Abstract
There is increasing evidence supporting an oral chemosensory detection system for free fatty acids (FFA). The presumptive transduction mechanisms have different ligand specificities. Psychophysical studies with FFA varying in chain length and saturation may aid in identifying the presence and functionality of these mechanisms in humans. Oral detection thresholds were measured for linoleic, stearic, lauric, and caproic acids in 32 healthy adults by an ascending, 3-alternative, forced-choice, sip and spit procedure. Thresholds were obtained for all fatty acids from all participants, but the distributions were wide and nonnormal. Thresholds were not correlated between fatty acids nor with thresholds for sucrose (taste), butanol (olfactory), mineral oil, or gum acacia (both somatosensory). These data demonstrate human oral sensitivity to short-, medium-, and long-chain FFA and suggest the presence of multiple transduction mechanisms. The findings are consistent with, but do not definitively demonstrate, a role for taste that may have a genetic basis.
Keywords: BMI, chemosensory, fat, gustatory, PROP, taste, taste threshold, taste transduction
Introduction
A number of candidate oral fatty acid “taste” transduction mechanisms have been identified through animal electrophysiological and behavioral studies. Each has unique ligand specificity, but collectively, the putative mechanisms are responsive to fatty acids varying in saturation and chain length. Delayed rectifying potassium channels (DRK) are blocked by cis long-chain unsaturated fatty acids leading to taste cell depolarization (Gilbertson et al. 1997; Hansen and Gilbertson 2005). Cluster of differentiation-36 (CD36) is a scavenger cell surface membrane receptor protein that binds not only long-chain unsaturated fatty acids but also saturated fatty acids. Its mode of action has not been established (e.g., transporter and docking protein), but compared with DRK, its presence extends the range of fatty acids that may be detected (Laugerette et al. 2005). G-protein–coupled receptors (GPCR) are a class of 7 transmembrane domain proteins in a large family of membrane proteins that bind extracellular compounds and then activate intracellular signaling systems. Several have been implicated in taste. GPCR120 further broadens the array of fatty acids that may be effective taste stimuli as it binds the upper ranges of medium-, as well as long-chain saturated and unsaturated fatty acids (Hirasawa et al. 2005). Expanding the types of effective stimuli to shorter medium-chain fatty acids is GPCR40, which binds these species, as well as long-chain unsaturated and saturated fatty acids (Itoh et al. 2003). Finally, GPCR41 and GPCR43 are reported receptors for short-chain fatty acids (Brown et al. 2003; LePoul et al. 2003; Xiong et al. 2004). Thus, current data suggest that if detection of FFA is receptor mediated and involves the proteins just listed, detection of short- and long-chain FFA would require the presence of at least 2 different transduction systems. However, it still has not been established that these proteins are actually taste receptors and are functional in humans. One important component of the evidence base needed to clarify the issue is data documenting human taste responses to fatty acids varying in structure.
Psychophysical studies of human oral fatty acid detection are limited. Whereas each of the above putative transduction mechanisms requires a FFA as a ligand, rather than triacylglycerol, one early trial measured detection thresholds for oil-in-water emulsions of bleached, deodorized soybean oil, medium-chain triglyceride (MCT) oil, and light mineral oil (Schiffman et al. 1998). Thresholds were measurable in the 12 young (5.3% v/v) and 12 elderly (15.8% v/v) participants and did not differ significantly when measured with and without nose clips, suggesting that olfaction was not the basis for detection. Threshold concentrations were higher for MCT than the soybean oil, and the choice of emulsifier led to 2- to 3-fold differences. Whether the basis of detection was taste or tactile cues cannot be determined nor can the effective stimulus. The intended stimuli were esterified fats, but some nonesterified fatty acids were likely present (Weiss 1970; Smith et al. 1986; Chow 1992; Metzger et al. 1995; Ministry of Agriculture, Fisheries and Food 1998; Wan et al. 1998; Bertran et al. 1999; Che Man et al. 1999) and not measured. A subsequent study determined detection thresholds for pure, 18-carbon FFA varying in saturation: linoleic (C18:2), oleic (C18:1), and stearic (C18:0) (Chale-Rush et al. 2007a, 2007b). The FFA were administered in a mixture with gum acacia and mineral oil to minimize textural cues the FFA may have imparted, and participants were tested under red light (to reduce visual cues), wearing nose clips (to eliminate olfactory cues), and having undergone oral desensitization with capsaicin (to further reduce tactile