Abstract
A history of melanoma is associated with increased risks of Parkinson's disease (PD). We examined whether hair color, one of the most important phenotypes of pigmentation and a risk factor for melanoma, was associated with PD risk in the Health Professionals Follow-up Study (HPFS) (1986-2002) and the Nurses' Health Study (NHS) (1980-2002). We included 38,641 men and 93,661 women, free of PD at baseline. Information on natural hair color in early adulthood (age 18-21 years) was assessed via a questionnaire. We also conducted a case-control study (298 PD cases) nested in these two cohorts to examine the association between the melanocortin1-receptor (MC1R) Arg151Cys polymorphism and PD risk. Relative risks (RRs) were estimated using Cox proportional hazards models in the cohort analyses and conditional logistic regression in the nested case-control study. Log RRs from the two cohorts were pooled using a fixed-effects model. PD risk increased with decreasing darkness of hair color. Pooled RRs for PD were 1(ref.), 1.40, 1.61, and 1.93 (95% CI: 1.1, 3.4) for black, brown, blonde, and red hair, after adjusting for age, smoking, ethnicity and other covariates. The associations between hair color and PD were particularly strong for younger onset of PD (<70 y) (adjusted RR for red vs. black hair=3.83; 95% CI: 1.7, 8.7). In the case-control study, participants with Cys/Cys genotype, which was associated with red hair, had a higher PD risk, relative to the Arg/Arg genotype (adjusted RR=3.15; 95% CI: 1.1, 9.4). These findings suggest a potential role of pigmentation in PD.
An increased risk of Parkinson's disease (PD) among individuals with melanoma has been reported in a few studies, 1-4 but not all.5 Conversely, individuals with PD seem to have an increased risk of melanoma,6 and questions have been raised over whether the association was, at one point, attributed to an adverse effect of PD medicines.7-9
Hair color, one of the most important phenotypes of pigmentation, is largely determined by the quantity, quality, and distribution of the melanin and individuals with red hair have an approximately three-fold higher risk of melanoma than those with black hair. 10, 11 We, therefore, examined the relationship between hair color and PD risk among 131,821 US men and women who participated in the two large ongoing prospective cohorts, the Health Professionals Follow-up Study (HPFS) and the Nurses' Health Study (NHS). In a secondary analysis, we examined the associations between the melanocortin 1 receptor (MC1R) Arg151Cys polymorphism, an important genetic determinant of hair color and risk factor for melanoma, 10, 12-18 and PD risk in a prospective case-control study nested within the HPFS and NHS cohorts.
Subjects and Methods
Study population
The HPFS was established in 1986, when 51,529 male US health professionals (dentists, optometrists, osteopaths, podiatrists, pharmacists, and veterinarians) aged 40-75 completed a mailed questionnaire regarding their medical history and lifestyle. The NHS cohort was established in 1976, when 121,700 female registered nurses responded to a similar questionnaire. The overall response rate is greater than 94% in the HPFS and the NHS follow-up has been 95% of potential person-years in the overall cohort.
Information on natural hair color in the HPFS was assessed in 1988 with the question “Which of the following most closely describes the nature color of your hair at age 18?”, with five possible responses: black, dark brown, light brown, blonde, or red. A similar question on natural hair color at age 21 was asked in the NHS in 1982.
Dietary intakes were assessed every four years with validated semi-quantitative food frequency questionnaires beginning in 1986 in the HFPS and 1980 in the NHS. 19, 20 Information on age, ethnicity, weight, height, smoking status, and use of non-aspirin non-steroid anti-inflammatory drugs was collected through biennial questionnaires. Body mass index (BMI) was calculated as weight (kg) / height (m) 2. We also asked the major ancestry in 1986 for the HPFS and 1992 for the NHS. Possible responses include southern European, Scandinavian, other Caucasian, African-American, Asian, and others.
