Abstract
Objectives. Lactoferrin is an iron-binding protein that is released from activated neutrophils at sites of inflammation and has anti-microbial as well as anti-inflammatory properties. This study set out to determine whether lactoferrin can delay neutrophil apoptosis and could act as a survival factor for neutrophils in SF.
Methods. Human peripheral blood and SF neutrophils were incubated with iron-free lactoferrin and apoptosis determined after 9 h. SF from patients with RA was added to isolated neutrophils, with or without immunodepletion of lactoferrin, and effects on neutrophil apoptosis determined. Levels of lactoferrin in SF were assessed and related to disease duration and markers of disease activity.
Results. Iron-free lactoferrin significantly delayed apoptosis of peripheral blood neutrophils, in a concentration-dependent manner after 9 h in culture (P < 0.04). Lactoferrin could also delay apoptosis of neutrophils isolated from SF of patients with RA. SF from patients with established RA delayed apoptosis of peripheral blood neutrophils and this effect was significantly reduced by depletion of lactoferrin (P < 0.03). Lactoferrin levels in SF from patients with established RA did not correlate with disease severity, but did correlate with markers of inflammation (CRP) and with the presence of RF. SF from patients with arthritis of <12 weeks duration did not contain significant levels of lactoferrin.
Conclusion. Lactoferrin contributes to extended neutrophil survival in the rheumatoid joint in the established phase of RA but not in very early arthritis.
Keywords: Lactoferrin, Neutrophil, Apoptosis, Rheumatoid arthritis
Introduction
Neutrophils are the most abundant yet the most short-lived of leucocytes, surviving for 24–48 h in the circulation before dying by apoptosis [1, 2]. Neutrophil apoptosis can be delayed at sites of infection in order to maximize neutrophil bactericidal function [3], but inappropriate extension of neutrophil survival can lead to chronic inflammation and pathology [4]. Tight regulation of neutrophil apoptosis is thus required to prevent chronic inflammatory diseases such as RA. We have shown previously that neutrophil apoptosis is suppressed in the SF of patients with established RA [5] and more recently also in those patients with disease of <3 months duration [6], contributing significantly to the persistence of inflammation. Several synovial components have already been identified as neutrophil survival factors, including type 1 IFN, GM-CSF and immune complexes [7–10]. The hypoxic environment of the synovium has also been proposed as a factor promoting neutrophil survival [11]. Identification of key microenvironmental influences on neutrophil life span within the inflamed joint may suggest further targets for therapy.
We and others have previously reported the importance of reactive oxygen species as mediators of neutrophil spontaneous apoptosis [12–14]. Neutrophils from patients with chronic granulomatous disease, a hereditary defect in reactive oxygen intermediate (ROI) production, show significant inhibition of apoptosis [12]. Mice that are hypomorphic for expression of the p47phox subunit of NADPH oxidase and show a minimal neutrophil superoxide burst are highly sensitive to pristane-induced arthritis [15]. Here we have investigated the role of the iron-binding protein lactoferrin in regulating neutrophil apoptosis in the synovial environment. Several iron chelators, including desferrioxamine (DFO) have been shown to delay neutrophil spontaneous apoptosis in vitro [13, 14, 16] and the physiological iron chelator lactoferrin is often present at significant levels in SF [17–19].
Lactoferrin is an iron-binding glycoprotein belonging to the transferrin family and is present in secondary granules in neutrophils and milk and is released upon neutrophil activation [20–22]. The concentration of lactoferrin in blood is normally low, i.e. 0.2–0.6 μg/ml, but at sites of inflammation this is significantly raised due to its release from neutrophils and can be as high as 200 μg/ml [23]. The effect of increased lactoferrin in the synovium has been thought to be primarily anti-inflammatory. Iron accumulates within the RA synovium and synovial macrophages release hydrogen peroxide and superoxide, which can then be converted to damaging hydroxyl radicals by free iron via the Fenton/Haber-Weiss reaction. Iron chelation by lactoferrin will thus remove a source of damaging ROI and indeed iron chelators, including lactoferrin, have been shown to reduce inflammation in animal models of inflammation, particularly septic arthritis [24] and in patients with acute inflammation [25]. However, if synovial lactoferrin also delays neutrophil apoptosis, it may have both pro-inflammatory and anti-inflammatory effects within the rheumatoid joint. Lactoferrin has been reported to have pro-inflammatory effects by enhancing neutrophil adhesion [26].
