Abstract
This review examines the ‘Quiet Embryo Hypothesis’ which proposes that viable preimplantation embryos operate at metabolite or nutrient turnover rates distributed within lower ranges than those of their less viable counterparts. The ‘quieter’ metabolism consistent with this hypothesis is considered in terms of (i) ‘functional’ quietness; the contrasting levels of intrinsic metabolic activity in different cell types as a consequence of their specialized functions, (ii) inter-individual embryo/cell differences in metabolism and (iii) loss of quietness in response to environmental stress. Data are reviewed which indicate that gametes and early embryos function in vivo at a lower temperature than core body temperature, which could encourage the expression of a quiet metabolism. We call for research to determine the optimum temperature for mammalian gamete/embryo culture. The review concludes by examining the key role of reactive oxygen species, which can induce molecular damage, trigger a cellular stress response and lead to a loss of quietness.
Introduction
The ‘quiet embryo hypothesis’ proposed that viable embryos have a ‘quieter’ metabolism than those which arrest (Leese, 2002). The term ‘quiet’ embryo was chosen as it reflects the range of metabolic activity consistent with a viable phenotype. The hypothesis was based on data largely derived from experiments on the net depletion or release of nutrients, such as pyruvate, glucose, lactate and amino acids, and on oxygen consumption, by mouse and human embryos, as well as on data from studies of somatic cells. In a development of the hypothesis, it was proposed that stochastic events and environmental stresses determine whether a zygote will develop (Baumann et al., 2007). Extrinsic noise can be influenced by environmental stresses that increase the heterogeneity of the cell population, e.g. nucleic acid damage and heat shock, and this heterogeneity can be inherited by daughter cells. For example, disproportionate inheritance of mitochondria has been observed in cleavage stage human embryos, which can lead to a daughter cell with reduced ATP generating capacity and developmental competence (van Blerkom et al., 2000). In a viable embryo, the genome, transcriptome and proteome are not overly compromised and will support development. This leads to a situation whereby less oxygen and nutrients need to be consumed. In contrast, less viable embryos have more molecular/cellular damage or are less well equipped at the transcriptome and proteome levels to cope with damage present. Such cells may attempt to carry out repair and/or resort to rescue strategies or undergo apoptosis with the consumption of a greater quantity of nutrients, reflected as a more ‘active’ metabolism. Two more propositions were made on the basis of data on livestock embryos (Leese et al., 2007): (i) the concept of quiet metabolism may be applied to the response of embryos to stress, as well as to their basal metabolism and (ii) the hypothesis can be extended to the embryos of mammals generally.
In this paper, we explore these concepts in more detail and introduce a further category of ‘quietness’ which we have termed ‘functional’, to account for the contrasting levels of intrinsic metabolic activity in different cell types and tissues as a consequence of their specialized functions. Each ‘category of quietness’ will be considered in turn, using the examples in Table I. We also speculate on the key role of reactive oxygen species (ROS) in triggering molecular/cellular damage leading to an active metabolic phenotype.
Table I.
Proposed categories of metabolic ‘Quietness’.
| Category of ‘Quietness’ | Examples |
|---|---|
| Functional Quietness | Cleavage stage embryos are quieter than blastocysts |
| Cells of the inner cell mass are quieter than trophectoderm | |
| Metabolic rate is dramatically reduced in embryonic diapause | |
| Inter-individual embryo/cell differences in quietness | Quiet embryos are more viable than active |
| Do quiet ICM cells go on to form embryonic stem cells in culture? | |
| Are quieter cells/blastomeres less likely to apoptose? | |
| Loss of Quietness, e.g. in response to environmental stress | In vivo-derived embryos are metabolically less active than in vitro-produced |
| Exposure to the environment in vitro elevates metabolic activity of in vivo embryos | |
| High plane of maternal nutrition/feeding leads to increased embryonic metabolism | |
| Serum and ammonium in culture medium increase metabolic activity | |
| Increased plasma ammonium levels (in response to diet) increase embryo metabolism | |
| Accelerated, precocious embryo development up-regulates metabolic activity | |
| Exposure to elevated oxygen may increase ROS production | |
| Gamete development occurs at a reduced body temperature |
Functional quietness: metabolic activity as a consequence of specialized function
Cleavage stage embryos are quieter than blastocysts
The early, cleavage stages of preimplantation development are relatively quiescent metabolically with ATP production sustained by the oxidation of substrates such as pyruvate, lactate and amino acids (Leese, 1991; Leese, 2003), a process that can broadly be estimated by measuring oxygen consumption. During this period, DNA replication and cell division occur but there is no overall increase in cellular volume or mass (Turner et al., 1992) such that the energy needs of the embryo are relatively low. By limiting oxygen consumption during early cleavage, embryos may also minimize ROS formation, especially during activation of the zygotic genome which begins at the 4–8-cell stage in the human. (For more detailed discussion of the influence of oxygen tension during preimplantation development, see Harvey et al., 2004, 2007a).