In the current study, we used 1986 as baseline for the HFPS and 1980 for the NHS. Participants who had been previously diagnosed with PD or those who did not report hair color information were excluded, leaving 38,641 men and 93,661 women for further analyses. Both studies were approved by the Human Research Committees at the Harvard School of Public Health and the Brigham and Women's Hospital.
Ascertainment of PD
We identified new PD cases by biennial self-reported questionnaires.21, 22 We then asked the treating neurologists to complete a questionnaire to confirm the diagnosis of PD or to send a copy of the medical records. A case was confirmed if a diagnosis of PD was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of PD made by a neurologist, or evidence of at least two of the three cardinal signs (rest tremor, rigidity, bradykinesia) in the absence of features suggesting other diagnoses. The review of medical records was conducted by the investigators, blind to the exposure status. Overall, the diagnosis was confirmed by the treating neurologist in 81% of the cases, by the treating internist without further support in the remaining 16%, and by review of the medical records in 3%. We also requested the death certificates of the deceased study participants and identified PD diagnoses that were not reported in the regular follow-up (less than 2 percent). If Parkinson's disease was listed as a cause of death on the death certificate, we requested permission from the family to contact the treating neurologist or physician and followed the same procedure as for the non-fatal cases. In this analysis, only definite and probable PD cases were included as in our previous studies. 22-24
Nested case-control study
We collected blood samples from 32,825 women in the NHS in 1989-90 and 18,018 samples from men in the HPFS during 1993-95. We have been using the “swish and spit” method with mouthwash to collect cheek cells from participants who did not provide blood samples in the NHS and HPFS since 2001.
Men and women with both a PD diagnosis and an available DNA sample were included as cases in this study. For each case, up to six controls were matched by cohort (and thus gender), birth year, and source of DNA (blood for 235 PD cases, and cheek smear for 63). Controls were randomly selected among cohort participants at risk of developing PD at the time of diagnosis of the matched case.
We used Taqman to genotype the Arg151Cys polymorphism which is the SNP most consistently associated with melanoma risk in previous studies.10, 12-18 This polymorphism is a loss of function variant resulting in an overproduction of pheomelanin and, consequently, red hair. Laboratory personnel were blinded to case-control status and blinded quality control samples were inserted to validate genotyping procedures; concordance for the blinded samples was 100%. A total of 298 cases and 1289 controls were available for genotyping. Of these, 272 cases and 1185 controls were successful genotyped and included in the current analyses.
Statistical analysis
In the cohort analyses, we computed the person-time of follow-up for each participant from the return date of the baseline questionnaire (1986 for the HPFS and 1980 for the NHS) to the date of the occurrence of the first symptoms of PD, the date of death, or the end of follow up (2002), whichever came first. Natural hair color at age 18-21 was categorized into four categories (black, brown, blonde, and red) and relative risks (RRs) were calculated by dividing the incidence rate in each category by the rate in the category of black hair color in each cohort. Multivariate adjusted RRs were derived from Cox proportional hazard models controlling for age (in months), smoking status (never smoker, past smoker, or current smoker: cigarettes/d, 1-14 or ≥ 15), ethnicity (Caucasian, African-American, and Asian and others), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), use of non-steroid anti-inflammatory drug (yes/no), and intake of alcohol(none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), caffeine (quintiles), and lactose (quintiles). Log RR from the two cohorts were pooled by a fixed-effects model, weighted by the inverse of their variances as significance tests did not suggest heterogeneity between cohorts (P>0.1). 25
We also examined potential interactions between hair color and age of onset, smoking status (never vs. ever), and caffeine intake (based on median intake) by adding multiplicative terms in the Cox models, adjusting for other potential confounders. To test robustness of our results, we conducted a sensitivity analysis by restricting to PD cases diagnosed by neurologists.
In the nested case-control study, we used conditional logistics regression models to estimate RRs of PD associated with the MC1R Arg151Cys variant and their 95% CIs. In addition to conditioning on the matching factors (sex and age), we further adjusted for other potential cofounders mentioned above. We used a χ2 test to assess whether the MC1R genotypes were in Hardy-Weinberg equilibrium among the controls. The SAS statistical package (version 9: SAS Institute, Cary, NC) was used for all analyses. All statistical tests are two-sided.