Although an increased level of lactoferrin in SF of patients with RA has been reported [17], no study has ever looked at its role in regulating neutrophil apoptosis, though several studies have shown that it has an anti-oxidant effect on these cells [27, 28]. As lactoferrin has been proposed as a possible therapy for RA [24], we felt it important to determine if it could delay neutrophil apoptosis. Our data show that lactoferrin was able to extend neutrophil life-span and that depletion of lactoferrin from SF reduced its ability to delay neutrophil apoptosis. Our results suggest that lactoferrin may not be a useful anti-inflammatory in all patients and perhaps its utility will be limited to those with excessive accumulation of iron within the joint or with septic arthritis in which extending neutrophil lifespan and function would be beneficial.
Methods
Isolation and culture of human neutrophils
Neutrophils were isolated from the peripheral blood of healthy volunteers as previously described [29] and all subjects gave their written informed consent. Briefly, blood was collected into tubes containing EDTA and erythrocytes sedimented with 2% dextran T-500 in normal saline. Neutrophils were isolated using a discontinuous density gradient of Percoll (Sigma-Aldrich, Poole, UK). After hypotonic lysis to eliminate any remaining erythrocytes, neutrophils were resuspended in a round-bottom polypropylene tube in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% heat-inactivated fetal bovine serum (Sera Laboratories International, Bolney, UK) and containing 2 mM glutamine, 100 U/ml penicillin and 100 ug/ml streptomycin (Sigma-Aldrich). The purity of isolated neutrophils was determined by Giemsa staining (Diff-Quick, Gamidor Technical Services, Didcot, UK), and light microscopy and was routinely >97%, with eosinophils as the main contaminating cell. For isolation of neutrophils from SF, the synovial sample was treated with 10 U/ml hyaluronidase for 20 min to reduce viscosity and then applied directly to the Percoll density gradient. Purity was routinely >98%.
Neutrophils were cultured in a humidified atmosphere under routine tissue culture conditions (5% CO2; ∼20% pO2), in the presence or absence of a range of concentrations of iron-free lactoferrin isolated from human milk (tissue culture grade, Sigma-Aldrich). Lactoferrin interferes with routine assays for endotoxins and for this reason all lactoferrin treatments also included 10 μg/ml polymyxin B (Sigma-Aldrich) to neutralize lipopolysaccharide (LPS) and ensure that any effects were not due to endotoxin contamination [30]. Neutrophils were also cultured with other factors known to delay their apoptosis, namely recombinant human GM-CSF (50 ng/ml) and IFN-β (800 IU/ml), both purchased from R&D Systems, Abingdon, UK, and LPS (Sigma-Aldrich).
SF
SF was collected from the joints of patients with established RA of >2 yrs duration, or from patients attending a very early arthritis clinic whose arthritis was of <3 months duration. These latter patients were followed to determine which ones went on to develop RA. All patients gave their written informed consent to their involvement in the study and the study was approved by South Birmingham Local Research Ethics Committee (Study Reference number LREC 04/Q2709/103). For immunodepletion of lactoferrin, SF was incubated with an anti-lactoferrin monoclonal antibody (Sigma-Aldrich) or an isotype matched control and the antibody removed using protein G linked to magnetic beads (µ beads, Miltenyi Biotec, Surrey, UK). The efficiency of depletion was confirmed by ELISA and any remaining lactoferrin was below the detection limit of the kit at 1 ng/ml.
Measurement of neutrophil apoptosis
To determine the effect of various treatments on spontaneous neutrophil apoptosis cytospin preparations (5 min, 300 rpm; Cytospin 2; Shandon, Pittsburgh, PA, USA) were made and differentially stained using a Giemsa stain and assessed for the classic apoptotic morphology of condensed nucleus, reduced cytoplasm and vacuolation [31]. Morphological assessments were confirmed by measurement of mitochondrial permeability transition using DiOC6 (Molecular Probes Inc., Leiden, The Netherlands) staining and flow cytometric analysis as described previously [32].
For experiments involving incubation of neutrophils with SF, apoptosis was determined by active caspase-3 staining and flow cytometry. Briefly, neutrophils were fixed and permeabilized using PermeaFix™ solution (Ortho Diagnostic Systems Inc., Raritan, NJ, US) for 45 min at room temperature. The fixed cells were washed twice in PBS/2% BSA (Sigma-Aldrich) and stained with an affinity-purified rabbit anti-human active caspase-3 mAb (BD Pharmingen, Oxford, UK) or normal rabbit immunoglobulin fraction (DakoCytomation, Ely, UK) as a negative control. Staining was detected with a FITC-conjugated goat anti-rabbit IgG antibody (Cambridge Bioscience, Cambridge, UK) and flow cytometry.
