Abstract
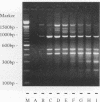
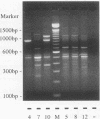
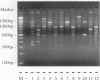
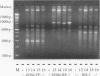
Investigations of the epidemiology of tuberculosis have been hampered by the lack of strain-specific markers that can be used to differentiate isolates of Mycobacterium tuberculosis. We report the development of a rapid protocol for random amplified polymorphic DNA analysis which included the use of a commercially available DNA extraction kit (GeneReleaser). This was applied to 14 strains of M. tuberculosis, including strains associated with temporal and geographical clusters of tuberculosis in the United Kingdom and those from India, Africa, and Saudi Arabia. Strains of M. tuberculosis could be discriminated in about 8 h by this method, which is therefore a rapid and simple alternative to restriction fragment length polymorphism analysis.
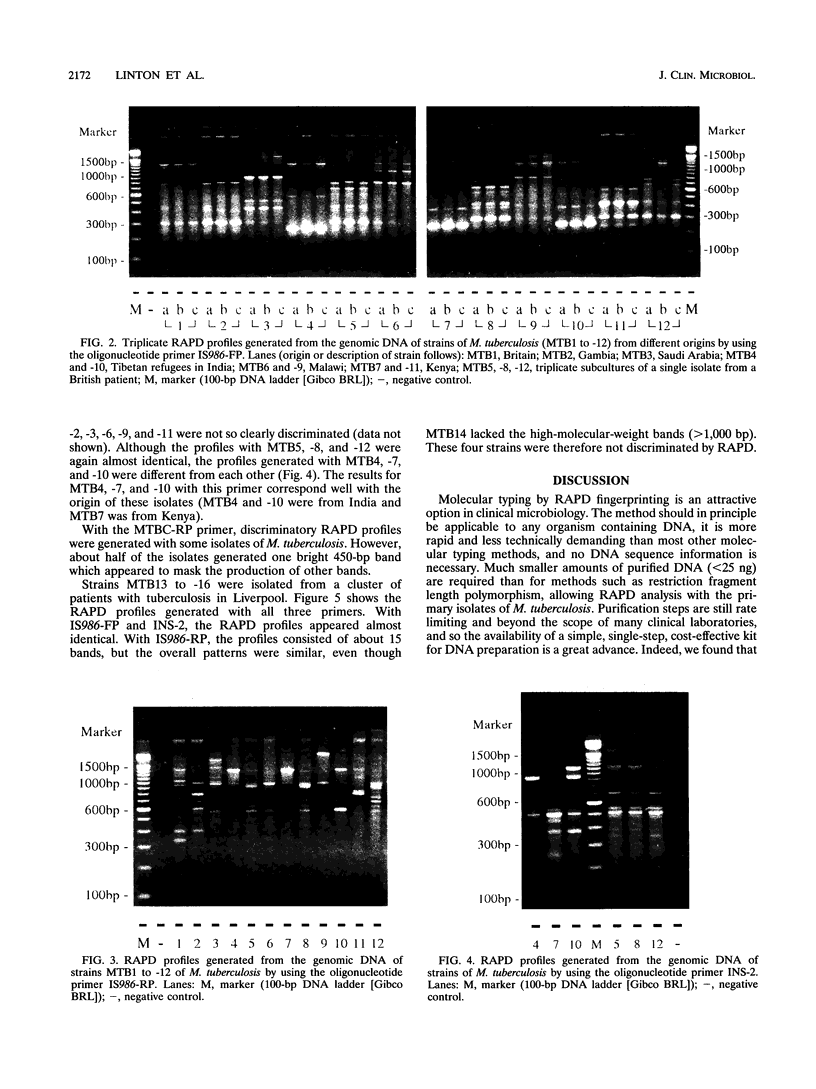
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyanz N., Bukanov N. O., Westblom T. U., Kresovich S., Berg D. E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992 Oct 11;20(19):5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufauvre-Brown A., Cohen J., Holden D. W. Use of randomly amplified polymorphic DNA markers to distinguish isolates of Aspergillus fumigatus. J Clin Microbiol. 1992 Nov;30(11):2991–2993. doi: 10.1128/jcm.30.11.2991-2993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. E., O'Hara L. C., Summersgill J. T. Rapid, simple method for treating clinical specimens containing Mycobacterium tuberculosis to remove DNA for polymerase chain reaction. J Clin Microbiol. 1992 May;30(5):1331–1334. doi: 10.1128/jcm.30.5.1331-1334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Bassam B. J., Gresshoff P. M. DNA amplification fingerprinting using very short arbitrary oligonucleotide primers. Biotechnology (N Y) 1991 Jun;9(6):553–557. doi: 10.1038/nbt0691-553. [DOI] [PubMed] [Google Scholar]
- Cancilla M. R., Powell I. B., Hillier A. J., Davidson B. E. Rapid genomic fingerprinting of Lactococcus lactis strains by arbitrarily primed polymerase chain reaction with 32P and fluorescent labels. Appl Environ Microbiol. 1992 May;58(5):1772–1775. doi: 10.1128/aem.58.5.1772-1775.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M. D., Eisenach K. D., McDermott P. F., Bates J. H., Crawford J. T. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 1991 Feb;5(1):73–80. doi: 10.1016/0890-8508(91)90040-q. [DOI] [PubMed] [Google Scholar]