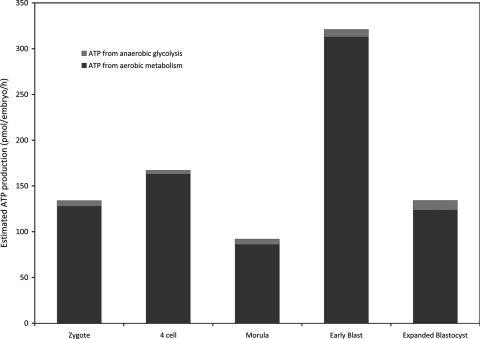
With the onset of morula formation (compaction) and during blastocyst formation (cavitation), energy demands, measured in terms of oxygen and nutrient consumption, increase dramatically due largely to two processes: (i) the pumping of sodium ions by the Na+,K+ATPase transport system located in the trophectoderm (TE) which leads to the formation of the blastocoel cavity and (ii) protein synthesis as the embryo initiates net growth for the first time. A feature of nutrient uptake by the blastocyst is the increased consumption of glucose, a high proportion of which is converted to lactate (Hardy et al., 1989; Conaghan et al., 1993). It has been proposed that increased glycolysis may prepare the embryo for the hypoxic environment it will encounter at implantation (Leese, 1989). While the ability to generate ATP by this means is undoubtedly critical, we would like to emphasize that the glycolytic pathway is an inefficient means of generating ATP (relative yield: 2 ATPs per glucose versus ∼32 ATPs for complete oxidation) and that the overall contribution of ‘aerobic glycolysis’ to ATP biosynthesis at the blastocyst stage is therefore small as our data for pig preimplantation embryos illustrate (Sturmey and Leese, 2003) (Fig. 1).
Figure 1:
ATP production by in vitro-derived porcine embryos calculated from lactate production and oxygen consumption.
There is a characteristic shift in the metabolic profile of early porcine embryos, with an increase in the amount of ATP produced but the relative contribution from glycolysis is minimal. The methods used in generating these data are described in Sturmey and Leese (2003).
Cells of the inner cell mass are quieter than the TE
The inner cell mass (ICM) is quieter metabolically than the TE in mouse (Houghton, 2006). TE cells, which comprise a transporting epithelium, are responsible for creating the environment within the blastocoel, consume more oxygen and nutrients and appear to have more mitochondria (as assessed semi-quantitatively by retention of Mitotracker Green) than those of the ICM which has a more glycolytic metabolism than the TE in mouse (Hewitson and Leese, 1993). The quiescence shown by the ICM may reflect pluripotency (Houghton, 2006).
There are numerous biochemical and molecular regulators of oxygen consumption: energy substrate supply, redox status, ADP/ATP ratio, mitochondrial abundance, and localization and differential expression of oxygen-regulated genes (Harvey et al., 2004, 2007b). Downstream regulation may be mediated by the signalling agent nitric oxide, which inhibits mitochondrial cytochrome oxidase and oxygen consumption in mouse blastocysts (Manser et al., 2004).
Metabolic rate is reduced in embryonic diapause
In embryonic diapause, also known as delayed implantation, the early embryo is arrested at the blastocyst stage; cell division, development and blastocyst expansion cease or are very considerably reduced and there is down-regulation of metabolism (Spindler et al., 1996; Renfree and Shaw, 2000; Lambert et al., 2001; Renfree, 2006). After a period in diapause, which may last up to 11 months in some species, the blastocyst is reactivated and resumes development. The biochemical mechanisms involved in this particularly intriguing example of quiet metabolism are relatively little understood though are now beginning to attract the attention they deserve (Hondo and Stewart, 2005). As well as its physiological and evolutionary significance, ‘embryonic diapause provides a wonderful system for understanding the control of early embryonic development’ (Renfree, 2006).
