207–9363) | 10922 (7495–19 971) | 0.001 |
| cGMP in 24 h urine (×1000 pmol/24 h), mean (SD) | 2879.4 (2093.2) | 2998.4 (1440.8) | 0.82 |
| cGMP filtered load (×1000 pmol/min), mean (SD) | 3.72 (2.96) | 3.49 (2.47) | 0.78 |
Figure 1.
Box plots of plasma cyclic guanosine monophosphate to B-type natriuretic peptide ratio (left) and urine cyclic guanosine monophosphate to plasma B-type natriuretic peptide (right) distributions in patients with and without adverse outcome events. Left panel: distribution of the plasma cyclic guanosine monophosphate to B-type natriuretic peptide ratio in patients who died or were hospitalized due to worsening heart failure during the 3-month follow-up and in patients who remained free of this outcome. The ratio was significantly lower in patients with worse prognosis (P = 0.007). Right panel: distribution of the urine cyclic guanosine monophosphate to plasma B-type natriuretic peptide ratio in patients who died or were hospitalized due to worsening heart failure during the 3-month follow-up and in patients who remained free of this outcome. The ratio was significantly lower in patients with worse prognosis (P = 0.001).
Discussion
Our study shows a significant ucGMP/BNP ratio decrease in chronic advanced HF patients with severe LVSD when compared with age- and sex-matched patients with severe LVSD who were symptomatically controlled by optimized medical therapy. These results are in line with previous reports from animal studies1,26 and support the role of renal hyporesponsiveness contributing to the progression of HF to a more symptomatic state. Consistent with this interpretation is the fact that the symptomatic patients were more fluid overloaded than the controls, even under higher diuretic dosages and that despite similar 24 h urinary volumes the cases did not excrete significantly higher amounts of cGMP.
BNP was significantly higher in the chronic advanced HF patients, and the same was true for pcGMP. There was no difference in the pcGMP/BNP ratio between cases and controls. This suggests that systemic attenuation is not associated with more intense and uncontrollable symptoms.
The 12 patients who died or were readmitted for HF worsening during follow-up had significantly lower pcGMP/BNP and ucGMP/BNP ratios. The pcGMP/BNP ratio can be considered as an in vivo marker of NP systemic signalling sensitivity24,26 and the ucGMP/BNP ratio as an in vivo marker of NP signalling sensitivity in the kidney.26,29 Thus, in event-free patients, there was no or less attenuation of the NP system both systemically and in the kidney.
No differences were detected in urine cGMP excretion and in the cGMP filtered load when comparing chronic advanced HF patients with controls nor did these variables differ in patients with and without adverse outcome events. Given that cases had higher BNP plasma concentrations, this is consistent with the achievement of a plateau in the renal cGMP production response in chronic HF and ineffectiveness of the NP system at the kidney level. To draw this conclusion, we assumed that ucGMP is a convenient ‘mirror’ of the intracellular signalling response to NPs; this probably represents an oversimplification of cGMP metabolism in the kidney. However, previous animal and human studies suggest this assumption is acceptable, since (i) after exogenous ANF administration, ucGMP increases progressively in the non-HF context;22,29 (ii) little alteration of ucGMP excretion has been documented in response to the administration of many other hormones including parathyroid hormone, glucagon, thyroxine, adrenocorticotropic hormone, glucocorticoids, vasopressin, and epinephrine;29–31 and (iii) NP receptors in the kidney have only been detected on tubular basolateral membranes.5 To our knowledge, this is the first study in humans in which simultaneous measurements of pcGMP and ucGMP allowed the determination of the urine cGMP excretion and of the cGMP filtered load in the kidney as well as ucGMP/BNP and pcGMP/BNP ratios.
NPs exert their effects through the second messenger cGMP. Comparing cGMP levels with NP levels, we can determine if chain disruption occurs upstream or downstream of the second messenger production. Our results suggest disruption at the production level or downstream.