- Chevrel-Dellagi D., Abderrahman A., Haltiti R., Koubaji H., Gicquel B., Dellagi K. Large-scale DNA fingerprinting of Mycobacterium tuberculosis strains as a tool for epidemiological studies of tuberculosis. J Clin Microbiol. 1993 Sep;31(9):2446–2450. doi: 10.1128/jcm.31.9.2446-2450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho H. L., Handley B. A., Kay H. E., Stevenson L., Beringer J. E. The effect of colony age on PCR fingerprinting. Lett Appl Microbiol. 1993 Dec;17(6):282–284. doi: 10.1111/j.1472-765x.1993.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Del Portillo P., Murillo L. A., Patarroyo M. E. Amplification of a species-specific DNA fragment of Mycobacterium tuberculosis and its possible use in diagnosis. J Clin Microbiol. 1991 Oct;29(10):2163–2168. doi: 10.1128/jcm.29.10.2163-2168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel H. W. Mycobacteriophages and phage typing. Ann Microbiol (Paris) 1978 Jan;129(1):75–90. [PubMed] [Google Scholar]
- Genewein A., Telenti A., Bernasconi C., Mordasini C., Weiss S., Maurer A. M., Rieder H. L., Schopfer K., Bodmer T. Molecular approach to identifying route of transmission of tuberculosis in the community. Lancet. 1993 Oct 2;342(8875):841–844. doi: 10.1016/0140-6736(93)92698-s. [DOI] [PubMed] [Google Scholar]
- Godfrey-Faussett P., Mortimer P. R., Jenkins P. A., Stoker N. G. Evidence of transmission of tuberculosis by DNA fingerprinting. BMJ. 1992 Jul 25;305(6847):221–223. doi: 10.1136/bmj.305.6847.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W. H., Butler W. R., Woodley C. L., Crawford J. T. Mixed-linker polymerase chain reaction: a new method for rapid fingerprinting of isolates of the Mycobacterium tuberculosis complex. J Clin Microbiol. 1993 May;31(5):1293–1298. doi: 10.1128/jcm.31.5.1293-1298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersulyte D., Woods J. P., Keath E. J., Goldman W. E., Berg D. E. Diversity among clinical isolates of Histoplasma capsulatum detected by polymerase chain reaction with arbitrary primers. J Bacteriol. 1992 Nov;174(22):7075–7079. doi: 10.1128/jb.174.22.7075-7079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991 Mar;72(1):1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- Kolk A. H., Schuitema A. R., Kuijper S., van Leeuwen J., Hermans P. W., van Embden J. D., Hartskeerl R. A. Detection of Mycobacterium tuberculosis in clinical samples by using polymerase chain reaction and a nonradioactive detection system. J Clin Microbiol. 1992 Oct;30(10):2567–2575. doi: 10.1128/jcm.30.10.2567-2575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. F., Lin D., Lasker B. A. Genotypic identification and characterization of species and strains within the genus Candida by using random amplified polymorphic DNA. J Clin Microbiol. 1992 Dec;30(12):3249–3254. doi: 10.1128/jcm.30.12.3249-3254.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGowan A. P., O'Donaghue K., Nicholls S., McLauchlin J., Bennett P. M., Reeves D. S. Typing of Listeria spp. by random amplified polymorphic DNA (RAPD) analysis. J Med Microbiol. 1993 May;38(5):322–327. doi: 10.1099/00222615-38-5-322. [DOI] [PubMed] [Google Scholar]