Measurement of neutrophil respiratory burst
Neutrophil respiratory burst in response to fMLP was determined in neutrophils incubated for 20 h in medium alone or in the presence of lactoferrin. Generation of superoxide was measured in a lucigenin-based assay as described previously [33] and results are expressed as integral counts per minute, determined from the area under the curve of the reaction for the first 10 min and adjusted for cell viability.
Measurement of lactoferrin in SF and serum
Lactoferrin was detected in serum and SF by ELISA, with paired capture and biotinylated detection monoclonal antibody, using a commercial kit (Calbiochem, Nottingham, UK). The lower limit of detection was <1 ng/ml. To block interference by RFs with the lactoferrin ELISA, blocking with 40% mouse serum (Sigma-Aldrich), 20% goat serum, 20% rabbit serum (DakoCytomation) and 20% sample diluting buffer was used [33].
Statistical analysis
Data were analysed by Spearman's correlation, Student's t-test or ANOVA followed by Dunnett's comparison with control, as appropriate. A P-value of <0.05 was considered to indicate a significant difference between groups.
Results
Lactoferrin levels in SF
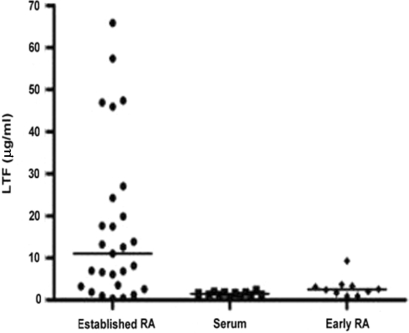
Lactoferrin was measured by ELISA in SFs aspirated from the joints of 26 patients with RA of >2 yrs duration and in matched sera from 11 patients (Fig. 1). Levels in SF varied greatly, ranging from 0.48 to 65.8 μg/ml (mean 17.7 ± 19.2 μg/ml) and were in broad agreement with previous reports for levels of lactoferrin in SF [23]. Levels of lactoferrin in serum from patients with established RA were very low (mean 1.5 ± 0.5 μg/ml), indicating that lactoferrin was produced within the synovial environment, again in agreement with previous reports [17]. We also measured lactoferrin in SF taken from the articular joints of 10 patients with an arthritis of <3 months disease duration who went on to develop RA. We have shown recently that neutrophil apoptosis was also delayed in the SF of these patients [6], despite the different cytokine content of the aspirate compared with that of established RA patients [33]. Interestingly, the SF from these very early arthritis patients did not contain significant levels of lactoferrin, with a mean value of 3.0 ± 2.4 μg/ml (Fig. 1).
Fig. 1.
Lactoferrin levels in RA SF. Lactoferrin concentrations were measured by ELISA in SFs from patients with RA of >2 yrs (n = 26) (•) or <3 months (♦) duration (n = 10) and in matched sera from 11 of the patients with established RA (▪). The median value is indicated by a bar.
Lactoferrin delays neutrophil spontaneous apoptosis
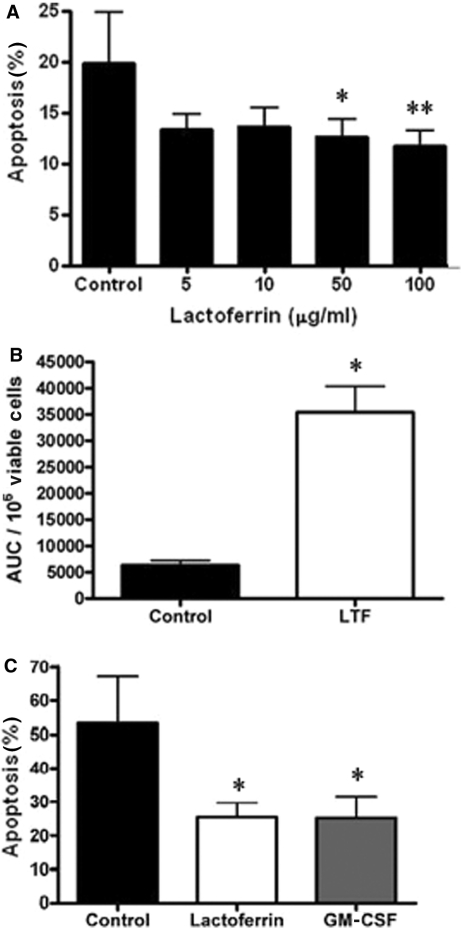
We have shown previously that the apoptosis of neutrophils was inhibited in the synovial microenvironment [5] and that the iron chelator desferrioxamine could delay neutrophil apoptosis in vitro [14]. We therefore determined whether lactoferrin, as a naturally occurring iron chelator present in the inflamed joint, could modulate neutrophil apoptosis in vitro. Freshly isolated neutrophils were cultured with or without iron free lactoferrin at a range of concentrations and apoptosis was measured by DiOC6 staining (Fig. 2A) after 9 h in culture. Lactoferrin significantly delayed neutrophil spontaneous apoptosis in a concentration-dependent manner (Fig. 2A), with significant effects seen at concentrations 50 μg/ml (P < 0.04) and 100 μg/ml (P < 0.02). The lactoferrin-rescued neutrophils were also fully functional, producing a robust superoxide burst in response to fMLP that was significantly greater (P < 0.01) than neutrophils cultured in medium alone (Fig. 2B). Lactoferrin alone did not induce superoxide generation but it did produce a modest increase in CD11b (data not shown), suggestive of a slight priming effect.