Results
We documented 264 PD cases in men and 275 in women during 16-22 years of follow-up. Participants with red hair were more likely to be Whites, to smoke, and consume larger amounts of alcohol and lactose, than those with black hair color (Table 1), but no differences were observed with respect to other covariates.
Table 1. Baseline characteristics according to reported hair color in the Health Professionals Follow-up Study (1986) and the Nurses' Health Study (1980).
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Hair color | Hair color | |||||||
| Black | Brown | Blonde | Red | Black | Brown | Blonde | Red | |
| n | 4401 | 28966 | 4220 | 1054 | 3979 | 75072 | 10703 | 3907 |
| Cases # | 26 | 191 | 37 | 10 | 7 | 227 | 25 | 16 |
| Age, y | 55.9 | 54.0 | 54.5 | 55.5 | 43.8 | 43.5 | 44.7 | 44.5 |
| African, % | 4.3 | 0.3 | 0 | 0 | 14.0 | 0.7 | 0 | 0.01 |
| Asian & other ethnicity, % | 14.5 | 1.6 | 1.3 | 1.5 | 16.7 | 1.5 | 1.1 | 1.1 |
| Current smokers, % | 8.0 | 8.5 | 10.1 | 9.1 | 25.6 | 27.4 | 28.3 | 29.6 |
| Past smokers, % | 40.9 | 42.0 | 42.6 | 42.7 | 24.4 | 27.6 | 27.5 | 28.2 |
| BMI, kg/m2 | 25.4 | 25.4 | 25.4 | 25.4 | 24.6 | 24.4 | 24.2 | 24.4 |
| Alcohol intake, g/d | 9.7 | 11.5 | 12.8 | 11.2 | 5.0 | 6.3 | 7.0 | 6.4 |
| Caffeine intake, mg/d | 230 | 238 | 246 | 231 | 368 | 396 | 401 | 397 |
| Lactose intake, g/d | 12.7 | 14.6 | 15.2 | 14.9 | 11.9 | 13.7 | 13.8 | 13.8 |
| Use of non-aspirin non-steroidal anti-inflammatory, % | 4.7 | 5.5 | 5.7 | 6.8 | 5.3 | 5.2 | 5.2 | 4.7 |
Values were standardized to the age distribution of the overall cohort.
Risk of PD increased monotonically with decreasing darkness of hair color (Table 2). When we further adjusted for ethnicity, the associations were attenuated but blonde (RR=1.61, 95% CI: 1.03, 2.51) and red hair color (RR=1.93, 95% CI: 1.08, 3.43) remained significantly associated with a higher PD risk, relative to those with black hair. Further adjustment for major ancestry of participants (southern European, Scandinavian, other Caucasian, African-American, and Asian and others) did not materially change these associations (data not shown).
Table 2. Relative risk of Parkinson's disease according to hair color in the Health Professional Follow-up Study (1986-2002) and the Nurses' Health Study (1980-2002).
| Hair color | |||||
|---|---|---|---|---|---|
| Black | Brown | Blonde | Red | P-trend | |
| Case # | 33 | 418 | 62 | 26 | |
| Person-years | 162806 | 2211838 | 313604 | 107353 | |
| Age- and Smoking-adjusted | 1(ref) | 1.48 (1.03,2.13) |
1.70 (1.10,2.62) |
2.08 (1.18,3.67) |
0.03 |
| Multivariate 1 | 1(ref) | 1.48 (1.03,2.13) |
1.69 (1.09,2.62) |
2.04 (1.16,3.61) |
0.03 |
| Further adjusting for ethnicity | 1(ref) | 1.40 (0.97,2.03) |
1.61 (1.03,2.51) |
1.93 (1.08, 3.43) |
0.06 |
Adjusted for sex, age (in month), smoking(never smoker, past smoker, or current smoker: cigarettes/d, 1-14 or ≥ 15), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), use of non-steroid anti-inflammatory drug (yes/no), and intakes of alcohol(none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), caffeine (quintiles), and lactose (quintiles).