Inter-individual embryo/cell differences in quietness: differences in metabolic activity between individual embryos or between the blastomeres of a given embryo
The relationship between ‘quiet metabolism’ of individual embryos and their subsequent viability, i.e. the capacity to form blastocysts in vitro and pregnancies in vivo, has been considered elsewhere (Leese, 2002; Baumann et al., 2007; Leese et al., 2007). This concept arose from data on the metabolism of individual embryos as opposed to those cultured in groups. The group-culture approach adopted historically was invaluable in establishing the general picture of early embryo metabolism but masked the considerable variation within and between cohorts of embryos. By measuring single embryos, we have been able to examine the heterogeneity within these data, and since most of our assays are non-invasive, to relate the values to embryo developmental capacity. Predictably, in embryos from inbred mice, heterogeneity in metabolic activity is very small since the vast majority of such embryos exhibit high developmental competence in line with their high genetic homogeneity; in contrast, human embryos vary greatly in genetic background and, not surprisingly, exhibit high heterogeneity in metabolic and developmental capacity. The embryos of farm animal species such as the cow, sheep and pig are intermediate in these terms, between mouse and human. A major question is how metabolic activity and developmental competence are linked; is there a causative effect; i.e. does aberrant metabolism impair development, or vice versa? This approach can be extended to the cellular level and further questions arise, for example.
Are quiet ICM cells more likely to form stem cells?
The ICM of the blastocyst contains the pluripotent embryo stem cell population, from which embryonic stem (ES) cell lines can be derived. As pointed out above, ICM cells have a lower or ‘quieter’ consumption of O2 and a more glycolytic energy metabolism, which is less reliant on O2 availability, compared with their differentiated counterparts in the TE. Animal embryos form blastocysts successfully in low (5%) O2 and, in the case of the bovine, produce more ICM cells under these conditions. Similarly, although human ES cell lines grow well in either hypoxic (3–5% O2) conditions or in air (21% O2), spontaneous loss of pluripotency, i.e. differentiation, as measured by loss of expression of the pluripotency marker Oct4, is reduced under hypoxia (Ezashi et al., 2005). These data suggest that culture under conditions which promote a lower level of energy metabolism and/or oxidative metabolism may be important for developing and maintaining the pluripotent cell state. Alternatively, hypoxia may maintain the pluripotent state directly via up-regulation of hypoxia-inducible factor (HIF2α) which binds directly to the Oct4 promoter and up-regulates its expression (Simon and Keith, 2008). Equally, as ROS signalling is important during differentiation of cells, reduced ROS generation under low O2 conditions may be an alternative but not inconsistent mechanism for suppressing differentiation and maintaining pluripotency. Either way, individual ICM cells which have a quiet metabolism may be primed to maintain pluripotency while retaining the ability to self-renew and proliferate; characteristics required both for the formation of a fetus and an ES cell line. However, very little work has been done to establish whether only a subset of ICM cells is capable of giving rise to ES cell lines, and the O2 dependency or otherwise of this process. It may be that an identifiable subset of quiet ICM cells provides the progenitors of ES cell lines and that it is advantageous to attempt to isolate these cells under conditions designed to maintain quiet metabolism.
Are quiet blastomeres less likely to undergo apoptosis?
There are cell biological consequences of ‘quiet’ and ‘active’ metabolism. The best candidate, and most studied, is apoptosis since this occurs alongside cell proliferation and may be essential in removing damaged cells from the embryo and ensuring an appropriate number and ratio of cells in the ICM and TE of the blastocyst; critical for subsequent embryo survival (Byrne et al., 1999). Apoptosis first occurs in preimplantation development at around the time the embryonic genome is activated (1–2-cell stage in mouse, 4–8-cell in human) and then at the blastocyst stage, where it is targeted primarily to the ICM cells. Embryos produced or cultured in vitro, in conditions which may favour non-quiet metabolism, i.e. 21% O2 or high concentrations of energy substrates, generally undergo more apoptosis at an earlier stage of development and contain more apoptotic cells at the blastocyst stage compared with their in vivo-derived counterparts (Brison and Schultz, 1997; Brison, 2000; Byrne et al., 1999; Jurisicova and Acton, 2004). In terms of biochemical mechanisms, there are well-defined links between energy metabolism and apoptosis. For example, phosphatidylinositol 3-kinase (PI3K)-activation by growth factors promotes glucose consumption and inhibits apoptosis; conversely, inhibition of the PI3K pathway leads to induction of apoptosis (Robey and Hay, 2005; Riley et al., 2006). Thus, glucose starvation stimulates apoptosis while paradoxically glucose at hyperglycaemic concentrations has a similar effect, due in this case to high glucose down-regulation of GLUT1 transporter protein (Moley et al., 1998) and reduced glucose uptake. Moreover, high, un-regulated oxidative metabolism leading to excess ROS is likely to increase DNA damage, reflected in elevated levels of apoptosis. This evidence suggests that metabolic perturbations induce dysregulation of apoptosis in early embryos; an effect exacerbated by ROS-induced DNA damage. We therefore propose that appropriate regulation of apoptosis is a further component of the quiet metabolism phenotype.