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides. PDEs 5, 6, and 9 are cyclic GMP specific.33 Increased cGMP degradation is well documented in animal models of sodium retention,12–14 and the therapeutic potential of PDE inhibitors in HF has been suggested.34–37
Study limitations
The small sample size precluded analysing for potential confounders. However, taking into account that this was an exploratory study and also the laboratory logistics required, the sample size was enough to prove the main study hypothesis and opens doors for further studies to be conducted in this area.
The inclusion of patients receiving nitrates may have influenced the results, since NO is a well-known inducer of cGMP production. Second messenger cGMP production in response to NO is accomplished via soluble guanylyl cyclase receptors.23,32 More cases (n = 12) than controls (n = 5) were receiving nitrates, and we can speculate that this may have contributed to an underestimation of the cGMP response attenuation in the chronic advanced HF cases.
The filtered load of cGMP was certainly overestimated, since creatinine clearance overestimates the glomerular filtration rate due to secretion of creatinine by the kidney tubules.
Routine procedures were followed for 24 h urine collection and urine was kept at 4°C for at least 24 h; the calculated 24 h cGMP excretion probably represents an underestimation of the true values. Notwithstanding, this was a random effect problem that affected the cases in the same way as the controls and did not preclude the detection of differences in the ucGMP/BNP ratio between the two groups.
Another important limitation that we must point out is that a commercially available assay—Abbott AxSym—was used to measure BNP levels. The immunoreactive BNP measured is a mixture of high- and low-molecular-weight forms with very little biologically active BNP1-32, as suggested by Hawkridge et al.16 In our study, we cannot distinguish the contribution of each BNP form to the final natriuretic effect. Both BNP and proBNP stimulate intracellular cGMP production in a dose-dependent way; however, proBNP is believed to be less potent than BNP.17 It may be argued that the cGMP/BNP ratio may not accurately reflect reduced bioactivity of BNP1-32, but it certainly reflects bioactivity of the mixture of different BNP forms in a particular patient and is a marker of sensitivity to the BNPs as a whole.
Conclusions
In conclusion, our results show an association between HF severity and NP system attenuation, both renally and systemically. The mechanisms underlying NP system attenuation and therapeutic approaches targeting NP’s second messenger cGMP require further evaluation.
Acknowledgements
We thank Gabriela Couto, Madalena Oliveira, Susana Silva, Bruno Ribeiro, and Adélia Mão-de-Ferro for their precious help. We also thank Dra Conceição Gonçalves for the careful handling of laboratory measurements.
Conflict of interest: none declared.
References
- 1.Margulies KB, Heublein DM, Perrella MA, Burnett JC. ANF-mediated renal cGMP generation in congestive heart failure. Am J Physiol. 1991;260:F562–F568. doi: 10.1152/ajprenal.1991.260.4.F562. [DOI] [PubMed] [Google Scholar]
- 2.Margulies KB, Barclay PL, Burnett JC., Jr The role of neutral endopeptidase in dogs with evolving congestive heart failure. Circulation. 1995;91:2036–2042. doi: 10.1161/01.cir.91.7.2036. [DOI] [PubMed] [Google Scholar]
- 3.Hirooka Y, Takeshita A, Imaizumi T, Suzuki S, Yoshida M, Ando S, Nakamura M. Attenuated forearm vasodilative response to intra-arterial atrial natriuretic peptide in patients with heart failure. Circulation. 1990;82:147–153. doi: 10.1161/01.cir.82.1.147. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Arakawa N, Yoshida H, Makita S, Niinuma H, Hiramori K. Vasodilatory effects of B-type natriuretic peptide are impaired in patients with chronic heart failure. Am Heart J. 1998;135:414–420. doi: 10.1016/s0002-8703(98)70316-3. [DOI] [PubMed] [Google Scholar]