Fig. 2.
Effect of lactoferrin on neutrophil apoptosis and function. (A) Peripheral blood neutrophils were incubated with medium alone or with medium containing iron-free lactoferrin at the concentrations shown and apoptosis measured by loss of DiOC6 staining after 9 h in culture. All incubations also contained 10 μg/ml polymyxin B. Data are mean ± s.d. (n = 5) and *P < 0.04, **P < 0.02. (B) Neutrophils were incubated with 100 μg/ml lactoferrin (LTF) for 20 h prior to stimulation with fMLP and measurement of superoxide generation. Data are expressed as mean ± s.d. of the area under the curve (AUC) per 106 viable cells (n = 3). *P < 0.01. (C) Neutrophils isolated from RA synovial fluid were incubated with medium alone, 50 ng/ml GM-CSF or 100 μg/ml lactoferrin. Apoptosis was measured by active caspase 3 staining after 9 h in culture. Data are mean ± s.d. (n = 3) and *P < 0.05. All data were analysed by Student's t-test.
We also determined whether lactoferrin could delay the apoptosis of neutrophils isolated from RA SF, to ensure that cells recruited to the joint were still susceptible to lactoferrin survival signals. Figure 2C shows that neutrophils isolated from SF entered apoptosis very rapidly with almost 50% loss of viability after 9 h in culture. However, lactoferrin could still significantly delay their apoptosis (P < 0.05). The established neutrophil survival factor GM-CSF, which is known to be present in SF, also gave a significant reduction in neutrophil apoptosis (P < 0.05).
Lactoferrin delays neutrophil apoptosis to a similar degree as pro-inflammatory cytokines
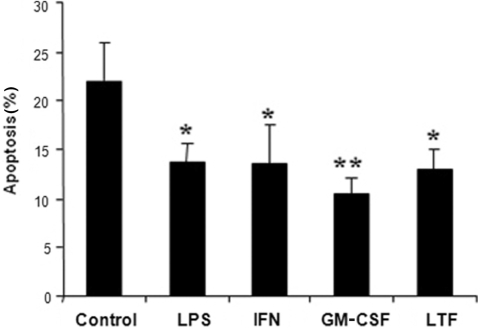
To compare the relative survival promoting effects of lactoferrin with other known neutrophil survival factors we incubated neutrophils with lactoferrin, GM-CSF, IFN-β and LPS. Figure 3 shows that GM-CSF was the most potent neutrophil survival factor (P < 0.01) but that lactoferrin was equivalent to IFN-β, achieving 70% of the inhibition seen with GM-CSF.
Fig. 3.
Comparison of lactoferrin effects with known neutrophil survival factors. Peripheral blood neutrophils were incubated with medium alone (control), 100 ng/ml LPS, 800 IU/ml IFN-β, 50 ng/ml GM-CSF, 100 μg/ml lactoferrin (LTF) and apoptosis measured by differential staining and nuclear morphology after 9 h in culture. Data are mean ± s.d. (n = 5) and were analysed by ANOVA followed by Dunnett's comparison with control. *P < 0.05, **P < 0.01.
