Abstract
Objective
Congenital muscular dystrophy type 1A (MDC1A) is an autosomal recessive disease that is caused by loss-of-function mutations in the laminin-α2 gene and results in motor nerve and skeletal muscle dysfunction. In a previous study, we used genetic modifications to show that inappropriate induction of apoptosis was a significant contributor to pathogenesis in a laminin-α2-deficient mouse model of MDC1A. To identify a possible pharmacological therapy for laminin-α2-deficency, this study was designed to determine if treatment with minocycline or doxycycline, which are tetracycline derivatives reported to have anti-apoptotic effects in mammals, would significantly increase lifespan and improve neuromuscular function in laminin-α2-deficient mice.
Methods
Mice that were homozygous for a targeted, inactivating mutation of the laminin-α2-gene were placed into control, minocycline-treated, or doxycycline-treated groups. Drug treatment began within two weeks of birth and the progression of disease was followed over time using behavioral, growth, histological, and molecular assays.
Results
We found that treatment with either minocycline or doxycycline increased the median lifespan of laminin-α2-null mice from ∼32 days to ∼70 days. Furthermore, doxycycline improved postnatal growth rate and delayed the onset of hindlimb paralysis. Doxycycline-treated laminin-α2-deficient muscles had increased Akt phosphorylation, decreased inflammation, and decreased levels of Bax protein, TUNEL-positive myonuclei, and activated caspase-3.
Interpretation
Doxycycline or other drugs with similar functional profiles may be a possible route to improving neuromuscular dysfunction due to laminin-α2-deficiency.
Introduction
Mutation of the human LAMA2 (mouse Lama2) gene that encodes laminin-α2 causes the most common, type 1A, form of congenital muscular dystrophy (MDC1A) (1 - 3). Loss of laminin-α2 function in this autosomal recessive disease results in severe neuromuscular dysfunction. A number of other diseases are accompanied by partial laminin-α2-deficiency, including Muscle-Eye-Brain disease, Fukuyama Congenital Muscular Dystrophy, and Walker-Warburg Syndrome, though these diseases are caused by primary mutations in genes other than LAMA2 (2). Therapies to ameliorate neuromuscular dysfunction in MDC1A are currently lacking.
Loss of laminin-α2 leads to impaired function in both skeletal muscle and motor nerves (4, 5). In skeletal muscle, laminin-α2 associates with the additional laminin subunits β1 and γ1 to form the heterotrimeric laminin-211 complex; and, in motor nerves, laminin-α2 is found in the Schwann cell basal lamina (4). Heterotrimeric laminins that include laminin-α2 have been termed merosins, and MDC1A has thus also been termed merosin-deficient congenital muscular dystrophy. Laminin-α2 plays a key role in linking cellular and extracellular components. An extracellular binding partner of laminin-α2 is entactin/nidogen, which in turn binds to collagen IV. In addition, laminin-α2 has at least two membrane-associated binding partners in skeletal muscle: α-dystroglycan and α7-integrin (1).
One significant pathogenetic mechanism by which loss of laminin-α2 leads to neuromuscular dysfunction appears to be inappropriate induction of apoptotic cell death in skeletal muscles and motor nerves (6 - 10). Laminin-α2-deficient skeletal muscles in both humans and mice show relatively abundant signs of apoptosis (8 - 10). Furthermore, we previously demonstrated that pathology in laminin-α2-deficient (Lama2-/-) mice is markedly lessened by inhibiting apoptosis through either (i) body-wide inactivation of the pro-apoptosis protein Bax or (ii) skeletal muscle-specific overexpression of the anti-apoptosis protein Bcl-2 (6, 7). Both of these genetic interventions produced a >2X increase in the lifespan of Lama2-/- mice, as well as improved growth and myofiber histology. Bax inactivation, but not skeletal muscle-specific Bcl-2, also delayed hindlimb paralysis of Lama2-/- mice, consistent with improvement in both motor nerve and muscle function.
To extend our genetic studies, we sought to identify pharmaceutical approaches to ameliorate disease due laminin-α2-deficiency. Because doxycycline, as well as other tetracycline derivatives including minocycline, had been reported to inhibit apoptosis in mammalian cells and to improve outcomes in models of diseases such as amyotrophic lateral sclerosis (ALS) and oculopharyngeal muscular dystrophy (OPMD) (11 - 16), we examined how disease progression in Lama2-/- mice was affected by doxycycline or minocycline. Here we show that treatment with doxycycline or minocycline significantly increased lifespan of Lama2-/- mice. Doxycycline-treated Lama2-/- mice also showed delayed paralysis, decreased inflammation, and increased activation of Akt. Appropriate pharmaceutical therapy is a possible route to ameliorating neuromuscular dysfunction due to laminin-α2-deficiency in humans.
Materials and Methods
Mice
Heterozygous Lama2dy-W/+ mice, which carry the targeted dy-W mutation in Lama2 (6, 21), have been maintained in our laboratory for >4 years by breeding with C57BL/6J mice. Genotypes were identified at weaning by PCR (6, 17).
Drug treatments
Minocycline (Sigma-Aldrich) was administered at 10 μg/g body weight by daily intraperitoneal injection beginning at 7 – 10 days after birth and pups within each litter were randomly assigned to minocycline or control groups. Doxycycline (Sigma-Aldrich, St. Louis MO) was administered to mothers and newborns in drinking water at 6 mg/ml in 5% sucrose beginning at 1 – 3 days after birth (15). Untreated litters received either normal water or 5% sucrose in water; and we found no significant differences in measured outcomes between Lama2-/- mice that received normal water and those that received 5% sucrose in water. Due to the necessity of administering doxycycline through drinking water, entire litters were randomly assigned to either the doxycycline or the untreated group. For both drug treatments, we carried out both a long-term study, which was designed for lifespan analysis and was continued until mice either died or were euthanized as they became unable to carry out daily functions, and a short-term study, which was designed for histological and molecular analyses and was halted by euthanasia at six weeks after birth. Mice were monitored daily and weighed at least once per week. Mice with symptoms that interfered with grooming or access to food were euthanized. Hindlimb function was assayed by counting how often mice stood up on their hindlimbs during their first five minutes in a new cage (6, 18). Protocols were approved by the Institutional Animal Use and Care Committee of the Boston Biomedical Research Institute.
Histology
Histological analyses of muscle sections were as described (6, 7, 19). Cross-sectional areas of individual myofibers were determined using the SPOT Advanced v4.6 program (Diagnostic Instruments, Sterling Heights MI). Statistical significance was assessed by the appropriate unpaired two-tailed t-test; Welch alternate t-test, or non-parametric Mann-Whitney test using InStat v2.03 (GraphPad Software).