cues). Thresholds were obtained in nearly all the 22 participants for each FFA. These thresholds did not correlate with those based on olfaction or nasal irritancy (Chale-Rush et al. 2007b). Nevertheless, because tactile cues were not definitively eliminated as a basis for detection, the data provide only presumptive evidence for human FFA taste sensitivity. Evidence for human oral sensitivity to FFA of medium- and short-chain length is lacking. Given the evidence for transduction mechanisms for these species, the aim of the present study was to determine oral detection thresholds for a short-, medium-, and long-chain FFA holding saturation constant. If detection of these compounds is possible after controlling contributions from nongustatory cues, it would suggest that humans have more than one of the reported transduction mechanisms, a mechanism not currently identified, or a nonspecific mechanism such as diffusion of FFA across taste cell membranes. With respect to the latter, diffusion of sweet and bitter substances across taste cell membranes with activation of intracellular signaling systems has been demonstrated (DeSimone 2000; Peri et al. 2000; Zubare-Samuelov et al. 2005), but not explored with FFA.
Materials and methods
Participants
Thirty-two men (N = 11) and women (N = 21) were recruited through public advertisements. Eligibility criteria included the following: 18–60 years of age, healthy, nonsmoker, normal taste, smell, and somatosensory function.
General protocol
Testing sessions
Eligibility status and baseline data on butanol olfactory, sucrose taste, mineral oil (lubricity), and gum acacia (viscosity) somatosensory thresholds were collected during a screening session. Recruited participants were then tested during two 60-min sessions per week for 4 weeks. Four FFA stimuli were assessed under 2 conditions (normal and capsaicin desensitized). Participants refrained from orosensory exposure to food, beverages, and oral care products for at least 2 h prior to testing.
Sensory stimuli
The FFA stimuli included linoleic acid, stearic acid, lauric acid, and caproic acid. The fatty acids were homogenized in deionized water containing 0.01% ethylenediaminetetraacetic acid, 5% w/w gum acacia, and 5% w/w mineral oil. Solutions were made daily and stored in tightly sealed, light protected bottles. Concentrations ranged from 0.00028% to 5% w/w with dilutions differing by 0.25 log units. Because stearic and lauric acids are solids at room temperature, they were presented at 67–69 °C. All other samples were presented at room temperature. Participants completed testing for a single condition for one fatty acid per session.
For olfactory testing, butanol concentrations in deionized water ranged from 0.0006% to 4.0% (v/v), with dilutions differing by a factor of 3. For sucrose taste assessment, concentrations ranged from 0.0001 to 1.0 M, with dilutions of 0.25 log units. Somatosensory stimuli included mineral oil and gum acacia concentrations ranging from 0.00028% to 5% w/w for both compounds with dilutions differing by 0.25 log units.
Sensory testing
Olfactory function was assessed using an ascending, 2-alternative, forced-choice procedure for butanol. Stimuli were sniffed from 250-mL plastic bottles containing 20 mL of sample at room temperature. Threshold sensitivity was estimated as the lowest concentration of a series where 5 successive correct choices were made. Taste thresholds for sucrose and somatosensory thresholds for mineral oil and gum acacia were assessed using an ascending, 3-alternative, forced-choice, sip and spit procedure. Stimuli were presented in 5-mL portions at room temperature following a rinse with deionized water. The participant's nares were closed during all trials. A correct identification was followed by presentation of the same concentration again. An incorrect identification was followed by presentation of the next higher concentration. Threshold sensitivity was defined as the lowest concentration correctly identified 3 consecutive times. Detection thresholds were obtained for all 4 fatty acids using the same procedures as were used for sucrose, except that testing was conducted with nares closed and under red light to minimize any olfactory and visual cues. They were also measured with and without prior oral desensitization with capsaicin. To desensitize participants, they were instructed to hold 20 mL samples of 20-ppm concentrations of capsaicin in their mouth for 30 s 5 times with a 60-s interval between each. They rated each rinse for perceived “hotness” on a visual analog scale with end anchors of “not at all hot” and “extremely hot.” After the fifth rinse, participants thoroughly rinsed their mouth with deionized water and waited 15 min. This was followed by a sixth capsaicin rinse to document the degree of desensitization that had occurred. FFA thresholds were then determined. The project was approved by the Purdue University Institutional Review Board.
