Abstract
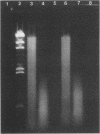
Two criteria must be met before mycobacterial specimens can be tested by DNA amplification methods: (i) the sample must be rendered noninfectious, and (ii) the organisms must be lysed to free the DNA. Previous publications reporting DNA amplification of mycobacteria have concentrated on lysis and amplification procedures and have not addressed the issue of sample safety. We have shown that heating of samples below 100 degrees C may not consistently kill mycobacteria; however, heating at 100 degrees C in a boiling-water bath or a forced-air oven for a minimum of 5 min kills mycobacteria, including Mycobacterium thermoresistibile. Furthermore, heating at 100 degrees C for 30 min consistently lyses mycobacteria to produce short fragments of DNA that are suitable for amplification by PCR and strand displacement amplification. This procedure works with clinical samples digested by the n-acetyl cysteine-NaOH method as well as with suspensions of organisms in phosphate buffer. This paper also demonstrates the feasibility of using strand displacement amplification with clinical specimens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose M., Chander A., Das R. H. A rapid and gentle method for the isolation of genomic DNA from mycobacteria. Nucleic Acids Res. 1993 May 25;21(10):2529–2530. doi: 10.1093/nar/21.10.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- De Wit D., Steyn L., Shoemaker S., Sogin M. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol. 1990 Nov;28(11):2437–2441. doi: 10.1128/jcm.28.11.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Sifford M. D., Cave M. D., Bates J. H., Crawford J. T. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis. 1991 Nov;144(5):1160–1163. doi: 10.1164/ajrccm/144.5.1160. [DOI] [PubMed] [Google Scholar]
- Fries J. W., Patel R. J., Piessens W. F., Wirth D. F. Detection of untreated mycobacteria by using polymerase chain reaction and specific DNA probes. J Clin Microbiol. 1991 Aug;29(8):1744–1747. doi: 10.1128/jcm.29.8.1744-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., Schuitema A. R., Van Soolingen D., Verstynen C. P., Bik E. M., Thole J. E., Kolk A. H., van Embden J. D. Specific detection of Mycobacterium tuberculosis complex strains by polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1204–1213. doi: 10.1128/jcm.28.6.1204-1213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. J., Fries J. W., Piessens W. F., Wirth D. F. Sequence analysis and amplification by polymerase chain reaction of a cloned DNA fragment for identification of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Mar;28(3):513–518. doi: 10.1128/jcm.28.3.513-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikaytis B. B., Eisenach K. D., Crawford J. T., Shinnick T. M. Differentiation of Mycobacterium tuberculosis and Mycobacterium bovis BCG by a polymerase chain reaction assay. Mol Cell Probes. 1991 Jun;5(3):215–219. doi: 10.1016/0890-8508(91)90043-j. [DOI] [PubMed] [Google Scholar]
- Rutala W. A., Cole E. C., Wannamaker N. S., Weber D. J. Inactivation of Mycobacterium tuberculosis and Mycobacterium bovis by 14 hospital disinfectants. Am J Med. 1991 Sep 16;91(3B):267S–271S. doi: 10.1016/0002-9343(91)90380-g. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Savić B., Sjöbring U., Alugupalli S., Larsson L., Miörner H. Evaluation of polymerase chain reaction, tuberculostearic acid analysis, and direct microscopy for the detection of Mycobacterium tuberculosis in sputum. J Infect Dis. 1992 Nov;166(5):1177–1180. doi: 10.1093/infdis/166.5.1177. [DOI] [PubMed] [Google Scholar]
- Schulze-Röbbecke R., Buchholtz K. Heat susceptibility of aquatic mycobacteria. Appl Environ Microbiol. 1992 Jun;58(6):1869–1873. doi: 10.1128/aem.58.6.1869-1873.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritharan V., Barker R. H., Jr A simple method for diagnosing M. tuberculosis infection in clinical samples using PCR. Mol Cell Probes. 1991 Oct;5(5):385–395. doi: 10.1016/s0890-8508(06)80011-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nagata A., Ono Y., Yamada T. Complete nucleotide sequence of the 16S rRNA gene of Mycobacterium bovis BCG. J Bacteriol. 1988 Jun;170(6):2886–2889. doi: 10.1128/jb.170.6.2886-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. T., Fraiser M. S., Schram J. L., Little M. C., Nadeau J. G., Malinowski D. P. Strand displacement amplification--an isothermal, in vitro DNA amplification technique. Nucleic Acids Res. 1992 Apr 11;20(7):1691–1696. doi: 10.1093/nar/20.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. T., Little M. C., Nadeau J. G., Shank D. D. Isothermal in vitro amplification of DNA by a restriction enzyme/DNA polymerase system. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):392–396. doi: 10.1073/pnas.89.1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Isolation of deoxyribonucleic acid from mycobacteria. J Bacteriol. 1968 Apr;95(4):1481–1482. doi: 10.1128/jb.95.4.1481-1482.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deWit D., Wootton M., Allan B., Steyn L. Simple method for production of internal control DNA for Mycobacterium tuberculosis polymerase chain reaction assays. J Clin Microbiol. 1993 Aug;31(8):2204–2207. doi: 10.1128/jcm.31.8.2204-2207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]