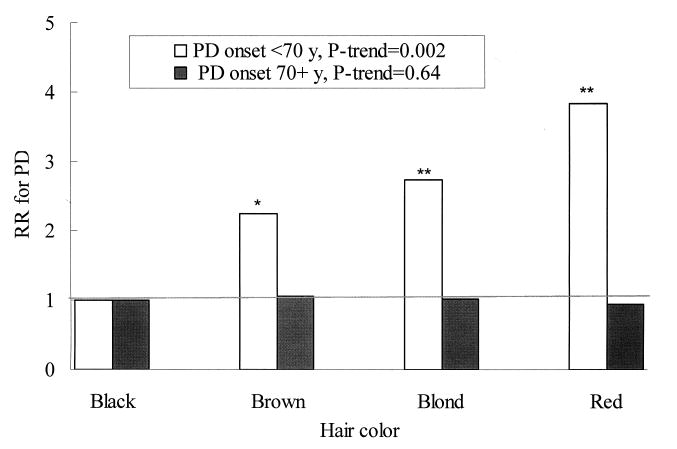
We found a significant interaction between hair color and age of onset of PD (P for interaction=0.01). The adjusted RRs for risk of PD onset <70 y (approximate median value of age of onset) were 1(ref.), 2.25, 2.73, and 3.83 (95% CI: 1.7, 8.7; P=0.001) for black, brown, blonde, and red hair color, respectively, after adjusting for ethnicity and other covariates (Figure 1). In contrast, hair color was not significantly associated with risk of PD onset at 70+ years. We did not find significant interactions between hair color and smoking, or caffeine intake on PD risk (P for interaction > 0.5 for both).
Figure 1.
Hair color and relative risk for Parkinson's disease onset < 70 or ≥ 70 years (approximate median value of age of onset). Adjusted for sex, age (in month), smoking(never smoker, past smoker, or current smoker: cigarettes/d, 1-14 or ≥ 15), ethnicity (Caucasian, African-American, and Asian and others), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), use of non-steroid anti-inflammatory drug (yes/no), and intakes of alcohol(none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), caffeine (quintiles), and lactose (quintiles). * P<0.05, ** P<0.01, relative to black hair. P for interaction=0.01
In the sensitivity analysis, we saw similar associations between hair color and PD risk when restricting to the PD cases diagnosed by neurologists. The RRs across categories of hair color were 1.0(ref.), 1.43, 1.49, and 2.05 (95%CI: 1.10, 3.81), after adjusting for ethnicity and other covariates. Restricting analyses to Caucasians generated similar associations between hair color and PD risk; the adjusted RRs across categories of hair color were 1.0(ref.), 1.32, 1.55, and 1.85(95%CI: 1.03, 3.34).
In the nested case-control study, the genotype distributions of the MC1R Arg151Cys were in Hardy-Weinberg equilibrium among controls (P=0.19). We observed that MC1R Cys/Cys variant, which leads to loss-of-function of the receptors, was significantly associated with red hair color; the RR comparing Cys/Cys homozygotes with Arg/Arg homozygotes was 57 (95% CI: 7.3, 457). The Cys/Cys genotype was associated with a higher PD risk, relative to the Arg/Arg genotype (adjusted RR=3.15; 95% CI: 1.06, 9.37) (Table 3).