Statistical analyses
The Kolmogorov–Smirnov test was used to explore data distribution normality. Because of a lack of normality, fatty acid threshold values were analyzed by nonparametric analysis of variance (i.e., Friedman test). Correlations between thresholds were assessed by Kendall's tau_b. The criterion for statistical significance was P < 0.05, 2 tailed. Testing was conducted with version 15.0 for Windows of SPSS statistical software.
Results
Participants included 11 male and 21 female adults (mean ± standard error = 25.8 ± 1.2 years of age). Their mean body mass index was 25.48 ± 0.86 kg/m2.
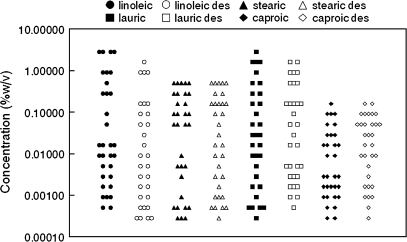
Two-thirds of participants reported ≥50% reductions in the intensity of the capsaicin rinse for the sixth exposure (indicative of desensitization), just prior to fatty acid sampling. Assessment of all participants or just those experiencing this degree of desensitization had minimal impact on the findings, so all participants were retained in the final analyses. There were wide ranges for each of the FFA thresholds; generally covering about 3–4 orders of magnitude (Figure 1). All distributions significantly deviated from normality except for caproic acid thresholds prior to desensitization.
Figure 1.
Distributions of fatty acid detection threshold values (N = 32). “des” = Capsaicin desensitized prior to threshold determination.
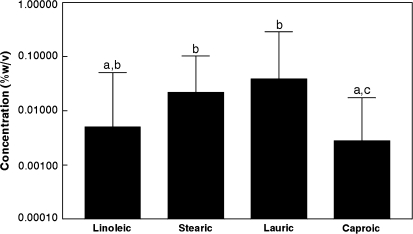
Detection thresholds for the FFA with nares closed, visual cues minimized, and following capsaicin desensitization are presented in Figure 2. Because of the nonnormal distributions, data are presented as medians with semi-interquartile ranges ([75th–25th%]/2) as the index of variance. The threshold for caproic acid was significantly lower than the thresholds for stearic acid (P = 0.023) and lauric acid (P = 0.003). The threshold for linoleic acid tended to be lower than the threshold for lauric acid (P = 0.094).
Figure 2.
Median fatty acid detection thresholds (N = 32).
Correlations between the fatty acids were not significant. The coefficients were as follows: 0.09 (linoleic–stearic), 0.09 (linoleic–lauric), −0.19 (linoleic–caproic), −0.01 (stearic–lauric), −0.004 (stearic–caproic), and −0.17 (lauric–caproic) (all P values >0.1). The FFA thresholds also were not significantly correlated with thresholds for sucrose, butanol, mineral oil, or acacia (all correlation coefficients ranged between −0.3 and 0.3).
Discussion
Only recently have FFA been recognized as important cell signaling molecules. They modulate a wide array of physiological processes (Milligan et al. 2006), in part, through binding to GPCR such as GPCR40/41/43/120 (Briscoe et al. 2003; Brown et al. 2003; Itoh et al. 2003; LePoul et al. 2003; Hirasawa et al. 2005). In the gastrointestinal tract, GPCR reportedly provide a nutrient-sensing role (Dyer et al. 2005), and fatty acid binding to GPCR leads to release of gut peptides such as Cholecystokinin (Tanaka et al. 2008), peptide YY (Aponte et al. 1985), and glucagon-like peptide-1 (Hirasawa et al. 2005) that regulate digestive processes and appetite (Woods 2005). These GPCR have also been identified in gustatory tissue of animal models (Hansen et al. 2006; Damak et al. 2007; Matsumura et al. 2007) and contribute to transduction of sweet, bitter, and umami tastes (Hoon et al. 1999; Nelson et al. 2001, 2002). Thus, on this basis alone, it may be posited that FFA also serve a signaling role in the oral cavity, as taste stimuli. However, signaling mechanisms not involving GPCR, that is, blockage of DRK (Gilbertson et al. 1997), and binding to CD36 (Fukuwatari et al. 1997; Laugerette et al. 2005) have also been proposed.