- Majiwa P. A., Maina M., Waitumbi J. N., Mihok S., Zweygarth E. Trypanosoma (Nannomonas) congolense: molecular characterization of a new genotype from Tsavo, Kenya. Parasitology. 1993 Feb;106(Pt 2):151–162. doi: 10.1017/s0031182000074941. [DOI] [PubMed] [Google Scholar]
- Mazurek G. H., Cave M. D., Eisenach K. D., Wallace R. J., Jr, Bates J. H., Crawford J. T. Chromosomal DNA fingerprint patterns produced with IS6110 as strain-specific markers for epidemiologic study of tuberculosis. J Clin Microbiol. 1991 Sep;29(9):2030–2033. doi: 10.1128/jcm.29.9.2030-2033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurier S. I., Wernars K. Typing of Listeria strains by random amplification of polymorphic DNA. Res Microbiol. 1992 Jun;143(5):499–505. doi: 10.1016/0923-2508(92)90096-7. [DOI] [PubMed] [Google Scholar]
- Myers L. E., Silva S. V., Procunier J. D., Little P. B. Genomic fingerprinting of "Haemophilus somnus" isolates by using a random-amplified polymorphic DNA assay. J Clin Microbiol. 1993 Mar;31(3):512–517. doi: 10.1128/jcm.31.3.512-517.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otal I., Martín C., Vincent-Lévy-Frebault V., Thierry D., Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol. 1991 Jun;29(6):1252–1254. doi: 10.1128/jcm.29.6.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palittapongarnpim P., Chomyc S., Fanning A., Kunimoto D. DNA fingerprinting of Mycobacterium tuberculosis isolates by ligation-mediated polymerase chain reaction. Nucleic Acids Res. 1993 Feb 11;21(3):761–762. doi: 10.1093/nar/21.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner G. A., Bush A., Wise R., Kim W., Domier L., Kasha K., Laroche A., Scoles G., Molnar S. J., Fedak G. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. PCR Methods Appl. 1993 May;2(4):341–345. doi: 10.1101/gr.2.4.341. [DOI] [PubMed] [Google Scholar]
- Ross B. C., Dwyer B. Rapid, simple method for typing isolates of Mycobacterium tuberculosis by using the polymerase chain reaction. J Clin Microbiol. 1993 Feb;31(2):329–334. doi: 10.1128/jcm.31.2.329-334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier P., Bourneix C., Prévost G., Andremont A. Random amplified polymorphic DNA assay is less discriminant than pulsed-field gel electrophoresis for typing strains of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993 Apr;31(4):982–985. doi: 10.1128/jcm.31.4.982-985.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierwater B., Ender A. Different thermostable DNA polymerases may amplify different RAPD products. Nucleic Acids Res. 1993 Sep 25;21(19):4647–4648. doi: 10.1093/nar/21.19.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Pauls K. P. Optimization of the PCR program for RAPD analysis. Nucleic Acids Res. 1992 May 25;20(10):2606–2606. doi: 10.1093/nar/20.10.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]