Cell culture
Cells of the mouse C2C12 line and of the laminin-α2-deficient B4.pLAM derivative of C2C12 (24) were grown as before (19, 20) on a gelatin substrate that did not contain laminin-α2. To determine if doxycycline inhibited staurosporine-induced apoptosis, C2C12 or B4.pLAM myoblast cultures were pre-treated for 3h with 2 μM doxycycline, at which time staurosporine was added to 100 nM or 500 nM while doxycycline was maintained at 2 μM. The percentage of apoptotic cells was then determined by visual analysis of nuclear shape or by Annexin V staining (20, 21)
Assays
Antibodies for total Akt, phosphoThr308-Akt, phosphoSer473-Akt, total p38MAPK, phospho(Thr180/Tyr182) p38MAPK, and activated caspase-3 were from Cell Signaling Technology (Beverly, MA). Mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was from Research Diagnostics (Flanders, NJ), goat anti-lactate dehydrogenase (LDH) was from Santa Cruz Biotechnology (Santa Cruz, Ca) rabbit anti-Bax was from Upstate Biotechnology (Lake Placid, NY), and rat anti-CD11b (Mac-1) clone M1/70 was from Invitrogen (Carlsbad, CA). Immunoblots were quantified using a LI-COR Odyssey infrared system for Alex-680 conjugates (LI-COR Biosciences, Lincoln, NE) or by chemiluminescence. TUNEL-positive nuclei were identified with the ApopTag system (Chemicon, Temecula CA) on sections stained with rabbit anti-pan-laminin (Sigma, St. Louis MO) and Alexa-594 conjugated secondary antibody (Invitrogen). Caspase-3 activity was measured with the CaspACE system (Promega, Madison WI)
PCR
Total RNA was isolated from 30 – 80 mg of muscle tissue and treated with Turbo DNAse I (Ambion). 1.5 μg DNAse I-treated RNA was converted into cDNA and used with pre-designed Taqman gene expression assays for CD11b and cyclophilin B (Applied Biosystems). Relative expression was determined by the delta-delta CT method.
Results
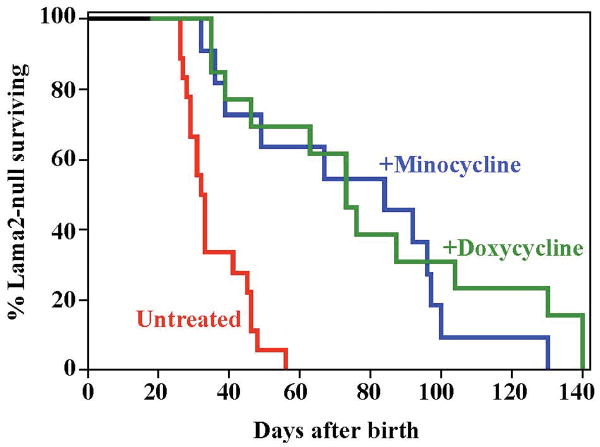
Treatment with doxycycline significantly increased the lifespan of Lama2-/- mice (Fig. 1). The time at which half of the mice died increased from ∼32 days for the untreated cohort up to ∼70 days for the doxycycline-treated mice (P<0.001 by log rank analysis). Doxycycline was administered in the drinking water at 6 mg/ml (15) beginning within one week of birth with the expectation that an effective concentration of the drug would be passed to offspring through milk until weaning (e.g., 22). Treatment with minocycline similarly increased the lifespan of Lama2-/- mice (Fig. 1). Minocycline, due to instability in water, was administered by daily intraperitoneal injections at 10 μg/g body weight beginning at 7 – 10 days after birth.
Fig. 1.
Treatment with doxycycline or minocycline increased the lifespan of laminin-α2-deficient, Lama2-/- mice. The control group of untreated Lama2-/- mice (red line; n=18) had a median lifespan of ∼32 days and all died by 56 days after birth, whereas the median lifespan of Lama2-/- mice was significantly (P<0.001 by log rank analysis) increased to ∼70 days by treatment with either minocycline (blue line; n=11; 10 μg/g body weight i.p. beginning at 7-10 days after birth) or doxycycline (green line; n=13; 6 mg/ml in drinking water beginning within 4 days of birth).
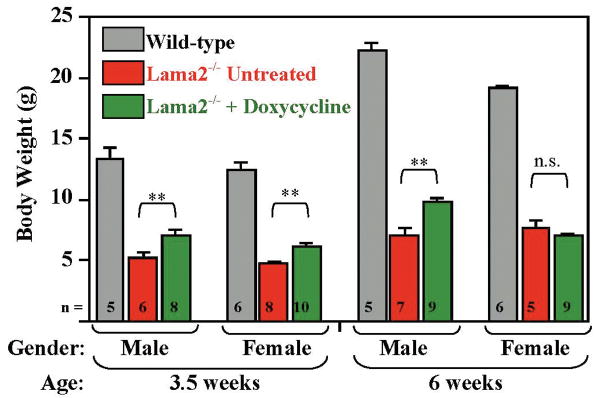
In addition to increasing lifespan, treatment with doxycycline improved the growth of Lama2-/- mice (Fig. 2). At 3.5 weeks after birth, doxycycline-treated male and female Lama2-/- mice weighed 30-40% more than untreated Lama2-/- mice, whereas at 6 weeks after birth, the doxycycline-treated male, but not female, Lama2-/- mice continued to weigh more than the small percentage of untreated Lama2-/- mice that survived this long (Fig. 2). The doxycycline-treated Lama2-/- mice still remained significantly smaller than healthy mice that had normal expression of laminin-α2 (Fig. 2). Doxycycline did not affect the growth rate of wild-type littermates (not shown). In contrast to doxycycline, treatment with minocycline did not consistently produce a significant increase in body weight of Lama2-/- mice (not shown). Because doxycycline was easier to administer and produced better growth than minocycline, we focused additional analyses on the effects of doxycycline on Lama2-/- mice.
Fig. 2.
Effect of doxycycline on growth of laminin-α2-deficient, Lama2-/- mice. At 3.5 and 6 weeks after birth, male Lama2-/- mice that were treated with doxycycline (green bars) weighed significantly more than male Lama2-/- mice that were not treated (red bars). Though doxycycline-treated female Lama2-/- mice were significantly larger than untreated Lama2-/- mice at 3.5 weeks, no difference was found at 6 weeks. At both ages, the doxycycline-treated male and female Lama2-/- mice remained significantly smaller than untreated wild-type (gray bars) littermates (P < 0.01 or better for all comparisons of wild-type to Lama2-/- mice). Treatment with doxycycline did not affect the weights of wild-type mice (not shown). **Weights of untreated and doxycycline-treated Lama2-/- mice were significantly different at P<0.01 by student's t-test; n.s. Weights of untreated and minocycline-treated Lama2-/- mice were not significantly different. Ave. ± SE, n as indicated.