The ligand specificity of each of the putative oral FFA receptors differs for chain length and degree of saturation. If the currently proposed FFA receptors are present and functional in humans, it may be predicted that humans should be capable of detecting a wide array of FFA without input from other sensory systems. In one trial attempting to isolate a taste component for FFA by masking textural cues and testing participants with nares closed and under red light, detection thresholds were obtained for linoleic, oleic, and stearic acids. These three 18-carbon fatty acids vary only in saturation. The present study used the same testing approach and now provides evidence for the human ability to detect FFA varying in chain length but with common saturation. This is consistent with evidence for multiple FFA taste transduction mechanisms and, more specifically, for GPCR40, 41, and/or 43 because they reportedly bind short- and medium-chain FFA. Contributions from different mechanisms may account for the weak correlations between FFA thresholds. The lack of correlation between thresholds for FFA and butanol, mineral oil, and acacia indicate detection was not based on these stimuli components. The similarity of thresholds for each FFA with and without prior capsaicin desensitization suggests that irritancy was not an important determinant of detection.
Alternatively, it is possible that an inadequately controlled somatosensory cue contributed to the detection of these fatty acids or that their transduction is not receptor mediated. As amphipatic molecules, FFA may cross cell membranes by diffusion and directly, or though a metabolite (Sidhu et al. 2000; Prentki et al. 2002), activate intracellular signaling pathways as demonstrated for selected sweet and bitter compounds (DeSimone 2000; Peri et al. 2000; Zubare-Samuelov et al. 2005). Although questions have been raised about the time course for this mechanism, in lipid bilayers and nongustatory tissue, millimolar concentrations of FFA may migrate across membranes in milliseconds (Hamilton and Kamp 1999), so it can be a rapid process. Indeed, permeability coefficients for octanoic (C8) and capric (C10) acids are more than a 1000-fold higher than water (Kamp and Hamilton 2006).
Although the sample size of this study was limited, the threshold distributions were extremely broad, bridging 3–4 orders of magnitude. This may be attributable to the use of participants who were inexperienced with the sensory testing regimen and rating of unfamiliar stimuli. However, the distributions also deviated significantly from normality. If the thresholds are stable, this suggests the possibility of inherent differences in FFA sensitivity. The existence of fatty acid tasters and nontasters has been proposed (Nasser et al. 2001; Kamphuis et al. 2003), but not confirmed. The earlier trials did not control for olfactory and all forms of tactile input so attribution of responses to taste is not possible. Olfactory cues were controlled here, but the thresholds still cannot be definitively classified as taste because capsaicin desensitization does not eliminate all trigeminal input and there may be other sensory cues from fats (e.g., mouth-coating and flavor partitioning) that were not controlled but aid detection. Further, FFA may directly stimulate trigeminal fibers (Gilbertson et al. 2004). Nevertheless, coupled with evidence for genetic contributions to intestinal lipid processing (e.g., triacylglycerol assembly and cholesterol absorption, Chen and Davidson 2006), the present findings provide a basis for exploring a genetic contribution to FFA detection.
Interpretation of the absolute detection threshold concentrations must be made cautiously. Detection thresholds are only probabilistic measures strongly influenced by the vehicle in which the stimulus is delivered. The FFA used in this study were added to a mixture of compounds designed to mask nongustatory cues the FFA may have provided to allow isolation of a likely taste mechanism. The aim was to document the ability to detect the FFA rather than their contribution to the flavor of any particular food. Further, the comparison of thresholds for the different FFA requires recognition that the stearic and lauric acids were presented in a warm solution to keep them liquid, whereas the caproic and linoleic acids were served at room temperature. The results did not reveal a simple detection discrepancy based on temperature, for example, the two 18-carbon FFA (linoleic and stearic) thresholds did not differ even though they were sampled at different temperatures. Still, the temperature of the stimuli may have altered the absolute threshold concentrations.