- 5.Charloux A, Piquard F, Doutreleau S, Brandenberger G, Geny B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur J Clin Invest. 2003;33:769–778. doi: 10.1046/j.1365-2362.2003.01222.x. [DOI] [PubMed] [Google Scholar]
- 6.Andreassi MG, Del Ry S, Palmieri C, Clerico A, Biagini A, Giannessi D. Upregulation of ‘clearance’ receptors in patients with chronic heart failure: a possible explanation for the resistance of biological effects of cardiac natriuretic hormones. Eur J Heart Fail. 2001;3:407–414. doi: 10.1016/s1388-9842(01)00161-1. [DOI] [PubMed] [Google Scholar]
- 7.Knecht M, Pagel I, Langenickel T, Philipp S, Scheuermann-Freestone M, Willnow T, Bruemmer D, Graf K, Dietz R, Willenbrock R. Increased expression of renal neutral endopeptidase in severe heart failure. Life Sci. 2002;71:2701–2712. doi: 10.1016/s0024-3205(02)01990-2. [DOI] [PubMed] [Google Scholar]
- 8.Tsutamoto T, Kanamori T, Morigami N, Sugimoto Y, Yamaoka O, Kinoshita M. Possibility of down-regulation of atrial natriuretic peptide receptor coupled to guanylate cyclase in peripheral vascular beds of patients with chronic severe heart failure. Circulation. 1993;87:70–75. doi: 10.1161/01.cir.87.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Bryan PM, Xu X, Dickey DM, Chen Y, Potter LR. Renal hyporesponsiveness to atrial natriuretic peptide in congestive heart failure results from reduced atrial natriuretic peptide receptor concentrations. Am J Physiol Renal Physiol. 2007;292:F1636–F1644. doi: 10.1152/ajprenal.00418.2006. [DOI] [PubMed] [Google Scholar]
- 10.Yechieli H, Kahana L, Haramati A, Hoffman A, Winaver J. Regulation of renal glomerular and papillary ANP receptors in rats with experimental heart failure. Am J Physiol. 1993;265:F119–F121. doi: 10.1152/ajprenal.1993.265.1.F119. [DOI] [PubMed] [Google Scholar]
- 11.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 12.Wilkins MR, Settle SL, Stockmann PT, Needleman P. Maximizing the natriuretic effect of endogenous atriopeptin in a rat model of heart failure. Proc Natl Acad Sci USA. 1990;87:6465–6469. doi: 10.1073/pnas.87.16.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Supaporn T, Sandberg SM, Borgeson DD, Heublein DM, Luchner A, Wei CM, Dousa TP, Burnett JC., Jr Blunted cGMP response to agonists and enhanced glomerular cyclic 3′,5′-nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int. 1996;50:1718–1725. doi: 10.1038/ki.1996.491. [DOI] [PubMed] [Google Scholar]
- 14.Lee EYW, Humphreys MH. Phosphodiesterase activity as a mediator of renal resistance to ANP in pathological salt retention. Am J Physiol. 1996;271:F3–F6. doi: 10.1152/ajprenal.1996.271.1.F3. [DOI] [PubMed] [Google Scholar]
- 15.Schiffrin EL. Decreased density of binding sites for atrial natriuretic peptide on platelets of patients with severe congestive heart failure. Clin Sci. 1988;74:213–218. doi: 10.1042/cs0740213. [DOI] [PubMed] [Google Scholar]
- 16.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci USA. 2005;102:17442–17447. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang F, O’Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–1078. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 18.Lam CS, Burnett JC, Jr, Costello-Boerrigter L, Rodeheffer RJ, Redfield MM. Alternate circulating pro-B-type natriuretic peptide and B-type natriuretic peptide forms in the general population. J Am Coll Cardiol. 2007;49:1193–1202. doi: 10.1016/j.jacc.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, Sugita K, Yamada K. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316:129–135. doi: 10.1016/s0009-8981(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 20.Seferian KR, Tamm NN, Semenov AG, Mukharyamova KS, Tolstaya AA, Koshkina EV, Kara AN, Krasnoselsky MI, Apple FS, Esakova TV, Filatov VL, Katrukha AG. The brain natriuretic peptide (BNP) precursor is the major immunoreactive form of BNP in patients with heart failure. Clin Chem. 2007;53:866–873. doi: 10.1373/clinchem.2006.076141. [DOI] [PubMed] [Google Scholar]