Loss of quietness in response to environmental stress
The metabolism of early embryos may be up-regulated in response to different environments (Leese et al. 2007). For example:
The female reproductive tract is likely to promote quietness while the in vitro environment induces a more active metabolism. Of particular interest is the transition, from the in vivo environment to the in vitro.
Up-regulation may follow enrichment of the periconceptual environment as a result of a high plane of maternal feeding.
The addition of serum and ammonium to culture media may promote an active metabolism.
Elevated plasma ammonium following urea feeding tends to up-regulate metabolism in ruminants.
Up-regulation is often associated with accelerated or precocious development.
Up-regulation may follow culture at oxygen concentrations above those present physiologically (e.g. under air (20% O2) versus 1–5% O2 in the female reproductive tract).
In considering these examples, it is clear that oocytes and embryos are not shielded from effects of maternal diet, human body mass index, or in the case of livestock, body condition parameters, and can suffer severely from the impact of such environmental perturbations. Such effects can be a function of heat stress, endocrine status and toxic by-products, such as ammonia in ruminants (McEvoy et al., 1997).
Animal studies have shown that the consequences can be as severe as embryo loss and that surviving embryos exposed to sub-optimal conditions may be susceptible to longer-term effects which extend to adulthood and even the next generation. As a response to the various stimuli, some of the resultant phenotypes exhibit up-regulated (active) metabolism which restores their normal developmental trajectory. In contrast, aberrations may arise, for example, through influences on imprinted genes, whereby up-regulated metabolism is sufficiently severe or persists long enough to interfere with normal development. Even so, and reflecting its remarkable resilience, the mammalian embryo is capable of normal development when culture conditions favour quiet metabolism, for example, minimal exposure to adverse stimuli such as excessive ROS, ammonia, heavy metal ions and other stressors. The challenge is therefore to devise culture conditions which expose early embryos to the minimum of stress. Of emerging systems, microfluidics technologies seem to offer a good prospect of achieving this.
Culture of gametes and embryos at core body temperature may lead to loss of quietness
It is well known that sperm develop at a temperature 1–2°C or more below core body temperature and that heat stress impairs spermatogenesis and semen quality. What is less appreciated is that the temperature within the ovarian follicle is also ∼1.5–2°C cooler than deep body temperature: by 1.4°C in rabbit (Grinsted et al., 1980), 2.4°C in pig (Hunter and Nichol, 1986) and up to 2.3°C in women (Grinsted et al., 1985). Hunter et al. (2006) elegantly illustrate this phenomenon in thermographic images of pig ovarian follicles and the surrounding stroma. For summaries of this work, see Einer-Jensen and Hunter (2005) Einer-Jensen and Hunter (2006) and Ye et al. (2007). Moreover, there is a temperature gradient along the Fallopian tube (David et al., 1971; Hunter and Nichol, 1986). Thus, Hunter and Nichol (1986) found in the pig that the pre-ovulatory isthmus was 0.43°C cooler than the ampulla (range 0.2–0.7), a difference which increased to 0.69°C (range 0.2–1.6) following mating. A similar pattern was reported for rabbit oviducts by Bahat et al. (2005) who found a temperature difference between the isthmus (the site of sperm storage) and ampulla (the site of fertilization) of 0.8°C preovulation, increasing to 1.6°C post-ovulation. The differences between these values for the rabbit and rectal temperature (∼38°C) were ∼−3.1 and −2.0°C for isthmus and ampulla, respectively. The reduced temperature in the isthmus might have a role in maintaining sperm in a quiescent state during storage, prior to hyperactivation at fertilization in the ampulla. It has also been speculated that sperm can sense these gradients and thereby be guided from the site of storage to that of fertilization (Bahat et al., 2003; Eisenbach and Giojalas, 2006).