The physiological relevance of fat taste has been questioned based on evidence of little or no lingual lipase in humans (Spielman et al. 1993; Schiffman et al. 1998) and the presumption that this would be required to generate a FFA signal in the oral cavity. However, although dietary fats are predominantly in the form of triglycerides, they also contain FFA in concentrations ranging up to 1–3% (Weiss 1970; Smith et al. 1986; Chow 1992; Metzger et al. 1995; Ministry of Agriculture, Fisheries and Food 1998; Wan et al. 1998; Bertran et al. 1999; Che Man et al. 1999). Thus, foods containing fats have adequate signal concentrations without the need for oral triacylglycerol hydrolysis.
In summary, the present data document the ability of humans to detect short-, medium-, and long-chain saturated fatty acids. This may be mediated by an uncharacterized somatosensory cue, nonspecific transport followed by activation of intracellular signaling pathways, or by receptor-mediated transduction. If the latter holds, these data call for the presence of multiple receptors and/or receptors capable of binding an array of FFA that currently exceeds the properties of any one of those currently proposed. Further, the data document the presence of marked individual variability in FFA sensitivity that raises the question about a genetic basis for these findings.
Funding
National Institutes of Health (PHS R01 DK45294).
References
- Aponte GW, Fink AS, Meyer JH, Tatemoto K, Taylor IL. Regional distribution of release of peptide YY with fatty acids of different chain length. Am J Physiol. 1985;249:G745–G750. doi: 10.1152/ajpgi.1985.249.6.G745. [DOI] [PubMed] [Google Scholar]
- Bertran E, Blanco M, Doello J, Iturriaga H, Maspoch S, Montoliu I. Determination of olive oil free fatty acid by fourier transform infrared spectroscopy. J Am Oil Chem Soc. 1999;76:611–616. [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Teheang L, Daniels D, Muir AI, Wigglesworth IK, Fraser NJ, Pike NB, et al. The orphan G protein-coupled receptors GPR 41 and GPR 43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Chale-Rush A, Burgess JR, Mattes RD. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem Senses. 2007a;32:423–431. doi: 10.1093/chemse/bjm007. [DOI] [PubMed] [Google Scholar]
- Chale-Rush A, Burgess JR, Mattes RD. Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol. 2007b;292:G1206–G1212. doi: 10.1152/ajpgi.00471.2006. [DOI] [PubMed] [Google Scholar]
- Che Man YB, Moh MH, van de Voort FR. Determination of free fatty acids in crude palm oil and refined-bleached-deodorized palm olein using fourier transform infrared spectroscopy. J Am Oil Chem Soc. 1999;76:485–490. [Google Scholar]
- Chen Z, Davidson NO. Genetic regulation of intestinal lipid transport and metabolism. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 4th ed. Boston: Academic Press; 2006. pp. 1711–1734. [Google Scholar]
- Chow CK. Fatty acids in foods and their health implications. New York: Marcel Dekker, Inc; 1992. [Google Scholar]
- Damak S, Le-Coutre J, Bezencon C, Cartoni C. International Application Published under the Patent Cooperation Treaty. 2007 Feb 8. Fat taste receptors and their methods of use. WO2007/014824 A1. [Google Scholar]
- DeSimone JA. Focus on “rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction”. Am J Physiol Cell Physiol. 2000;278:C13–C16. doi: 10.1152/ajpcell.2000.278.1.C13. [DOI] [PubMed] [Google Scholar]
- Dyer J, Salmon KSH, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–305. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–464. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Klein JT, Farmer-George M, Simon SA. Sarasota, FL: Presented at the Association for Chemoreception Sciences XXVIth Annual Meeting; 2004 Apr. Free fatty acids inhibit delayed rectifying K channels in isolated trigeminal neurons [abstract] [Google Scholar]
- Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 1999;48:2255–2269. doi: 10.2337/diabetes.48.12.2255. [DOI] [PubMed] [Google Scholar]
- Hansen DR, Gilbertson TA. Expression of delayed rectifying K channels in taste cells from obesity-prone and -resistant rats. Chem Senses. 2005;30:A51. [Google Scholar]
