Abstract
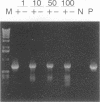
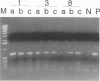
A reverse transcription-PCR (RT-PCR) assay was developed and compared with enzyme immunoassay (EIA) and virus isolation for detecting LaCrosse virus (LAC) in mosquito pools. All three techniques were able to detect a single LAC-infected mosquito in a pool of 99 negative mosquitoes. Virus isolation was the most sensitive of the three techniques; it was possible to isolate virus immediately following intrathoracic inoculation of mosquitoes. RT-PCR was second in sensitivity; LAC RNA was detected 1 day postinfection. EIA detected LAC antigen 2 days postinfection. Additionally, RT-PCR and EIA were able to detect LAC RNA and protein, respectively, from mosquito samples which were subjected to seven freeze-thaw cycles, and RT-PCR was able to detect LAC RNA from mosquito samples which remained at room temperature for up to 7 days.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akashi H., Bishop D. H. Comparison of the sequences and coding of La Crosse and snowshoe hare bunyavirus S RNA species. J Virol. 1983 Mar;45(3):1155–1158. doi: 10.1128/jvi.45.3.1155-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty B. J., Thompson W. H. Emergence of La Crosse virus from endemic foci. Fluorescent antibody studies of overwintered Aedes triseriatus. Am J Trop Med Hyg. 1975 Jul;24(4):685–691. doi: 10.4269/ajtmh.1975.24.685. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dykers T. I., Brown K. L., Gundersen C. B., Beaty B. J. Rapid diagnosis of LaCrosse encephalitis: detection of specific immunoglobulin M in cerebrospinal fluid. J Clin Microbiol. 1985 Nov;22(5):740–744. doi: 10.1128/jcm.22.5.740-744.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F., Shope R. E., Calisher C. E., Nathanson N. Characterization of monoclonal antibodies against the G1 and N proteins of LaCrosse and Tahyna, two California serogroup bunyaviruses. Virology. 1982 Jul 15;120(1):42–53. doi: 10.1016/0042-6822(82)90005-8. [DOI] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J. Detection of eastern equine encephalomyelitis virus and Highlands J virus antigens within mosquito pools by enzyme immunoassay (EIA). I. A laboratory study. Am J Trop Med Hyg. 1984 Sep;33(5):965–972. doi: 10.4269/ajtmh.1984.33.965. [DOI] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J. Economic comparison of enzyme immunoassay and virus isolation procedures for surveillance of arboviruses in mosquito populations. J Clin Microbiol. 1987 Jun;25(6):976–981. doi: 10.1128/jcm.25.6.976-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J., Maxfield H. K., Gilfillan R. F., Rosenau B. J. Detection of eastern equine encephalomyelitis virus and Highlands J virus antigens within mosquito pools by enzyme immunoassay (EIA). II. Retrospective field test of the EIA. Am J Trop Med Hyg. 1984 Sep;33(5):973–980. doi: 10.4269/ajtmh.1984.33.973. [DOI] [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J., Meegan J. M., Frazier C. L., Shope R. E. Detection of La Crosse arbovirus antigen in mosquito pools: application of chromogenic and fluorogenic enzyme immunoassay systems. J Clin Microbiol. 1982 May;15(5):879–884. doi: 10.1128/jcm.15.5.879-884.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnback T. L., Beaty B. J., Hildreth S. W., Brown K. L., Gundersen C. B. Capture immunoglobulin M system for rapid diagnosis of La Crosse (California encephalitis) virus infections. J Clin Microbiol. 1982 Sep;16(3):577–580. doi: 10.1128/jcm.16.3.577-580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti R. S., Calisher C. H., Gubler D. J., Chang G. J., Vorndam A. V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992 Mar;30(3):545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklasson B. S., Gargan T. P., 2nd Enzyme-linked immunosorbent assay for detection of Rift Valley fever virus antigen in mosquitoes. Am J Trop Med Hyg. 1985 Mar;34(2):400–405. doi: 10.4269/ajtmh.1985.34.400. [DOI] [PubMed] [Google Scholar]
- Olson K. E., Blair C. D., Beaty B. J. Detection of dengue viral RNA in mosquito vectors by mixed phase and solution hybridization. Mol Cell Probes. 1990 Aug;4(4):307–320. doi: 10.1016/0890-8508(90)90022-r. [DOI] [PubMed] [Google Scholar]
- THOMPSON W. H., KALFAYAN B., ANSLOW R. O. ISOLATION OF CALIFORNIA ENCEPHALITIS GROUP VIRUS FROM A FATAL HUMAN ILLNESS. Am J Epidemiol. 1965 Mar;81:245–253. doi: 10.1093/oxfordjournals.aje.a120512. [DOI] [PubMed] [Google Scholar]
- Tsai T. F., Bolin R. A., Montoya M., Bailey R. E., Francy D. B., Jozan M., Roehrig J. T. Detection of St. Louis encephalitis virus antigen in mosquitoes by capture enzyme immunoassay. J Clin Microbiol. 1987 Feb;25(2):370–376. doi: 10.1128/jcm.25.2.370-376.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]