Treatment with doxycycline delayed the onset of hindlimb paralysis in Lama2-/- mice. In addition to skeletal muscle defects, most Lama2-/- mice develop paralysis of the hindlimbs by 4 - 6 weeks after birth (1, 5, 7, 8, 18, 23, 24). In marked contrast to the early paralysis seen in all untreated Lama2-/- mice, the majority of the doxycycline-treated Lama2-/- mice did not show gait disorders until 10 -12 weeks after birth, and the hindlimbs of some Lama2-/- mice, though appearing much weaker than wild-type, were not completely paralyzed at even >15 weeks after birth.
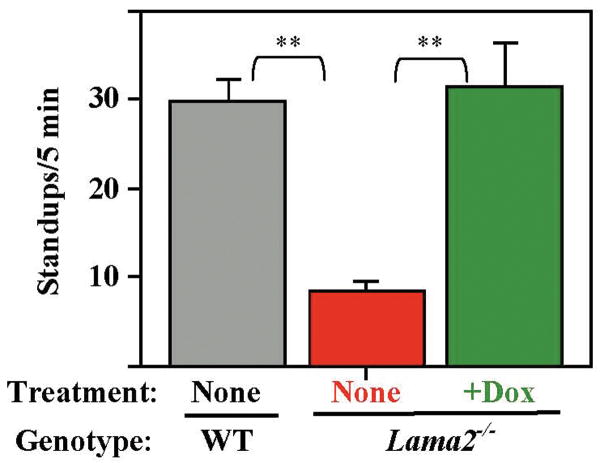
Further evidence of improved hindlimb function came from our observation that doxycycline treatment increased the spontaneous standing movements of Lama2-/- mice. When placed in a new cage, normal mice show exploratory behavior, including repeated standing up on hindlimbs with forepaws in the air or against the wall. At 6 weeks after birth, we found that surviving Lama2-/- mice stood up less than one-third as often as normal mice, consistent with impaired ability of the hindlimbs to function in weight-bearing activity (Fig. 3). In contrast, Lama2-/- mice treated with doxycycline showed approximately the same level of spontaneous standing behavior as wild-type mice (Fig. 3).
Fig. 3.
Doxcycline treatment improved motor behavior in Lama2-/- mice. Exploratory behavior, measured by the number of times the mice stood up on their hindlegs during the first five minutes in a new cage, was significantly decreased in 6 week old Lama2-/- mice (red bar, n = 9) compared to wild-type mice (WT, gray bar, n = 5). Doxycycline-treated Lama2-/- mice (green bar, n = 8) showed exploratory behavior that was near wild-type. Error bars = SE. ** P < 0.01 by t-test.
Individual muscles from doxycycline-treated Lama2-/- male mice weighed significantly more than those from untreated Lama2-/- mice. For example, at six weeks of age, the gastrocnemius/soleus muscle complex from male doxycycline-treated Lama2-/- mice weighed an average of 36.0 ± 5.3 mg (n=5), whereas those from male untreated Lama2-/- mice weighed 20.9 ± 2.7 mg (n=9), which was a significant difference (ave ± SE; P < 0.02 by Wilcoxon test). Doxycycline treatment also increased the size of additional Lama2-/- muscles, including the Tibialis Anterior (TA, untreated = 11.2 ± 2.5 mg, n=8; doxycycline-treated = 15.9 ± 0.6, n=4; P < 0.05, Wilcoxon test) and quadriceps (untreated = 26.6 ± 4.0 mg, n=6; doxycycline-treated = 50.7 ± 6.4 mg, n=4; P < 0.02, Wilcoxon test). The doxycycline-treated Lama2-/- muscles remained much smaller, however, than muscles from wild-type mice in which the gastrocnemius/soleus complex weighed ∼100 mg, the TA ∼40 mg, and the quadriceps ∼135 mg. The maximum cross-sectional areas of the TA and soleus muscles were also higher in doxycycline-treated than in untreated mice (P<0.05 for TA treated = 2.1 ± 0.10 mm2, n=4 vs. TA untreated = 1.7 ± 0.13 mm2, n=5; and P<0.01 for soleus treated = 0.65 ± 0.012 mm2, n=4 vs. soleus untreated = 0.42 ± 0.034 mm2, n=7).
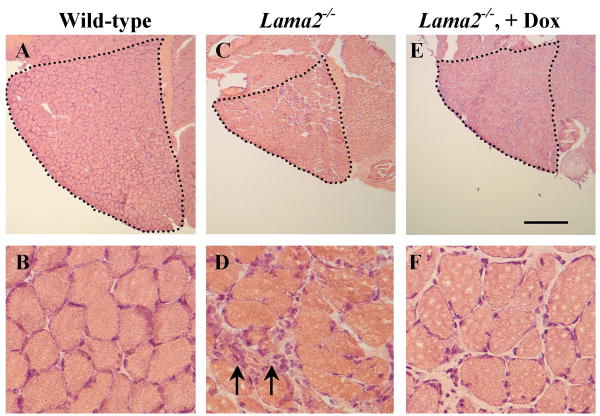
TA and soleus muscles from doxycycline-treated male Lama2-/- mice also tended to have more and larger myofibers than untreated muscles. For example, in the TA, which is a fast muscle composed largely of fast Type IIB and IIX fibers, the average cross-sectional area of myofibers in doxycycline-treated mice was 1472 ± 190 μm2 (ave ± SE, n=3), but only 1037 ± 61 μm2 (n=6) in untreated mice (P<0.05). Though the number of myofibers in the treated TA also tended to be higher than in the untreated TA, this difference did not reach significance (TA treated = 1234 ± 161 fibers, n=4; TA untreated = 1118 ± 57 fibers, n=5). In the soleus, which is a mixed muscle composed of ∼30% slow Type I fibers with the remaining fibers largely fast Type IIA, the average cross-sectional area of the fast myofibers in doxycycline-treated mice was 741 ± 30 μm2 (n=4), but only 545 ± 35 μm2 (n=6) in untreated mice (P<0.01). Though slow myofibers in the treated soleus tended to be larger than in untreated, this difference did not reach significance (soleus treated = 1926 ± 144 μm2 n=4, soleus untreated = 1625 ± 110 μm2 n=7). Doxcycline-treated soleus muscles also contained 469 ± 47 (n=4) myofibers compared to only 328 ± 28 myofibers/soleus in untreated muscle. (n=7; P=0.023). Wild-type soleus muscles have ∼1000 myofibers as noted previously (6). We found no significant differences between untreated and doxycycline-treated Lama2-/- soleus muscles in percentages of fast and slow myofibers. Also, using the percentage of myofibers with centrally located nuclei to identify newly regenerated myofibers (5, 6, 25, 26), we found that ∼10 –15% of the total myofibers in the soleus and other muscles of Lama2-/- mice had centrally located nuclei and that treatment with doxycycline did not produce any consistent difference in this percentage (not shown). Examples of cross-sections of male soleus muscles from wild-type, untreated Lama2-/- mice, and doxycycline-treated Lama2-/- mice are shown in Fig. 4.