In addition to the potential significance of these temperature differences for sperm function, it is tempting to propose that they could have a role in promoting functional quiet metabolism in the oocyte and early embryo. The increase or decrease in the rate of most chemical processes in response to a 10°C change in temperature, i.e. the ‘Q10’, is between 2- and 3-fold. There are no data on the temperature within the human Fallopian tube with respect to core body temperature, but it seems reasonable to expect a gradient and therefore to assume a value similar to that in other species (Eisenbach and Giojalas, 2006). On a conservative estimate, this gives a value post-ovulation ∼1.5°C cooler than deep body temperature. If this is the case, then the metabolism of human oocytes and early human embryos will be ∼15% lower than that measured at normal body temperature (37°C), or well within the range being considered for ‘quiet metabolism’. This raises the question as to whether human IVF and related procedures should be carried out, at, say, 35.5–36°C rather than at 37°C. In this context, one of us (McEvoy et al., 2000) suggested that ‘One feature of in vitro maturation technology that is out of step with the in vivo reality is the incubation temperature employed; this has conventionally been set to reflect the animal’s core body temperature but it is now known that, in the cow and pig for example, the temperature of the maturing oocyte’s follicular environment in the ovary is ∼2–3°C lower’ (Grondahl et al., 1996; Hunter et al., 1997). In preliminary experiments (Sturmey et al., unpublished), we have found that when bovine blastocysts are grown at a lowered temperature (37°C as opposed to the conventional 39°C) metabolic activity, in terms of amino acid consumption and production, is reduced, consistent with a quiet metabolism, while blastocyst rates remain largely unchanged. We are currently pursuing these potentially significant observations and urge others to do the same. We consider it is important to characterize these effects in animal embryos and spare human embryos before contemplating reducing the incubation temperature in clinical IVF. Useful markers for temperature effects on gametes and early embryos may derive from the valuable work of Hansen’s group on the effects of heat stress on embryonic survival (Hansen, 2007).
Reactive oxygen species: a trigger and consequence of lack of metabolic quietness?
Reactive oxygen species are an unavoidable by-product of oxidative phosphorylation resulting from in situ leakage from the mitochondrial electron transport chain (Burton et al., 2003). Due to the presence of an unpaired electron (Halliwell and Gutteridge, 2007), these molecules can damage cellular components including nucleic acids, proteins and membranes. By maintaining a quiet aerobic metabolism, the generation of excess ROS may be minimized. Baumann et al. (2007) put forward a catalogue of molecular components, damage to which would require the activation of repair mechanisms in oocytes and early embryos and an increase in metabolic activity, i.e. loss of quietness. Nucleic acids are key components in many cellular processes and are easily damaged by ROS. DNA and RNA damage can take the form of lesions or strand breaks (SBs). Lesions are an alteration to the chemical and/or physical structure of the DNA or RNA at the base level as a result of reactions between the bases and exogenous chemicals and agents.
The presence of modified bases can lead to point mutations or physical distortion in the DNA helix, preventing transcription and/or replication. ROS are a common cause of DNA lesions typically reacting with purines to generate 8-oxoguanine, the most abundant lesion in DNA (Poulsen, 2005) and 2-oxoadenine. SBs are physical breaks in the DNA and can take two forms; those present in one strand of the DNA (single-strand break), or in both strands (double-strand breaks, DSB). The presence of a single DSB is sufficient to trigger cell death. The ability to cope with DNA damage is vital for the normal functioning of cells and with regard to the early embryo, Johnson and Nasr-Esfhani (1994) argued persuasively that ROS-induced damage could, in part, be responsible for impaired development of preimplantation embryos in vitro. The pathways and strategies to cope with DNA damage in the early embryo are reviewed by Jaroudi and SenGupta (2007).