Table 3. MC1R Arg151Cys polymorphism and PD risk.
| Genotype of Arg151Cys | ||||
|---|---|---|---|---|
| Arg/Arg | Arg/Cys | Cys/Cys | P-trend | |
| Case/control | 224/1015 | 42/160 | 6/10 | |
| Crude Relative risk | 1 (ref) | 1.19 (0.82,1.72) |
2.55 (0.91, 7.12) |
0.11 |
| Multivariate1 | 1(ref) | 1.23 (0.84,1.82) |
3.15 (1.06, 9.37) |
0.06 |
| Red hair 1 | P-interaction | |||
| No | 1(ref) | 1.25 (0.81, 1.92) |
-- | 0.23 |
| Yes | 0.67 (0.19, 2.32) |
0.95 (0.30, 2.97) |
4.93 (1.50, 16.2) |
|
Conditional logistic regressions were used to estimate relative risks.
Adjusted for smoking(never smoker, past smoker, or current smoker: cigarettes/d, 1-14 or ≥ 15), ethnicity (Caucasian, African-American, and Asian and others), BMI (<23, 23-24.9, 25-26.9, 27-29.9, or ≥30 kg/m2), use of non-steroid anti-inflammatory drug (yes/no), and intakes of alcohol(none, 1-4.9, 5-9.9, 10-14.9, or ≥15 g/d for women; none, 1-9.9, 10-19.9, 20-29.9, or ≥30 g/d for men), caffeine (quintiles), and lactose (quintiles).
We further examined the combined effects of red hair color and the MC1R Arg151Cys polymorphism. Participants with red hair plus carrying Cys/Cys variant were more than four times (RR=4.9, 95% CI: 1.5, 16.2) more likely to have PD relative to those who did not have red hair and did not carry the Cys allele (Table 3). The interaction between Arg151Cys polymorphism and red hair was not significant (P=0.23).
Discussion
In this prospective study including 132,302 men and women, we found that lighter hair color was associated with a higher PD risk; participants with red hair had approximately a two-fold higher risk of PD relative to those with black hair. The observed associations were independent of ethnicity, smoking, caffeine intake, and other known risk factors for PD and the associations were particularly strong for younger onset of PD (<70 y). We also found that participants homozygous for the MC1R Arg151Cys variant allele had a significantly increased risk of PD.
Our findings suggest that the higher frequency of melanoma occurring among individuals with PD may be at least in part explained by the associations of both diseases with a lighter hair color and the MC1R Arg151Cys variant allele, rather than being an adverse effect of levodopa treatment as previously suggested. 7, 8 In a recent prospective case-control study including 8090 PD cases based on the national Danish Hospital Register, Olsen et al found an increased prevalence of melanoma before a diagnosis of PD 1. The OR was 1.44 (95% CI: 1.0, 2.0) for developing PD relative to population-based controls.1 In the PD patients who participated in the DATATOP (deprenyl and tocopherol antioxidative therapy of parkinsonism) trial, the observed incidence of melanoma during ∼4.5 years of follow-up was significantly higher than expected in the general population, with a standardized event ratio of 3.3 (95%CI: 1.1, 7.8) and the increased incidence of melanoma was not associated with levodopa therapy.2 In a case-control study based on the Rochester Epidemiology Project (PD case =196), Elbaz et al reported that PD patients tended to have a higher risk of melanoma (OR=1.5, 95% CI: 0.3, 9.0).4 However, most of these studies did not control for ethnicity and other ethnicity-relevant dietary habits and lifestyle factors, which may confound the observed melanoma-PD associations.