Fig. 4.
Doxycycline improved histology of soleus muscles from male Lama2-/- mice. Frozen sections through the gastrocnemius/soleus muscles were prepared from muscles obtained from 6 week old male wild-type (A, B), untreated Lama2-/- (C, D), and doxycycline-treated (+Dox) Lama2-/- (E, F) mice. Sections were stained with hematoxylin and eosin, and representative sections are shown at low (A, C, E) and high (B, D, F) magnification. At low magnification, the soleus muscles are outlined to show that doxycycline increased the overall size and numbers of myofibers in the Lama2-/- soleus muscle (see text for quantitative analyses). At high magnification, more mononucleate inflammatory cells (arrows) were found in the untreated than in the doxycycline-treated Lama2-/- muscles, a finding documented further in Fig. 6. See text for quantification. Scale bar in panel E equals 400 μm for A, C, and E, and 40 μm for B, D, and F.
We next carried out further analyses to identify cellular and molecular mechanisms that could underlie the positive effects of doxycycline treatment.
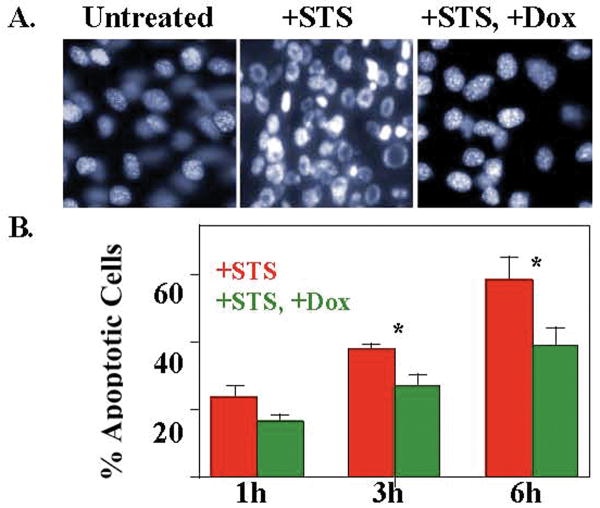
We first found that doxycycline inhibited staurosporine-induced apoptosis of myogenic cells in culture. Treatment of mouse C2C12 myoblasts with staurosporine caused abnormally shaped nuclei (indicating apoptosis) to appear within one hour and to increase in number so that most nuclei were abnormal by six hours after the start of treatment (Fig. 5). When C2C12 myoblasts were first pre-incubated for 3h with 2 μM doxycycline and then treated with a combination of staurosporine and 2 μM doxycycline, however, the percentage of apoptotic nuclei was significantly reduced at 3h and 6h after staurosporine addition (Fig. 5). Using a similar protocol but with Annexin V staining as the assay for apoptotic cells, we found that doxycycline also inhibited staurosporine-induced apoptosis in myoblasts of the laminin-α2-deficient B4.pLAM variant of the C2C12 line (27). In two independent experiments, we found that Annexin V-positive cells amounted to 34.1% and 31.2% of the cells after six hours of staurosporine without doxycycline, whereas only 21.1% and 18.5% of the cells were Annexin V-positive when doxycycline was present at 2 μM in the staurosporine-treated B4.pLAM cultures. Thus, at least in culture, doxycycline can act directly on myogenic cells to inhibit apoptosis.
Fig. 5.
Doxycycline inhibited staurosporine-induced apoptosis in myogenic C2C12 cells. A. Myoblasts of the mouse C2C12 cell line were either untreated, treated with 100 nM staurosporine (+STS), or treated with both 100 nM staurosporine and 2μM doxycycline (+STS, +Dox) after a 3h pre-incubation with 2 μM doxycycline. Doxycycline decreased the number of abnormally shaped nuclei (indicative of apoptosis) in staurosporine-treated cultures. B. Quantitative analysis of experiments performed as in panel A demonstrated that doxycycline decreased the percentage of abnormal, apoptotic nuclei induced by staurosporine. Error bars = SE, n=4. *P < 0.05 by t-test.
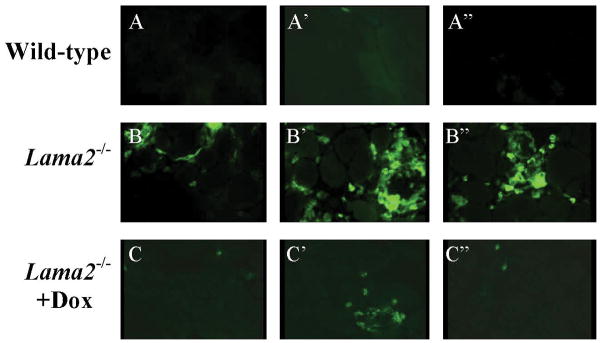
We next found that doxycycline reduced the number of inflammatory cells in Lama2-/- muscles. Lama2-/- muscles contained a large number of inflammatory (CD11b-positive) cells, which is consistent with a previous study (28), whereas healthy wild-type muscles contained almost none, and doxycycline-treated Lama2-/- muscles contained an intermediate number (Fig. 6). The percentage of soleus muscle cross-sectional areas in which muscle tissue was replaced with inflammatory cells was low in wild-type muscles at 1.1% ± 0.17 (ave. ± SE, n=3), much higher in untreated Lama2-/- muscles at 14.3% ± 1.8 (n=4), and intermediate in doxycycline-treated Lama2-/- muscles at 7.2% ± 0.59 (n=4) (all values differ from each other at P<0.05 or better). By quantitative PCR, we found that CD11b mRNA in untreated Lama2-/- muscles was an average of 3.1 ± 0.34 times (n=5) higher than in wild-type muscles, but was only 1.7 ± 0.06 times (n=4) higher than wild-type in doxycycline-treated Lama2-/- muscles (P < 0.02 for untreated vs. doxycycline-treated).
Fig. 6.
