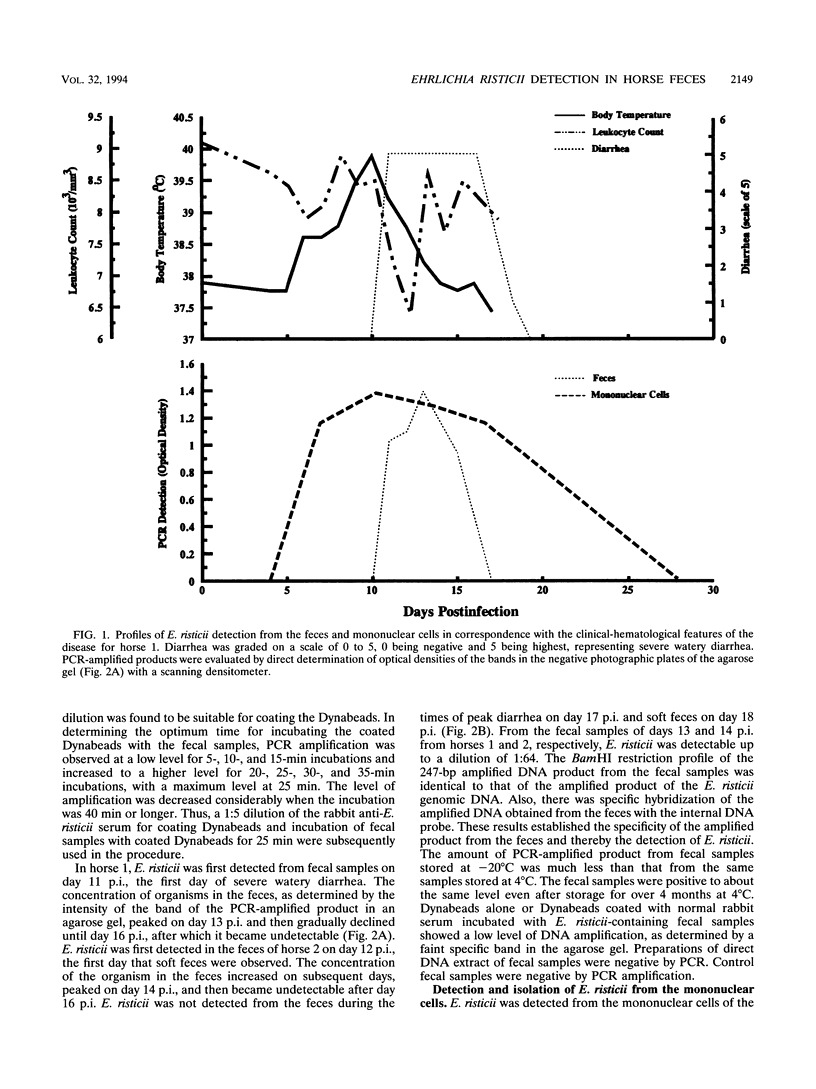
Abstract
Potomac horse fever, caused by Ehrlichia risticii, is an important disease of equines. The major features of the disease are fever, leukopenia, and diarrhea. The organism has been detected from the blood mononuclear cells of infected horses, but its presence in the feces has not been known. A method for immunomagnetic separation of E. risticii from the feces of infected horses was developed, and the separated organisms were detected by PCR. Coating immunomagnetic beads (Dynabeads) with a 1:5 dilution of rabbit anti-E. risticii serum and incubating the Dynabeads with fecal samples for 25 min at room temperature gave optimum results. E. risticii was detected from the feces during the course of diarrhea from two experimentally infected horses. In horse 1, watery diarrhea occurred from days 11 to 16 postinfection (p.i.), after which the feces became soft on day 17 p.i. and then returned to normal. The organisms were first detected from the feces on day 11 p.i., peaked on day 13 p.i., and then gradually decreased until day 16 p.i., after which they became undetectable. In horse 2, first, on day 12 p.i., there was soft feces which continued and progressed to diarrhea on day 17 p.i. The feces became normal after day 18 p.i. The organisms in the feces of this horse were first detected on day 12 p.i. and peaked on day 14 p.i., after which they declined until day 16 p.i. and then became undetectable. In both horses, the number of organisms in the mononuclear cells peaked on days 10 and 11 p.i., respectively, 3 days prior to the respective peaks in the feces. E. risticii was not detected from the plasma samples obtained from these horses. There was a drastic reduction in PCR amplification of E. risticii DNA for fecal samples stored frozen at -20 degrees C in comparison with those stored at 4 degrees C. The presence of the organism in the feces only during the soft- or diarrheal-feces phase supports the previous hypothesis that the diarrhea is caused by the organisms replicating in cells lining the intestines. This rapid simple method of detection of the organisms from the feces will be helpful in diagnostic and epidemiologic studies of Potomac horse fever.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biswas B., Mukherjee D., Mattingly-Napier B. L., Dutta S. K. Diagnostic application of polymerase chain reaction for detection of Ehrlichia risticii in equine monocytic ehrlichiosis (Potomac horse fever). J Clin Microbiol. 1991 Oct;29(10):2228–2233. doi: 10.1128/jcm.29.10.2228-2233.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D. O., Perry B. D., Rikihisa Y., Chickering W. R. Enterocolitis caused by Ehrlichia sp. in the horse (Potomac horse fever). Vet Pathol. 1986 Jul;23(4):471–477. doi: 10.1177/030098588602300418. [DOI] [PubMed] [Google Scholar]