The anti-apoptotic effect of RA SF is reduced by removal of lactoferrin
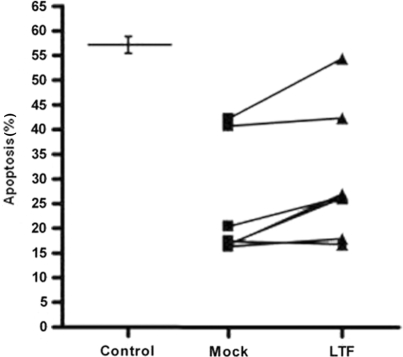
As the rheumatoid synovial environment contains several potential neutrophil survival factors it was important to establish the relative contribution made by lactoferrin. SFs from seven patients with established RA, with lactoferrin levels ranging from 6.11 to 65.82 μg/ml, were treated with either a monoclonal antibody to lactoferrin (Lactoferrin depletion), or an isotype-matched control antibody (mock depletion) and incubated with peripheral blood neutrophils and apoptosis determined. Depletion of lactoferrin was confirmed by ELISA and was below the detection limit of the assay at <1 ng/ml (data not shown). Figure 4 shows that mock-depleted RA SF significantly delayed neutrophil apoptosis, from the level of 56% seen in the untreated neutrophils to 16–42% in the treated cells. Depletion of lactoferrin reduced the survival effect of the SF in four out of seven samples (P < 0.03), suggesting that in the majority of samples lactoferrin contributed significantly to the survival effect of SF on human neutrophils.
Fig. 4.
The anti-apoptotic effect of synovial fluid is reduced by depletion of lactoferrin. Peripheral blood neutrophils were incubated with medium alone (control), or with synovial fluid from seven different RA patients which had been immunodepleted for lactoferrin (LTF) using an anti-lactoferrin monoclonal antibody (LTF) or mock-depleted using an isotype control immunoglobulin (Mock). Apoptosis was measured by active caspase 3 staining after 20 h in culture. Control and immunodepleted data were compared using a paired Student's t-test and *P < 0.03.
Lactoferrin levels correlate with serum CRP and RF
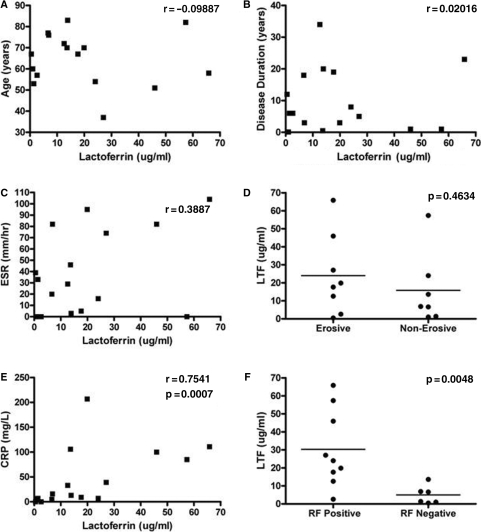
In RA, lactoferrin accumulates in the SF (Fig. 1), most probably following its release from activated neutrophils. As the actions of lactoferrin may result in either pro- or anti-inflammatory outcomes, we determined if there was any correlation between lactoferrin levels and markers of inflammation or disease duration in 24 patients with established RA. There was no correlation between the age of the patient, the duration of their disease, ESR or degree of joint erosion (Fig. 5A–D). However, there was a very significant positive correlation between serum CRP levels and synovial lactoferrin levels (P = 0.008; Fig. 5E). Interestingly, there was also a very striking association between RF positivity and lactoferrin levels (P = 0.017; Fig. 5F).
Fig. 5.
Lactoferrin levels correlate with serum CRP and RF status. Lactoferrin levels were measured in synovial fluid of 24 patients with RA and correlated with (A) age, (B) disease duration, (C) serum ESR, (D) presence of joint erosion, (E) serum CRP and (F) seropositivity for RF. Data were analysed by Spearman's correlation.
Discussion
In this study we report for the first time that the physiological iron-binding protein lactoferrin was able to delay spontaneous apoptosis of both peripheral blood and SF neutrophils. This effect was equivalent to the rescue effect of other known neutrophil survival factors such as GM-CSF and type 1 IFNs. We confirm that lactoferrin is present at significant levels in the SF of patients with established RA, but also show that it was not found at similar levels in the SF of patients whose disease duration was <3 months. Furthermore, depletion of lactoferrin from SF significantly reduced its ability to delay neutrophil apoptosis. We thus conclude that lactoferrin contributes to the extended life span of neutrophils within the rheumatoid joint of patients with established RA, but does not contribute to reduced neutrophil apoptosis in the very early stages of RA [6].