The exact mechanisms underlying the increased risk of PD associated with light hair or MC1R gene variants is not known. However, it is plausible that pigmentation metabolism, and genes that encode these proteins, may be involved in the pathogenesis of PD. Melanin, like dopamine, is synthesized from the amino acid tyrosine. A major pathological feature of PD is an abnormal loss of neuromelanin-containing cells within the substantia nigra. Neuromelanin has a chemical structure similar to that of melanin. A large body of evidence suggests that neuromelanin could be neuroprotective by scavenging redox active metals (e.g., iron), toxic metals (e.g., mercury and lead), and organic toxic compounds(e.g., pesticides).26 However, neuromelanin synthesis could be regulated by different enzymes than those in peripheral melanogenesis. 27 Alternatively, skin melanin could have a protective role by binding environmental toxins.28
Melanin is a mixture of polymers with different physicochemical properties.29 Mammalian melanocytes can produce two basic types of melanin, brown/black eumelanin and yellow/red pheomelanin. Hair color is one of most important phenotype of pigmentation, with a recessive trait.30 The MC1R gene encodes a 317-amino acid seven-pass-transmembrane G protein coupled receptor.30 Homozygote or compound heterozygote variant MC1R genotype carriers are generally red haired because of overproduction of pheomelanin.30, 31
Difference in pigmentation also reflects differences in ethnicity as well as ancestry. This could explain partially the observed relationship between hair color, MC1R polymorphism and PD risk. Africans and Asians carry less MC1R variants responsible for red hair and in some studies, 32-34 but not all,35, 36 Africans or Asians were found to have a lower PD prevalence than Caucasians. However, the difference in PD prevalence could be due to ascertainment biases or differences in demographic structure. 34, 36 Two studies, 37, 38 which compared PD incident rates among different ethnic groups living in the same region, generated conflicting results. Among members of the Kaiser Permanente Medical Care Program of Northern California, Van Den Eeden et al. found that PD incidence was lower in African Americans compared with non-Hispanics Whites and Hispanics, 37 whereas Mayeux et al. reported that Blacks had a significant higher incident rate of PD relative to Whites living in Northern Manhattan, 38 although the period of follow-up (three years) of the latter study was rather short to obtain stable estimates of PD incidence. Even among individuals of the same race, however, a lighter skin color was associated with a higher PD risk.39 In a case-control study including 509 newly diagnosed PD in Northern California, Tanner et al found that darker skin color was inversely associated with PD risk among Caucasians (OR=0.46; 95% CI: 0.28, 0.78) and African-Americans (OR=0.60; 95% CI: 0.38, 0.94).39 Although the association between hair color and PD risk remained significant after further controlling for ethnicity and major ancestry, a possibility of residual confounding cannot be ruled out.
Alternately, the MC1R gene variants could also contribute excessive PD via mechanisms other than its effects on pigmentation. For example, α-melanocyte-stimulating hormone (α-MSH), the MC1R ligand, has anti-inflammatary effects in the brain and immune modulating effects. 40, 41 It reduces NFκB activity and therefore, down-regulates production of nitric oxide, oxygen peroxide, and several cytokines, such as IL-6.40, 41 These have been suggested to have an unfavorable role in PD.42-44 MC1R has been detected in human periaqueductal gray matter,45 and the MC1R red-hair variants have been associated with a higher risk and severity of multiple sclerosis.46 Administration of α-MSH (intraperitoneally) has also been shown to prevent damage in brainstem ischemia and rescues neurons from excitotoxic cell death induced by kainic acid.47-49 These studies, together with our findings, suggest that MC1R or related genes could have a role in neurodegenerative diseases.
We observed a stronger association between hair color and PD risk for relative younger onset of PD (<70 y, based on median value) than those with age of onset greater than 70 years. This observation suggests a possible important role of pigmentation genes in the etiology of PD. However, we were not able to examine whether hair color is associated with early-onset PD (<50 y), which has been shown to be strongly associated with genetic factors.50
A limitation of our study is reliance on self-reported hair color, which could introduce non-differential misclassification and attenuate the association between hair color and PD risk. Although known PD risk factors were adjusted in our analysis, we cannot exclude the possibility of confounding by unknown risk factors. Also, the clinical diagnosis of PD is not perfect, and some degree of diagnostic error is thus likely. In a large clinicopathological study, however, the positive predictive value of a clinical diagnosis of PD has been found to be 90% or higher,51 and bias from this source is thus likely to be modest. We observed a similar significant association between hair color and PD risk when we restricted to PD cases diagnosed by neurologists.