Unfiltered radiation, temperature extremes and ROS can also induce RNA damage in a cell. This affects the integrity of both mRNA and the translation machinery. Protein synthesis is regulated at all stages: initiation, elongation and termination. In fact, synthesis is reprogrammed during cell stress, primarily through translational silencing at the initiation stage (Yamasaki and Anderson, 2008). This is important in the early embryo prior to genome activation as this reprogramming can conserve anabolism, preserve essential mRNAs and promote the repair of molecular damage.
In recent work, we have shown for the blastocysts of three species; bovine, porcine and human that the profile of amino acid depletion/appearance measured non-invasively, correlates with total DNA damage measured in individual blastomeres using a modified Comet assay; those embryos with the highest DNA damage are the most active metabolically (Sturmey et al., 2008). It is unclear whether the elevated level of DNA damage in metabolically active embryos arises as a result of up-regulated metabolism, or whether the presence of significant levels of DNA damage drives an increase in nutrient turnover to fuel repair processes or initiate apoptosis. It is hoped that the framework provided by the quiet embryo hypothesis will promote the search for answers to these and related questions.
Funding
Original research at SAC, carried out by T.G.M. and colleagues and referred to in this review, was funded by the Scottish Government, the UK Department for Environment, Food and Rural Affairs and the UK Meat and Livestock Commission.
Acknowledgements
H.J.L. acknowledges support from the Leverhulme Trust; R.G.S. from the Department of Health Sciences, University of York.
References
- Bahat A, Tur-Kaspa I, Gakamsky A, Giojalas LC, Breitbart H, Eisenbach M. Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat Med. 2003;9:149–150. doi: 10.1038/nm0203-149. [DOI] [PubMed] [Google Scholar]
- Bahat A, Eisenbach M, Tur-Kaspa I. Periovulatory increase in temperature difference within the rabbit oviduct. Hum Reprod. 2005;20:2118–2121. doi: 10.1093/humrep/dei006. [DOI] [PubMed] [Google Scholar]
- Baumann CG, Morris DG, Sreenan JM, Leese HJ. The quiet embryo hypothesis: molecular characteristics favoring viability. Mol Reprod Dev. 2007;74:1345–1353. doi: 10.1002/mrd.20604. [DOI] [PubMed] [Google Scholar]
- Brison DR. Apoptosis in mammalian preimplantation embryos: regulation by survival factors. Hum Fertil (Camb) 2000;3:36–47. doi: 10.1080/1464727002000198671. [DOI] [PubMed] [Google Scholar]
- Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor α1. Biol Reprod. 1997;56:1088–1096. doi: 10.1095/biolreprod56.5.1088. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Hempstock J, Jauniaux E. Oxygen, early embryonic metabolism and free radical-mediated embryopathies. Reprod Biomed Online. 2003;6:84–96. doi: 10.1016/s1472-6483(10)62060-3. [DOI] [PubMed] [Google Scholar]
- Byrne AT, Southgate J, Brison DR, Leese HJ. Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. J Reprod Fertil. 1999;117:97–105. doi: 10.1530/jrf.0.1170097. [DOI] [PubMed] [Google Scholar]
- Conaghan J, Hardy K, Handyside AH, Winston RM, Leese HJ. Selection criteria for human embryo transfer: a comparison of pyruvate uptake and morphology. J Assist Reprod Genet. 1993;10:21–30. doi: 10.1007/BF01204436. [DOI] [PubMed] [Google Scholar]
- David A, Vilensky A, Nathan H. Temperature changes in different parts of the rabbit oviduct. Preliminary report. Harefuah. 1971;80:180–182. [PubMed] [Google Scholar]
- Einer-Jensen N, Hunter R. Counter-current transfer in reproductive biology. Reproduction. 2005;129:9–18. doi: 10.1530/rep.1.00278. [DOI] [PubMed] [Google Scholar]
- Einer-Jensen N, Hunter RH. Reproductive health in domestic animals. J Fam Plann Reprod Health Care. 2006;32:245–248. doi: 10.1783/147118906778586543. [DOI] [PubMed] [Google Scholar]