- Dawson J. E., Ristic M., Holland C. J., Whitlock R. H., Sessions J. Isolation of Ehrlichia risticii, the causative agent of Potomac horse fever, from the fetus of an experimentally infected mare. Vet Rec. 1987 Sep 5;121(10):232–232. doi: 10.1136/vr.121.10.232. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Myrup A. C., Rice R. M., Robl M. G., Hammond R. C. Experimental reproduction of Potomac horse fever in horses with a newly isolated Ehrlichia organism. J Clin Microbiol. 1985 Aug;22(2):265–269. doi: 10.1128/jcm.22.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Penney B. E., Myrup A. C., Robl M. G., Rice R. M. Disease features in horses with induced equine monocytic ehrlichiosis (Potomac horse fever). Am J Vet Res. 1988 Oct;49(10):1747–1751. [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Cole A. I., Johnson P., Baker G., Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985 Feb 1;227(4686):522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- Hornes E., Wasteson Y., Olsvik O. Detection of Escherichia coli heat-stable enterotoxin genes in pig stool specimens by an immobilized, colorimetric, nested polymerase chain reaction. J Clin Microbiol. 1991 Nov;29(11):2375–2379. doi: 10.1128/jcm.29.11.2375-2379.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam D., Lindberg A. A. Detection of Shigella dysenteriae type 1 and Shigella flexneri in feces by immunomagnetic isolation and polymerase chain reaction. J Clin Microbiol. 1992 Nov;30(11):2801–2806. doi: 10.1128/jcm.30.11.2801-2806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsvik O., Popovic T., Skjerve E., Cudjoe K. S., Hornes E., Ugelstad J., Uhlén M. Magnetic separation techniques in diagnostic microbiology. Clin Microbiol Rev. 1994 Jan;7(1):43–54. doi: 10.1128/cmr.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Johnson G. C., Wang Y. Z., Reed S. M., Fertel R., Cooke H. J. Loss of absorptive capacity for sodium and chloride in the colon causes diarrhoea in Potomac horse fever. Res Vet Sci. 1992 May;52(3):353–362. doi: 10.1016/0034-5288(92)90037-3. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankarappa B., Dutta S. K., Sanusi J., Mattingly B. L. Monoclonal antibody-mediated, immunodiagnostic competitive enzyme-linked immunosorbent assay for equine monocytic ehrlichiosis. J Clin Microbiol. 1989 Jan;27(1):24–28. doi: 10.1128/jcm.27.1.24-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker S. R., Dutta S. K., Adhya S. L., Mattingly-Napier B. L. Molecular cloning of Ehrlichia risticii and development of a gene probe for the diagnosis of Potomac horse fever. J Clin Microbiol. 1990 Sep;28(9):1963–1967. doi: 10.1128/jcm.28.9.1963-1967.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjojoatmodjo M. N., Fluit A. C., Torensma R., Verdonk G. P., Verhoef J. The magnetic immuno polymerase chain reaction assay for direct detection of salmonellae in fecal samples. J Clin Microbiol. 1992 Dec;30(12):3195–3199. doi: 10.1128/jcm.30.12.3195-3199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. K. High-throughput purification of M13 templates for DNA sequencing. Biotechniques. 1993 Sep;15(3):414-6, 418-20, 422. [PubMed] [Google Scholar]