Doxycycline treatment reduced inflammation in muscles of Lama2-/- mice. A, A′, A″. Very few inflammatory cells were found in healthy wild-type muscles. B, B′, B″. Untreated Lama2-/- muscles contained large numbers of inflammatory cells. C, C′, C″. Doxycycline-treated Lama2-/- muscles contained fewer inflammatory cells than untreated Lama2-/- muscles. Leg muscles from 5-6 week old mice from each experimental group were stained with anti-CD11b (Mac-1) to identify inflammatory cells of the monocyte/macrophage lineage. Fields from three separate muscles are shown for each group. See text for quantification.
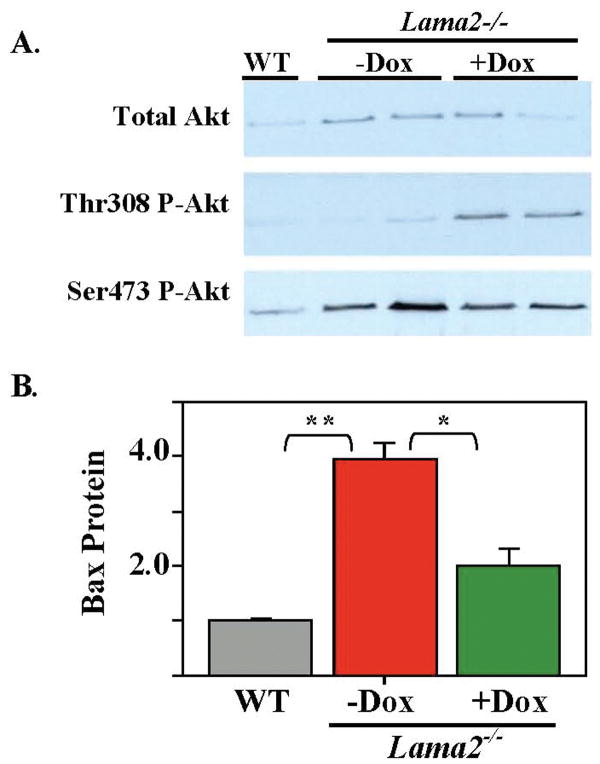
We next examined how laminin-α2-deficiency and doxycycline affected signaling molecules, including Akt, p38MAPK, and ERK1/2 that control cell growth and/or survival. For Akt, we found that, relative to wild-type, Lama2-/- muscles had increased amounts of both total Akt protein and phosphoSer473-Akt, but approximately the same amount of phosphoThr308-Akt (Fig. 7A). Doxycycline treatment caused a large increase in the amount of phosphoThr308-Akt in Lama2-/- muscles, but had little further effect on total Akt or phosphoSer473-Akt (Fig. 7A). For p38MAPK, we found that total p38MAPK protein levels were approximately the same in wild-type, untreated Lama2-/-, and doxycycline-treated Lama2-/- muscles, whereas the level of phospho-p38MAPK in Lama2-/- muscles was less than half that in wild-type muscles, though doxycycline did not consistently produce a further change in phospho-p38MAPK in Lama2-/- muscles (not shown). Also, wild-type and Lama2-/- muscles had similar levels of ERK1/2 and phospho-ERK1/2 (not shown).
Fig. 7.
Doxycycline treatment increased phosphorylation of Thr308 on Akt and decreased expression of Bax in muscles of Lama2-/- mice. A. Leg muscles were obtained from a wild-type (WT) mouse, as well as two untreated Lama2-/- (-Dox) and two doxycycline-treated Lama2-/- (+Dox) mice; and muscle extracts were analyzed by immunoblotting with antibodies specific for total Akt, phosphoThr308-Akt, and phosphoSer473-Akt as indicated. Diseased muscles had more total Akt and more phosphoSer473-Akt than wild-type muscles, and doxycycline treatment increased the amount of phosphoThr308-Akt in Lama2-/- muscles. Equal amounts of total muscle protein were analyzed for each group (10 μg for total Akt, 125 μg for phosphorylated Akt forms) and equal loading was verified by GAPDH staining (not shown). B. Quantitative immunoblots showed that Bax protein was more abundant in muscles obtained from untreated Lama2-/- mice than in muscles from healthy wild-type mice and that doxycycline treatment lowered the amount of Bax protein in Lama2-/- muscles to nearer the wild-type level. Equal amounts of protein (50 μg) were analyzed for each sample, and equal loading was verified by immunoblotting for GAPDH (not shown). Relative amounts of Bax are plotted with the Bax/GAPDH ratio for wild-type set equal to one. Errors bars = SE; n=3. **P<0.01. * P<0.02.
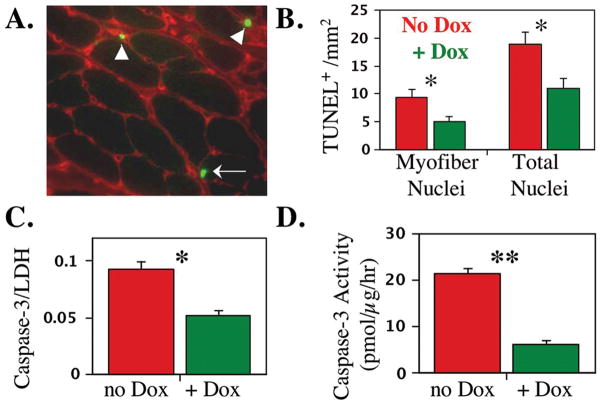
Finally, we found that doxycycline treatment decreased markers of ongoing apoptosis in Lama2-/- muscles. First, although the level of Bax protein was higher in Lama2-/- muscles than in wild-type muscles (consistent with increased cell death), we found that doxycycline treatment lowered the amount of Bax in Lama2-/- muscles to a level closer to that of wild-type muscles (Fig. 7B). Similarly, myotubes formed by myoblasts of the laminin-α2-deficient B4.pLAM variant of the C2C12 line (27) that were cultured in the presence of doxycycline had less Bax protein than untreated myotubes. In two independent experiments, the amount of Bax protein in doxycycline-treated cultures was reduced to only 25% and 44% of the amount in untreated cultures, as measured by quantitative immunoblotting using lactate dehydrogenase for normalization. In addition, we found that, compared to untreated Lama2-/- muscles, doxycycline treatment significantly decreased the number of TUNEL-positive nuclei in Lama2-/- soleus myofibers, as well as both the amount of the 17kDa activated form of the caspase-3 protein and the amount of caspase-3 enzyme activity in Lama2-/- diaphragm muscles (Fig. 8).
Fig. 8.