- 21.Chen HH. Heart Failure A state of brain natriuretic peptide deficiency or resistence or both! J Am Coll Cardiol. 2007;49:1089–1091. doi: 10.1016/j.jacc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Gerzer R, Witzgall H, Tremblay J, Gutkowska J, Hamet P. Rapid increase in plasma and urinary cyclic GMP after bolus injection of atrial natriuretic factor in man. J Clin Endocrinol Metab. 1985;61:1217–1219. doi: 10.1210/jcem-61-6-1217. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi M, Takeda S, Kurokawa S, Kubo T, Fukuda N, Izumi T. Cyclic GMP production by ANP, BNP and NO during worsening and improvement of chronic heart failure. Jpn Heart J. 2003;44:713–724. doi: 10.1536/jhj.44.713. [DOI] [PubMed] [Google Scholar]
- 24.Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, Ohnishi M, Sugimoto Y, Kinoshita M. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure. Circulation. 1997;96:509–516. doi: 10.1161/01.cir.96.2.509. [DOI] [PubMed] [Google Scholar]
- 25.Kawai K, Hata K, Tanaka K, Kuboka Y, Inoue R, Masuda E, Miyazaki T, Yokoyama M. Attenuation of biologic compensatory action of cardiac natriuretic peptide system with aging. Am J Cardiol. 2004;93:719–723. doi: 10.1016/j.amjcard.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 26.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49:1079–1088. doi: 10.1016/j.jacc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 27.Metra M, Ponikowsky P, Dickstein K, McMurray JJ, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohacsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M. Advanced chronic heart failure: a position statement from the study group on advanced heart failure of the heart failure association of the European society of cardiology. Eur J Heart Fail. 2007;9:684–694. doi: 10.1016/j.ejheart.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Cody RJ. The need for a sodium retention score in clinical trials of heart failure. Clin Pharmacol Ther. 1993;54:7–10. doi: 10.1038/clpt.1993.100. [DOI] [PubMed] [Google Scholar]
- 29.Wong KR, Xie MH, Shi LB, Liu FY, Huang CL, Gardner DG, Cogan MG. Urinary cGMP as biological marker of the renal activity of atrial natriuretic factor. Am J Physiol. 1988;255:F1220–F1224. doi: 10.1152/ajprenal.1988.255.6.F1220. [DOI] [PubMed] [Google Scholar]
- 30.Broadus AE, Kaminsky NI, Northcutt RC, Hardman JG, Sutherland EW, Liddle GW. Effects of glucagons on adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate in human plasma and urine. J Clin Invest. 1970;49:2237–2245. doi: 10.1172/JCI106442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardman JG, Davis JD, Sutherland EW. Effects of some hormonal and other factors on the excretion of guanosine 3′,5′-monophosphate and adenosine 3′,5′-monophosphate in rat urine. J Biol Chem. 1969;244:6354–6362. [PubMed] [Google Scholar]
- 32.Tsutamoto T, Kinoshita M, Ohbayashi Y, Wada A, Maeda Y, Adachi T. Plasma arteriovenous cGMP difference as a useful indicator of nitrate tolerance in patients with heart failure. Circulation. 1994;90:823–829. doi: 10.1161/01.cir.90.2.823. [DOI] [PubMed] [Google Scholar]
- 33.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147:S252–S257. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel MD, Kats SD. Phosphodiesterase 5 inhibition in chronic heart failure and pulmonary hypertension. Am J Cardiol. 2005;96:M47–M51. doi: 10.1016/j.amjcard.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercice haemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 36.Kats SD. Potential role of type 5 phosphosdiesterase inhibition in the treatment of congestive heart failure. Congest Heart Fail. 2003;9:9–15. doi: 10.1111/j.1527-5299.2002.00288.x. [DOI] [PubMed] [Google Scholar]
- 37.Lewis GD, Smigran MJ. The emerging role for type 5 phosphodiesterase inhibition in heart failure. Curr Heart Fail Rep. 2006;3:123–128. doi: 10.1007/s11897-006-0011-0. [DOI] [PubMed] [Google Scholar]