This observation for the first time suggests that in the early and late stages of disease different factors are involved in the regulation of neutrophil survival.
The question of whether lactoferrin is a pro- or anti-inflammatory factor in vivo is not merely one of scientific curiosity. Iron often accumulates in the synovium of patients with RA, but if iron is bound by lactoferrin it cannot react with superoxide to generate harmful hydroxyl radicals that cause significant tissue damage in RA. In this context, the presence of lactoferrin would be expected to be beneficial. Lactoferrin has been injected intra-articularly in animal models of inflammation and has been shown to suppress inflammation modestly [24, 34], leading to proposals that local administration of lactoferrin may be useful in the treatment of RA [24]. However, lactoferrin also promotes neutrophil adhesion [26], which could lead to increased recruitment and retention of neutrophils and our data shown here also reveal that it was able to significantly extend neutrophil lifespan and function. Each of these would help to increase the number of neutrophils infiltrating the inflamed joint and contribute to the persistence of inflammation. In the case of septic arthritis, it is probable that extension of neutrophil lifespan within the joint would be beneficial, increasing the bactericidal capacity of the neutrophil. Indeed, Staphylococcus aureus-induced arthritis in mice was significantly reduced by local injection of lactoferrin, whereas CIA was improved only to a modest extent and histological analysis of inflamed paws revealed extensive inflammation and erosion in control and lactoferrin-treated mice [24]. Lactoferrin may therefore have clinical utility, but we would suggest that this be restricted to infective arthritis and that its wider usage in RA cannot be argued with the same confidence at present.
Analysis of patient clinical history and markers of disease activity revealed a strong association between lactoferrin levels and CRP and RF positivity. The former is not surprising as inflammation will increase neutrophil recruitment to the joint and lead to release of lactoferrin from neutrophil granules. What these data do suggest is that the raised level of lactoferrin has not been able to lead to reduced systemic inflammation and this is in agreement with animal studies that have shown that the effects of lactoferrin were localized to the inflamed joint and do not extend to the periphery [24]. The association with the presence of RF in the serum is more intriguing. One possible explanation is that patients positive for RFs and thus a more serological disease would also have significant levels of immune complexes in their SF [35]. Immune complexes can induce degranulation of neutrophils [10, 36], which would then drive further release of lactoferrin in to the joint.
The ability of the iron chelators to enter neutrophils could determine their ability to chelate the intracellular labile iron pool and delay neutrophil spontaneous apoptosis. Desferrioxamine is a relatively small iron chelating molecule (656.8 Da) that has been shown to enter cells [37] and delay neutrophil apoptosis [14]. Lactoferrin is a large biological iron-binding protein (78 000–80 000 Da) that cannot enter cells by passive diffusion. Transferrin is also present in the rheumatoid synovium and has been shown to enter cells through receptor-mediated endocytosis. However, transferrin receptors are expressed mainly on proliferating cells and mature neutrophils do not express this receptor [38]. In contrast, neutrophils have been reported to express specific receptors for lactoferrin making receptor-mediated endocytosis possible [39]. Furthermore, in contrast to transferrin, lactoferrin can bind iron at much lower pH, allowing lactoferrin function even within an acidic environment such as the endocytic vacuoles. Lactoferrin may therefore be the dominant functional iron chelator in RA SF.
In conclusion, our findings unveil a previously unrecognized effect of lactoferrin on neutrophil biology. We predict that lactoferrin could play a significant role in the regulation of neutrophil apoptosis in vivo. High concentrations of lactoferrin have been found in the rheumatoid joint [18, 23], which might contribute to a lack of apoptotic neutrophils in SF of patients with long-standing disease. However, the iron saturation state of lactoferrin at the site of inflammation will determine outcome and we propose that its use clinically should be considered only for septic arthritis in which extension of neutrophil lifespan could enhance the microbicidal efficacy of infiltrating neutrophils.