Doxycycline-treated Lama2-/- muscles had fewer TUNEL-positive nuclei and less activated caspase-3 than untreated Lama2-/- muscles, which is consistent with decreased apoptosis in the treated muscles. A. Representative section of a Lama2-/- soleus muscle assayed for TUNEL-positive nuclei (green) and pan-laminin isoforms (red). The arrow indicates an example of a TUNEL-positive myofiber nucleus located within the laminin sheath is indicated and the arrowheads indicate two examples of interstitial TUNEL-positive nuclei. B. Quantitative analysis of myofiber and total (myofiber + interstitial) TUNEL-positive nuclei in Lama2-/- soleus muscles showed that doxycycline-treated muscles (green bars) had significantly fewer myofiber and total TUNEL-positive nuclei than untreated muscles. C. Quantitative immunoblots showed that doxycycline-treated Lama2-/- diaphragm muscles had significantly less of the 17kDa activated form of caspase-3 than untreated muscles. Equal amounts of protein (50 μg) were analyzed for each sample, and equal loading was verified by immunoblotting for lactate dehydrogenase (LDH, not shown). The 17kDa caspase-3/LDH ratio is plotted. D. Caspase-3 enzyme activity assays similarly showed that doxycycline-treated Lama2-/- diaphragm muscles had significantly less caspase-3 activity than untreated muscles expressed as pmol product produced/μg tissue extract/hour. Errors bars = SE; **P<0.01. * P<0.05.
Discussion
We found that treatment with doxycycline significantly ameliorated pathology in Lama2-/- mice, which are a model for the human disease Congenital Muscular Dystrophy Type 1A. Doxycycline increased the median lifespan of Lama2-/- mice by at least two-fold and also increased body weight and delayed hindlimb paralysis. Furthermore, doxycycline lessened inflammation, inhibited staurosporine-induced apoptosis of cultured myogenic cells, decreased expression of several markers of ongoing apoptosis in diseased muscles, and increased likely survival signaling through Akt.
In skeletal muscle, loss of laminin-α2 is accompanied by disrupted cross-linking of the basal lamina to the plasma membrane and also by abnormally high levels of apoptosis in myofibers (1, 6 – 10, 29). In previous studies, we showed that either inactivation of the pro-apoptotic Bax or muscle-specific overexpression of the anti-apoptotic Bcl-2 significantly ameliorates pathology in Lama2-/- mice (6, 7). Thus, excessive apoptosis, perhaps induced by disrupted integrin and/or dystroglycan signaling (30), is a significant contributor to pathogenesis in Lama2-/- mice. It was because of this pathogenetic role for apoptosis that we tested doxycycline and the related molecule minocycline, which had been shown to have apparently anti-apoptotic effects in other mouse disease models (11, 12, 14, 15), as possible pharmaceutical therapies that might ameliorate disease in Lama2-/- mice.
Our study demonstrated that disease due to loss of laminin-α2 was indeed significantly ameliorated by oral administration of doxycycline or injection of minocycline, though only doxycycline was non-invasive to administer. Doxycycline may have decreased pathology of both muscle (as shown by improved histology) and motor nerve (as shown by delayed paralysis), though neither was completely corrected. Paralysis in Lama2-/- mice has been reported to be due to defects in motor nerves that may include loss of basal lamina, Schwann cells, and/or motor neurons with subsequent conduction defects (1, 5, 8, 23); and such motor nerve involvement is supported by the finding that muscle-specific expression of laminin-α2, modified agrin, or Bcl-2 improves skeletal muscles, but does not prevent paralysis (5, 7, 18, 24). Thus, treatment with doxycycline, which reaches tissues throughout the body, appeared to delay the appearance of functional defects in motor nerves, as well as in skeletal muscles, of Lama2-/- mice. This result is similar to our previous study in which body-wide inactivation of Bax also both improved skeletal muscle function and delayed paralysis.
Lama2-/- mice are one of a growing number of mouse models of human neuromuscular diseases, including oculopharyngeal muscular dystrophy and spinal muscular atrophy (SMA), in which apoptosis is important to pathology and anti-apoptotic therapies can ameliorate pathology (12, 31-35). On the other hand, overexpression of apoptosis inhibiting proteins, either ARC or Bcl-2, in dystrophin-deficient mdx mouse muscles did not ameliorate pathology (7, 36). Though apoptosis may thus be more important to pathogenesis in some neuromuscular diseases than in others, it still appears that a single anti-apoptosis therapy might prove to be useful in multiple neuromuscular diseases. Indeed, Bax inactivation is effective in both SMA and Lama2-/- mice, and doxycycline is effective in both OPMD and Lama2-/- mice (6, 15, 32, this study).
Doxycycline and related molecules produce a large number of molecular and cellular changes in mammals (16, 37), and we found changes in doxycycline-treated Lama2-/- mice that likely contributed to disease amelioration. For example, doxycycline lessened inflammation in Lama2-/- muscles; and a previous study (38) showed that inactivation of complement or treatment with oral prednisolone improved muscle strength in Lama2-/- mice. Whether this decrease in inflammation was due to a direct effect of doxycycline on inflammatory cells or was indirectly due to decreased inflammatory signals from healthier muscles (39) remains to be determined. Our culture studies showed that doxycycline can act directly on myogenic cells to inhibit apoptosis, consistent with the possibility that myofibers are targets of doxycycline in vivo.
We also found increased Akt phosphorylation that was consistent with an anti-apoptotic effect in muscles of doxycycline-treated Lama2-/- mice. An earlier study, for example, showed that activation of Akt inhibits the apoptosis of cultured myotubes that is induced by disrupting laminin-α2 binding to dystroglycan (30). We found that doxycycline specifically increased the amount of phosphoThr308-Akt in Lama2-/- muscles, but had little further effect on total Akt or phosphoSer473-Akt, both of which were already elevated. Even though phosphorylation of both sites is necessary for full activation, phosphorylation of only Thr308 is sufficient to increase Akt activity, whereas phosphorylation of only Ser473 is not (40). Phosphorylation of Thr308 is mediated by 3′-phosphoinositide-dependent kinase 1 (41). Differential phosphorylation of Thr308 and Ser473 in the brain in response to hypoglycemia also suggested that phosphorylation of Thr308 is anti-apoptotic (42). Our finding that doxycycline decreases the levels of Bax, TUNEL-positive myofiber nuclei, and activated caspase-3 in muscles of Lama2-/- mice is also consistent with an anti-apoptotic effect of doxycycline.
An important remaining question is whether doxycycline or similar therapies will remain effective if started later in life. A recent study found that expression of agrin after disease progression had begun still lessened pathology in laminin-α2-deficient mice (24). Further work, which was beyond the scope of the present study, is also necessary to identify the important direct targets through which doxycycline acts to ameliorate pathology in Lama2-/- mice.