- Eisenbach M, Giojalas LC. Sperm guidance in mammals—an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–285. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J, Blendstrup K, Andreasen MP, Byskov AG. Temperature measurements of rabbit antral follicles. J Reprod Fertil. 1980;60:149–155. doi: 10.1530/jrf.0.0600149. [DOI] [PubMed] [Google Scholar]
- Grinsted J, Kjer JJ, Blendstrup K, Pedersen JF. Is low temperature of the follicular fluid prior to ovulation necessary for normal oocyte development? Fertil Steril. 1985;43:34–39. doi: 10.1016/s0015-0282(16)48314-7. [DOI] [PubMed] [Google Scholar]
- Grondahl C, Greve T, Schmidt M, Hunter RH. Bovine preovulatory follicles are cooler than ovarian stroma and deep rectal temperature. Theriogenology. 1996;45:289. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th edn. Oxford: Oxford University Press; 2007. [Google Scholar]
- Hansen PJ. To be or not to be—determinants of embryonic survival following heat shock. Theriogenology. 2007;68(Suppl 1):S40–S48. doi: 10.1016/j.theriogenology.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Hardy K, Hooper MAK, Handyside AH, Rutherford AJ, Winston RML, Leese HJ. Non-invasive measurement of glucose and pyruvate uptake by individual oocytes and preimplantation embryos. Human Reproduction. 1989;4:188–191. doi: 10.1093/oxfordjournals.humrep.a136869. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG. Oxygen-regulated gene expression in bovine blastocysts. Biol Reprod. 2004;71:1108–1119. doi: 10.1095/biolreprod.104.028639. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Thompson JG. Regulation of gene expression in bovine blastocysts in response to oxygen and the iron chelator desferrioxamine. Biol Reprod. 2007;a 77:93–101. doi: 10.1095/biolreprod.106.058826. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Navarrete SA, Kirstein M, Kind KL, Fischer B, Thompson JG. Differential expression of oxygen-regulated genes in bovine blastocysts. Mol Reprod Dev. 2007;b 74:290–299. doi: 10.1002/mrd.20617. [DOI] [PubMed] [Google Scholar]
- Hewitson LC, Leese HJ. Energy metabolism of the trophectoderm and inner cell mass of the mouse blastocyst. J Exp Zool. 1993;267:337–343. doi: 10.1002/jez.1402670310. [DOI] [PubMed] [Google Scholar]
- Hondo E, Stewart CL. Profiling gene expression in growth-arrested mouse embryos in diapause. Genome Biol. 2005;6:202. doi: 10.1186/gb-2004-6-1-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton FD. Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation. 2006;74:11–18. doi: 10.1111/j.1432-0436.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- Hunter RHF, Nichol R. A preovulatory temperature gradient between the isthmus and ampulla of pig oviducts during the phase of sperm storage. J Reprod Fertil. 1986;77:599–606. doi: 10.1530/jrf.0.0770599. [DOI] [PubMed] [Google Scholar]
- Hunter RH, Grondahl C, Greve T, Schmidt M. Graafian follicles are cooler than neighbouring ovarian tissues and deep rectal temperatures. Hum Reprod. 1997;12:95–100. doi: 10.1093/humrep/12.1.95. [DOI] [PubMed] [Google Scholar]
- Hunter RHF, Einer-Jensen N, Greve T. Presence and significance of temperature gradients among different ovarian tissues. Microsc Res Tech. 2006;69:501–507. doi: 10.1002/jemt.20308. [DOI] [PubMed] [Google Scholar]
- Jaroudi S, SenGupta S. DNA repair in mammalian embryos. Mutat Res. 2007;635:53–77. doi: 10.1016/j.mrrev.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Nasr-Esfhani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- Jurisicova A, Acton BM. Deadly decisions: the role of genes regulating programmed cell death in human preimplantation embryo development. Reproduction. 2004;128:281–291. doi: 10.1530/rep.1.00241. [DOI] [PubMed] [Google Scholar]
- Lambert RT, Ashworth CJ, Beattie L, Gebbie FE, Hutchinson JSM, Kyle DJ, Racey PA. Temporal changes in reproductive hormones and conceptus-endometrial interactions during embryonic diapause and reactivation of the blastocyst in European roe deer (Capreolus capreolus) Reproduction. 2001;121:863–871. [PubMed] [Google Scholar]