- Hansen DR, McKenna L, Shah BP, Gilbertson TA. Sarasota, FL: ACHEMS Annual Meeting; 2006 Apr 26–30. Expression of fatty acid-activated G protein coupled receptors in chemosensory cells [abstract] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretion glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba JN, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogl K, Hosoya M, Tanaka Y, Uejima H, et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature. 2003;122:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids. 2006;75:149–159. doi: 10.1016/j.plefa.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kamphuis MMJW, Saris WHM, Westerterp-Plantenga MS. The effect of addition of linoleic acid on food intake regulation in linoleic acid tasters and linoleic acid non-tasters. Br J Nutr. 2003;90:199–206. doi: 10.1079/bjn2003858. [DOI] [PubMed] [Google Scholar]
- Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur J-P, Besnard P. CD 36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePoul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, VanDamme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Mizushige T, Yoneda T, Iwanaga T, Tsuzuki S, Inoue K, Fushiki T. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. doi: 10.2220/biomedres.28.49. [DOI] [PubMed] [Google Scholar]
- Metzger K, Angres G, Maier H, Lehmann WD. Lipoxygenase products in human saliva: patients with oral cancer compared to controls. Free Radic Biol Med. 1995;18:185–194. doi: 10.1016/0891-5849(94)00108-v. [DOI] [PubMed] [Google Scholar]
- Milligan G, Stoddard LA, Brown AJ. G protein-coupled receptors for free fatty acids. Cell Signal. 2006;18:1360–1365. doi: 10.1016/j.cellsig.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture, Fisheries and Food. 1998. Fatty acids, supplement to McCance and Widdowson's the composition of foods. Cambridge (UK): The Royal Society of Chemistry; [Google Scholar]
- Nasser JA, Kissileff HR, Boozer CN, Chou CJ, Pi-Sunyer FX. PROP taster status and oral fatty acid perception. Eat Behav. 2001;2:237–245. doi: 10.1016/s1471-0153(01)00031-9. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Peri I, Mamrud-Brains H, Rodin S, Krizhanovsky V, Shai Y, Nir S, Naim M. Rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction. Am J Physiol Cell Psysiol. 2000;278:C17–C25. doi: 10.1152/ajpcell.2000.278.1.C17. [DOI] [PubMed] [Google Scholar]
- Prentki M, El-Assad W, Joly E, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002;51(3):S405–S413. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG, Sattely-Miller EA, Warwick ZS. Orosensory perception of dietary fat. Curr Dir Psychol Sci. 1998;7(5):137–143. [Google Scholar]
- Sidhu SS, Thompson DG, Warhurst G, Case RM, Benson RSP. Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in enteroendocrine cell lines, STC-1 and GLUTag. J Physiol. 2000;528:165–176. doi: 10.1111/j.1469-7793.2000.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Clifford AJ, Hamblin CL, Creveling TK. Changes in physical and chemical properties of shortenings used for commercial deep fat frying. J Am Oil Chem Soc. 1986;63:1017–1023. [Google Scholar]
- Spielman S, D'Abundo RB, Field RB, Schmale H. Protein analysis of human von Ebner saliva and a method for its collection from the foliate papillae. J Dent Res. 1993;72(9):1331–1335. doi: 10.1177/00220345930720091301. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Katsuma S, Adachi T, Koshimizu T, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- Wan PJ, Pakarinen DR, Wakelyn PJ. Concerns for the determination of free fatty acids in cottonseed. J Am Oil Chem Soc. 1998;75:1321–1324. [Google Scholar]
- Weiss TJ. Food oils and their uses. AVI Publishing; 1970. pp. 22–23. [Google Scholar]
- Woods SC. Signals that influence food intake and body weight. Physiol Behav. 2005;86:709–716. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubare-Samuelov M, Shaul ME, Peri I, Aliluiko A, Trosh O, Naim M. Inhibition of signal termination-related kinases by membrane-permeant bitter and sweet tastants: potential role in taste signal termination. Am J Physiol Cell Physiol. 2005;289:C483–C492. doi: 10.1152/ajpcell.00547.2004. [DOI] [PubMed] [Google Scholar]