In addition to doxycycline and genetic inhibition of apoptosis, previous studies have shown that muscle pathology in Lama2-/- mice can be prevented by transgenic expression of laminin-α1, laminin-α2, or a modified agrin protein that can replace laminin (4, 5, 18). Partial amelioration of one or more aspects of laminin-α2-deficiency in mice is also produced by feeding a 50% protein diet, inactivating the complement system, or by administering insulin-like growth factor 1, clenbuterol, or prednisolone (26, 43 - 45). Whether these treatments reduce pathology through inhibition of apoptosis, another mechanism, or some combination of mechanisms remains to be determined.
Though doxycycline significantly improved outcomes in Lama2-/- mice, we think it is unlikely that our results represent the best possible outcome to be expected from a pharmacological therapy. The many effects attributed to doxycycline in mammals (16, 37) raises the possibility that the positive outcomes are limited by counterbalancing negative effects. For example, doxycycline and minocycline appear to inhibit angiogenesis in an in vitro model, perhaps through inhibition of matrix metalloproteinases (46), but successful angiogenesis is required for extensive repair of injured skeletal muscles (47). Whether another tetracycline derivative will be significantly better than minocycline or doxycycline at ameliorating pathology in Lama2-/- mice remains to be tested. The need for additional molecular understanding of this therapeutic approach is highlighted by two recent clinical trials in which (i) minocycline appeared to have an adverse affect in ALS patients (48), whereas (ii) doxycycline in combination with beta-interferon in a small, open-label trial appeared to improve outcomes in patients with multiple sclerosis (49). Additional pharmacological inhibitors of apoptosis, particularly those that are more specifically targeted such as caspase inhibitors, should be considered as candidates to ameliorate Lama2-/- disease. A pharmacological therapy that significantly slows disease progression in human MDC1A could be of considerable benefit.
Acknowledgments
We thank Dr. Eva Engvall (Burnham Institute, La Jolla CA) for providing Lama2+/- mice; Dr. Pierre Vachon (Université de Sherbrooke, Sherbrooke Québec Canada) for the laminin-α2-deficient B4.pLAM variant of the C2C12 cell line; and Dr. Ajay Kumar for quantitative PCR analyses. Supported by a Development Grant to M.G. from the Muscular Dystrophy Association of the U.S.A. and by grants to J.B.M. from the Muscular Dystrophy Association of the U.S.A. and the National Institutes of Health (AR049496; HL064641).
Footnotes
Conflicts of Interest The authors declare that they have no conflicts of interest.
References
- 1.Miyagoe-Suzuki Y, Nakagawa M, Takeda I. Merosin and congenital muscular dystrophy. Microsc Res Tech. 2000;48:181–191. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<181::AID-JEMT6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Allamand V, Guicheney P. Merosin-deficient congenital muscular dystrophy, autosomal recessive (MDC1A, MIM#156225, LAMA2 gene coding for alpha2 chain of laminin) Eur J Hum Genet. 2002;10:91–94. doi: 10.1038/sj.ejhg.5200743. [DOI] [PubMed] [Google Scholar]
- 3.Khurana TS, Davies KE. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- 4.Gawlik KI, Li JY, Petersen A, Durbeej M. Laminin alpha1 chain improves laminin alpha2 chain deficient peripheral neuropathy. Hum Mol Genet. 2006;15:2690–2700. doi: 10.1093/hmg/ddl201. [DOI] [PubMed] [Google Scholar]
- 5.Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient congenital muscular dystrophy: Partial genetic correction in two mouse models. J Clin Invest. 1999;102:844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girgenrath M, Dominov JA, Kostek CA, Miller JB. Inhibition of apoptosis improves outcome in a model of congenital muscular dystrophy. J Clin Invest. 2004;114:1635–1639. doi: 10.1172/JCI22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominov JA, Kravetz AJ, Ardelt M, Kostek CA, Beermann ML, Miller JB. Muscle-specific BCL2 expression ameliorates muscle disease in laminin-α2-deficient, but not dystrophin-deficient, mice. Hum Mol Gen. 2005;14:1029–1040. doi: 10.1093/hmg/ddi095. [DOI] [PubMed] [Google Scholar]
- 8.Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin laminin 2-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 9.Mukasa T, Momoi T, Momoi MY. Activation of caspase-3 apoptotic pathways in skeletal muscle fibers in laminin alpha2-deficient mice. Biochem Biophys Res Commun. 1999;260:139–142. doi: 10.1006/bbrc.1999.0829. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi YK, Tezak Z, Momoi T, Nonaka I, Garcia CA, Hoffman EP, Arahata K. Massive muscle cell degeneration in the early stage of merosin-deficient congenital muscular dystrophy. Neuromuscul Disord. 2001;11:350–359. doi: 10.1016/s0960-8966(00)00203-0. [DOI] [PubMed] [Google Scholar]
- 11.Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 12.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu du C, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 13.Pi R, Li W, Lee NT, Chan HH, Pu Y, Chan LN, Sucher NJ, Chang DC, Li M, Han Y. Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. J Neurochem. 2004;91:1219–1230. doi: 10.1111/j.1471-4159.2004.02796.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem. 2004;279:9948–9954. doi: 10.1074/jbc.M313629200. [DOI] [PubMed] [Google Scholar]
- 15.Davies JE, Wang L, Garcia-Oroz L, Cook LJ, Vacher C, O'Donovan DG, Rubinsztein DC. Doxycycline attenuates and delays toxicity of the oculopharyngeal muscular dystrophy mutation in transgenic mice. Nat Med. 2005;11:672–677. doi: 10.1038/nm1242. [DOI] [PubMed] [Google Scholar]
- 16.Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci USA. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girgenrath M, Kostek CA, Miller JB. Diseased muscles that lack dystrophin or laminin-alpha2 have altered compositions and proliferation of mononucleate cells. BMC Neurol. 2005;5(paper 7):1–10. doi: 10.1186/1471-2377-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moll J, Barzaghi P, Lin S, Bezakova G, Lochmüller H, Engvall E, Müller U, Ruegg MA. An agrin minigene rescues dystrophic symptoms in a mouse model for congenital muscular dystrophy. Nature. 2001;413:302–307. doi: 10.1038/35095054. [DOI] [PubMed] [Google Scholar]