- Leese HJ. Energy metabolism of the blastocyst and uterus at implantation. In: Yoshinaga K, editor. Blastocyst Implantation. Boston: Adams Publishing Group Ltd; 1989. pp. 39–46. [Google Scholar]
- Leese HJ. Metabolism of the preimplantation mammalian embryo. Oxf Rev Reprod Biol. 1991;13:35–72. [PubMed] [Google Scholar]
- Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–849. doi: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- Leese HJ. What does an embryo need? Hum Fertil (Camb) 2003;6:180–185. doi: 10.1080/1464770312331369463. [DOI] [PubMed] [Google Scholar]
- Leese HJ, Sturmey RG, Baumann CG, McEvoy TG. Embryo viability and metabolism: obeying the quiet rules. Hum Reprod. 2007;22:3047–3050. doi: 10.1093/humrep/dem253. [DOI] [PubMed] [Google Scholar]
- Manser RC, Leese HJ, Houghton FD. Effect of inhibiting nitric oxide production on mouse preimplantation embryo development and metabolism. Biol Reprod. 2004;71:528–533. doi: 10.1095/biolreprod.103.025742. [DOI] [PubMed] [Google Scholar]
- McEvoy TG, Robinson JJ, Aitken RP, Findlay PA, Robertson IS. Dietary excesses of urea influence the viability and metabolism of preimplantation sheep embryos and may affect fetal growth among survivors. Anim Reprod Sci. 1997;47:71–90. doi: 10.1016/s0378-4320(96)01627-2. [DOI] [PubMed] [Google Scholar]
- McEvoy TG, Sinclair KD, Young LE, Wilmut I, Robinson JJ. Large offspring syndrome and other consequences of ruminant embryo culture in vitro: relevance to blastocyst culture in human ART. Hum Fertil (Camb) 2000;3:238–246. doi: 10.1080/1464727002000199061. [DOI] [PubMed] [Google Scholar]
- Moley KH, Chi MM-Y, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol. 1998:E38–E47. doi: 10.1152/ajpendo.1998.275.1.E38. [DOI] [PubMed] [Google Scholar]
- Poulsen HE. Oxidative DNA modifications. Exp Toxicol Pathol. 2005;57(Suppl 1):161–169. doi: 10.1016/j.etp.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Renfree MB. Society for Reproductive Biology Founders’ Lecture 2. Reprod Fertil Dev. 2006;18:721–734. doi: 10.1071/rd06072. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Shaw G. Diapause. Annu Rev Physiol. 2000;62:353–375. doi: 10.1146/annurev.physiol.62.1.353. [DOI] [PubMed] [Google Scholar]
- Riley JK, Carayannopoulos MO, Wyman AH, Chi M, Moley KH. Phosphatidylinositol 3-kinase activity is critical for glucose metabolism and embryo survival in murine blastocysts. J Biol Chem. 2006;281:6010–6019. doi: 10.1074/jbc.M506982200. [DOI] [PubMed] [Google Scholar]
- Robey RB, Hay N. Mitochondrial hexokinases: guardians of the mitochondria. Cell Cycle. 2005;4:654–658. doi: 10.4161/cc.4.5.1678. [DOI] [PubMed] [Google Scholar]
- Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler RE, Renfree MB, Gardner DK. Carbohydrate uptake by quiescent and reactivated mouse blastocysts. J Exp Zool. 1996;276:132–137. doi: 10.1002/(SICI)1097-010X(19961001)276:2<132::AID-JEZ6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Leese HJ. Energy metabolism in pig oocytes and early embryos. Reproduction. 2003;126:197–204. doi: 10.1530/rep.0.1260197. [DOI] [PubMed] [Google Scholar]
- Sturmey RG, Hawkhead JA, Barker EA, Leese HJ. DNA damage and metabolic activity in the preimplantation embryo. Hum Reprod. 2008 doi: 10.1093/humrep/den346. doi:10.1093/humrep/den346. [DOI] [PubMed] [Google Scholar]
- Turner K, Goldstein DJ, Rogers AW. Variation in the dry mass of mouse embryos throughout the preimplantation period. Hum Reprod. 1992;7:112–116. doi: 10.1093/oxfordjournals.humrep.a137541. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15:2621–2633. doi: 10.1093/humrep/15.12.2621. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Anderson P. Reprogramming mRNA translation during stress. Curr Opinion Cell Biol. 2008;20:222–226. doi: 10.1016/j.ceb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Coleman J, Hunter MG, Craigon J, Campbell KH, Luck MR. Physiological temperature variants and culture media modify meiotic progression and developmental potential of pig oocytes in vitro. Reproduction. 2007;133:877–886. doi: 10.1530/REP-06-0318. [DOI] [PubMed] [Google Scholar]