Acknowledgements
Funding: This work was supported by an Arthritis Research Campaign (ARC) programme grant to M.S. and J.M.L., an ARC Career Development Fellowship to D.S.-T. and a Medical Research Council PhD studentship to N.F.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Whyte M, Renshaw S, Lawson R, Bingle C. Apoptosis and the regulation of neutrophil lifespan. Biochem Soc Trans. 1999;27:802–7. doi: 10.1042/bst0270802. [DOI] [PubMed] [Google Scholar]
- 2.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GB, Albright BN, Caswell JL. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infect Immun. 2003;71:1643–9. doi: 10.1128/IAI.71.4.1643-1649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson GP. Resolution of chronic inflammation by therapeutic induction of apoptosis. Trends Pharmacol Sci. 1996;17:438–42. doi: 10.1016/s0165-6147(96)01004-8. [DOI] [PubMed] [Google Scholar]
- 5.Scheel-Toellner D, Wang K, Henriquez NV, et al. Cytokine-mediated inhibition of apoptosis in non-transformed T cells and neutrophils can be dissociated from protein kinase B activation. Eur J Immunol. 2002;32:486–93. doi: 10.1002/1521-4141(200202)32:2<486::AID-IMMU486>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Raza K, Scheel-Toellner D, Lee CY, et al. Synovial fluid leukocyte apoptosis is inhibited in patients with very early rheumatoid arthritis. Arthritis Res Ther. 2006;8:R120. doi: 10.1186/ar2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheel-Toellner D, Akbar AN, Pilling D, et al. Type I interferons inhibit the resolution of chronic inflammation. Symp Soc Exp Biol. 2000;52:277–88. [PubMed] [Google Scholar]
- 8.Brach MA, deVos S, Gruss HJ, Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood. 1992;80:2920–4. [PubMed] [Google Scholar]
- 9.Ottonello L, Cutolo M, Frumento G, et al. Synovial fluid from patients with rheumatoid arthritis inhibits neutrophil apoptosis: role of adenosine and proinflammatory cytokines. Rheumatology. 2002;41:1249–60. doi: 10.1093/rheumatology/41.11.1249. [DOI] [PubMed] [Google Scholar]
- 10.Fossati G, Bucknall RC, Edwards SW. Insoluble and soluble immune complexes activate neutrophils by distinct activation mechanisms: changes in functional responses induced by priming with cytokines. Ann Rheum Dis. 2002;61:13–9. doi: 10.1136/ard.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross A, Barnes T, Bucknall RC, Edwards SW, Moots RJ. Neutrophil apoptosis in rheumatoid arthritis is regulated by local oxygen tensions within joints. J Leukoc Biol. 2006;80:521–8. doi: 10.1189/jlb.0306178. [DOI] [PubMed] [Google Scholar]
- 12.Kasahara Y, Iwai K, Yachie A, et al. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 1997;89:1748–53. [PubMed] [Google Scholar]
- 13.Rollet-Labelle E, Grange MJ, Elbim C, Marquetty C, Gougerot-Pocidalo MA, Pasquier C. Hydroxyl radical as a potential intracellular mediator of polymorphonuclear neutrophil apoptosis. Free Radic Biol Med. 1998;24:563–72. doi: 10.1016/s0891-5849(97)00292-x. [DOI] [PubMed] [Google Scholar]
- 14.Scheel-Toellner D, Wang K, Craddock R, et al. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557–64. doi: 10.1182/blood-2004-01-0191. [DOI] [PubMed] [Google Scholar]
- 15.Hultqvist M, Holmdahl R. Ncf1 (p47phox) polymorphism determines oxidative burst and the severity of arthritis in rats and mice. Cell Immunol. 2005;233:97–101. doi: 10.1016/j.cellimm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Mecklenburgh KI, Walmsley SR, Cowburn AS, et al. Involvement of a ferroprotein sensor in hypoxia-mediated inhibition of neutrophil apoptosis. Blood. 2002;100:3008–16. doi: 10.1182/blood-2002-02-0454. [DOI] [PubMed] [Google Scholar]
- 17.Caccavo D, Sebastiani GD, Di MC, et al. Increased levels of lactoferrin in synovial fluid but not in serum from patients with rheumatoid arthritis. Int J Clin Lab Res. 1999;29:30–5. doi: 10.1007/s005990050059. [DOI] [PubMed] [Google Scholar]
- 18.Caccavo D, Garzia P, Sebastiani GD, et al. Expression of lactoferrin on neutrophil granulocytes from synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 2003;30:220–4. [PubMed] [Google Scholar]
- 19.Guillen C, McInnes IB, Brock JH. Iron in synovial fluid. Removal by lactoferrin and relationship to iron regulatory protein (IRP) activity. Adv Exp Med Biol. 1998;443:161–5. [PubMed] [Google Scholar]