- 19.Nowak JA, Malowitz J, Girgenrath M, Kostek CA, Kravetz AJ, Dominov JA, Miller JB. Immortalization of myogenic cells does not require loss of p16INK4a, p19ARF, or p53 and is accelerated by inactivation of Bax. BMC Cell Biol. 2004;5(paper 1):1–14. doi: 10.1186/1471-2121-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen MD, Weil M, Raff MC. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominov JA, Dunn JJ, Miller JB. Bcl-2 expression identifies an early stage of myogenesis and promotes clonal expansion of muscle cells. J Cell Biol. 1998;142:537–544. doi: 10.1083/jcb.142.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Q, Zhang Y, Uray IP, Hill JL, Kim HT, Lu C, Young MR, Gunther EJ, Hilsenbeck SG, Chodosh LA, Colburn NH, Brown PH. The AP-1 transcription factor regulates postnatal mammary gland development. Dev Biol. 2006;295:589–603. doi: 10.1016/j.ydbio.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa M, Miyagoe-Suzuki Y, Ikezoe K, Miyata Y, Nonaka I, Harii K, Takeda S. Schwann cell myelination occurred without basal lamina formation in laminin alpha2 chain-null mutant (dy3K/dy3K) mice. Glia. 2001;35:101–110. doi: 10.1002/glia.1075. [DOI] [PubMed] [Google Scholar]
- 24.Meinen S, Barzaghi P, Lin S, Lochmüller H, Ruegg MA. Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages. J Cell Biol. 2007;176:979–993. doi: 10.1083/jcb.200611152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang W, Xu H, Vilquin JT, Engvall E. Activation of the Lama2 gene in muscle regeneration: abortive regeneration in laminin alpha2-deficiency. Lab Invest. 1999;79:1601–1613. [PubMed] [Google Scholar]
- 26.Connolly AM, Keeling RM, Mehta S, Pestronk A, Sanes JR. Three mouse models of muscular dystrophy: the natural history of strength and fatigue in dystrophin-, dystrophin/utrophin-, and laminin alpha2-deficient mice. Neuromuscul Disord. 2001;11:703–712. doi: 10.1016/s0960-8966(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 27.Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li ZF, Shelton GD, Engvall E. Elimination of myostatin does not combat muscular dystrophy in dy mice but increases postnatal lethality. Am J Pathol. 2005;166:491–497. doi: 10.1016/S0002-9440(10)62271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jannapureddy SR, Patel ND, Hwang W, Boriek AM. Merosin deficiency leads to alterations in passive and active skeletal muscle mechanics. J Appl Physiol. 2003;94:2524–2533. doi: 10.1152/japplphysiol.01078.2002. [DOI] [PubMed] [Google Scholar]
- 30.Langenbach KJ, Rando TA. Inhibition of dystroglycan binding to laminin disrupts the PI3K/AKT pathway and survival signaling in muscle cells. Muscle Nerve. 2002;26:644–653. doi: 10.1002/mus.10258. [DOI] [PubMed] [Google Scholar]
- 31.Miller JB, Girgenrath M. Role of apoptosis in neuromuscular diseases and potential usefulness of anti-apoptosis therapy. Trends Mol Med. 2006;12:279–286. doi: 10.1016/j.molmed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Tsai MS, Chiu YT, Wang SH, Hsieh-Li HM, Lian WC, Li H. Abolishing Bax-dependent apoptosis shows beneficial effects on spinal muscular atrophy model mice. Mol Ther. 2006;13:1149–1155. doi: 10.1016/j.ymthe.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- 34.Friedlander RM, Brown RH, Gagliardini V, Wang J, Yuan J. Inhibition of ICE slows ALS in mice. Nature. 1997;388:31. doi: 10.1038/40299. [DOI] [PubMed] [Google Scholar]
- 35.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 36.Abmayr S, Crawford RW, Chamberlain JS. Characterization of ARC, apoptosis repressor interacting with CARD, in normal and dystrophin-deficient skeletal muscle. Hum Mol Genet. 2004;13:213–221. doi: 10.1093/hmg/ddh018. [DOI] [PubMed] [Google Scholar]
- 37.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Connolly AM, Keeling RM, Streif EM, Pestronk A, Mehta S. Complement 3 deficiency and oral prednisolone improve strength and prolong survival of laminin alpha2-deficient mice. J Neuroimmunol. 2002;127:80–87. doi: 10.1016/s0165-5728(02)00104-2. [DOI] [PubMed] [Google Scholar]
- 39.Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, Winkles JA, Michaelson JS, Allaire N, Schneider P, Scott M, Hsu YM, Yagita H, Flavell RA, Miller JB, Burkly LC, Zheng TS. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 2006;25:5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 41.Kondapaka SB, Zarnowski M, Yver DR, Sausville EA, Cushman SW. 7-hydroxystaurosporine (UCN-01) inhibition of Akt Thr308 but not Ser473 phosphorylation: a basis for decreased insulin-stimulated glucose transport. Clin Cancer Res. 2004;10:7192–7198. doi: 10.1158/1078-0432.CCR-04-0772. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang YB, Zhang XH, He QP, Wang GX, Siesjö BK, Hu BR. Differential phosphorylation at Ser473 and Thr308 of Akt-1 in rat brain following hypoglycemic coma. Brain Res. 2000;876:191–195. doi: 10.1016/s0006-8993(00)02618-4. [DOI] [PubMed] [Google Scholar]
- 43.Zdanowicz MM, Slonim AE, Bilaniuk I, O'Connor MM, Moyse J, Teichberg S. High protein diet has beneficial effects in murine muscular dystrophy. J Nutr. 1995;125:1150–1158. doi: 10.1093/jn/125.5.1150. [DOI] [PubMed] [Google Scholar]
- 44.Hayes A, Williams DA. Examining potential drug therapies for muscular dystrophy utilising the dy/dy mouse: I. Clenbuterol. J Neurol Sci. 1998;157:122–128. doi: 10.1016/s0022-510x(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 45.Lynch GS, Cuffe SA, Plant DR, Gregorevic P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul Disord. 2001;11:260–268. doi: 10.1016/s0960-8966(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 46.Yao JS, Shen F, Young WL, Yang GY. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem Int. 2007;50:524–530. doi: 10.1016/j.neuint.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messina S, Mazzeo A, Bitto A, Aguennouz M, Migliorato A, De Pasquale MG, Minutoli L, Altavilla D, Zentilin L, Giacca M, Squadrito F, Vita G. VEGF overexpression via adeno-associated virus gene transfer promotes skeletal muscle regeneration and enhances muscle function in mdx mice. FASEB J. 2007;21:3737–3746. doi: 10.1096/fj.07-8459com. [DOI] [PubMed] [Google Scholar]
- 48.Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R, Western ALS Study Group Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 49.Minagar A, Alexander JS, Schwendimann RN, Kelley RE, Gonzalez-Toledo E, Jimenez JJ, Mauro L, Jy W, Smith SJ. Combination Therapy With Interferon Beta-1a and Doxycycline in Multiple Sclerosis: An Open-Label Trial. Arch Neurol. 2008;65:199–204. doi: 10.1001/archneurol.2007.41. [DOI] [PubMed] [Google Scholar]