- 20.Baker EN, Lindley PF. New perspectives on the structure and function of transferrins. J Inorg Biochem. 1992;47:147–60. doi: 10.1016/0162-0134(92)84061-q. [DOI] [PubMed] [Google Scholar]
- 21.Furmanski P, Li ZP. Multiple forms of lactoferrin in normal and leukemic human granulocytes. Exp Hematol. 1990;18:932–5. [PubMed] [Google Scholar]
- 22.Crocker IP, Baker PN, Fletcher J. Neutrophil function in pregnancy and rheumatoid arthritis. Ann Rheum Dis. 2000;59:555–64. doi: 10.1136/ard.59.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillen C, McInnes IB, Kruger H, Brock JH. Iron, lactoferrin and iron regulatory protein activity in the synovium; relative importance of iron loading and the inflammatory response. Ann Rheum Dis. 1998;57:309–14. doi: 10.1136/ard.57.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guillen C, McInnes IB, Vaughan D, Speekenbrink AB, Brock JH. The effects of local administration of lactoferrin on inflammation in murine autoimmune and infectious arthritis. Arthritis Rheum. 2000;43:2073–80. doi: 10.1002/1529-0131(200009)43:9<2073::AID-ANR19>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Kruzel ML. [Role of lactoferrin in development of inflammation.] Postepy Hig Med Dosw. 2003;57:377–404. [PubMed] [Google Scholar]
- 26.Oseas R, Yang HH, Baehner RL, Boxer LA. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981;57:939–45. [PubMed] [Google Scholar]
- 27.Britigan BE, Serody JS, Hayek MB, Charniga LM, Cohan MS. Uptake of lactoferrin by mononuclear phagocytes inhibits their ability to form hydroxyl radical and protects them from membrane autoperoxidation. J Immunol. 1991;147:4271–7. [PubMed] [Google Scholar]
- 28.Gutteridge JM, Paterson SK, Segal AW, Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981;199:259–61. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afford SC, Pongracz J, Stockley RA, Crocker J, Burnett D. The induction by human interleukin-6 of apoptosis in the promonocytic cell line U937 and human neutrophils. J Biol Chem. 1992;267:21612–6. [PubMed] [Google Scholar]
- 30.Cardoso AS, Araujo MI, Goes AM, Pacifico LG, Oliveira RR, Oliveira SC. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb Cell Fact. 2007;6 doi: 10.1186/1475-2859-6-1. article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khwaja A, Tatton L. Caspase-mediated proteolysis and activation of protein kinase Cdelta plays a central role in neutrophil apoptosis. Blood. 1999;94:291–301. [PubMed] [Google Scholar]
- 32.Wang K, Scheel-Toellner D, Wong SH, et al. Inhibition of neutrophil apoptosis by type 1 IFN depends on cross-talk between phosphoinositol 3-kinase, protein kinase C-delta, and NF-kappa B signaling pathways. J Immunol. 2003;171:1035–41. doi: 10.4049/jimmunol.171.2.1035. [DOI] [PubMed] [Google Scholar]
- 33.Raza K, Curnow J, Taylor E, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–95. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trif M, Guillen C, Vaughan DM, et al. Liposomes as possible carriers for lactoferrin in the local treatment of inflammatory diseases. Exp Biol Med. 2001;226:559–64. doi: 10.1177/153537020122600608. [DOI] [PubMed] [Google Scholar]
- 35.Mathsson L, Lampa J, Mullazehi M, Ronnelid J. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther. 2006;8:R64. doi: 10.1186/ar1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naucler C, Grinstein S, Sundler R, Tapper H. Signaling to localized degranulation in neutrophils adherent to immune complexes. J Leukoc Biol. 2002;71:701–10. [PubMed] [Google Scholar]
- 37.Glickstein H, Ben El R, Shvartsman M, Cabantchik ZI. Intracellular labile iron pools as direct targets of iron chelators. A fluorescence study of chelator action in living cells. Blood. 2005;106:3342–50. doi: 10.1182/blood-2005-02-0460. [DOI] [PubMed] [Google Scholar]
- 38.Otaki Y, Nakanishi T, Hasuike Y, et al. Defective regulation of iron transporters leading to iron excess in the polymorphonuclear leukocytes of patients on maintainance hemodialysis. Am J Kidney Dis. 2004;43:1030–9. doi: 10.1053/j.ajkd.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Boxer LA, Haak RA, Yang HH, et al. Membrane-bound lactoferrin alters the surface properties of polymorphonuclear leukocytes. J Clin Invest. 1982;70:1049–57. doi: 10.1172/JCI110692. [DOI] [PMC free article] [PubMed] [Google Scholar]