In summary, we found that individuals with light hair and those homozygous for the MC1R Arg151Cys allele of Cys have an increased risk of PD. Our findings suggest a potential important role of pigmentation or ethnicity in PD, and may in part explain the higher than expected co-occurrence of PD and melanoma.
Acknowledgments
The study was supported by NIH/NINDS grant R01 NS048517, and Parkinson Study Group Mentored Clinical Research Award. None of the sponsors participated in the design of this study or in the collection, analysis, or interpretation of the data. None of the authors had a financial conflict of interest in relation to this study.
References
- 1.Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology. 2006;17:582–587. doi: 10.1097/01.ede.0000229445.90471.5e. [DOI] [PubMed] [Google Scholar]
- 2.Constantinescu R, Romer M, Kieburtz K. Malignant melanoma in early Parkinson's disease: the DATATOP trial. Mov Disord. 2007;22:720–722. doi: 10.1002/mds.21273. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira J, Silva JM, Freire R, et al. Skin cancers and precancerous lesions in Parkinson's disease patients. Mov Disord. 2007;22:1471–1475. doi: 10.1002/mds.21575. [DOI] [PubMed] [Google Scholar]
- 4.Elbaz A, Peterson BJ, Yang P, et al. Nonfatal cancer preceding Parkinson's disease: a case-control study. Epidemiology. 2002;13:157–164. doi: 10.1097/00001648-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 5.D'Amelio M, Ragonese P, Morgante L, et al. Tumor diagnosis preceding Parkinson's disease: a case-control study. Mov Disord. 2004;19:807–811. doi: 10.1002/mds.20123. [DOI] [PubMed] [Google Scholar]
- 6.Jansson B, Jankovic J. Low cancer rates among patients with Parkinson's disease. Ann Neurol. 1985;17:505–509. doi: 10.1002/ana.410170514. [DOI] [PubMed] [Google Scholar]
- 7.Kochar AS. Development of malignant melanoma after levodopa therapy for Parkinson's disease. Report of a case and review of the literature. Am J Med. 1985;79:119–121. doi: 10.1016/0002-9343(85)90555-8. [DOI] [PubMed] [Google Scholar]
- 8.Skibba JL, Pinckley J, Gilbert EF, Johnson RO. Multiple primary melanoma following administration of levodopa. Arch Pathol. 1972;93:556–561. [PubMed] [Google Scholar]
- 9.Zanetti R, Rosso S. Levodopa and the risk of melanoma. Lancet. 2007;369:257–258. doi: 10.1016/S0140-6736(07)60125-1. [DOI] [PubMed] [Google Scholar]
- 10.Han J, Kraft P, Colditz GA, et al. Melanocortin 1 receptor variants and skin cancer risk. Int J Cancer. 2006;119:1976–1984. doi: 10.1002/ijc.22074. [DOI] [PubMed] [Google Scholar]
- 11.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Palmer JS, Duffy DL, Box NF, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66:176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy C, ter Huurne J, Berkhout M, et al. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 14.Landi MT, Kanetsky PA, Tsang S, et al. MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population. J Natl Cancer Inst. 2005;97:998–1007. doi: 10.1093/jnci/dji176. [DOI] [PubMed] [Google Scholar]
- 15.Matichard E, Verpillat P, Meziani R, et al. Melanocortin 1 receptor (MC1R) gene variants may increase the risk of melanoma in France independently of clinical risk factors and UV exposure. J Med Genet. 2004;41:e13. doi: 10.1136/jmg.2003.011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Box NF, Duffy DL, Irving RE, et al. Melanocortin-1 receptor genotype is a risk factor for basal and squamous cell carcinoma. J Invest Dermatol. 2001;116:224–229. doi: 10.1046/j.1523-1747.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer T, Stankovich JM, Blizzard L, et al. Does the addition of information on genotype improve prediction of the risk of melanoma and nonmelanoma skin cancer beyond that obtained from skin phenotype? Am J Epidemiol. 2004;159:826–833. doi: 10.1093/aje/kwh120. [DOI] [PubMed] [Google Scholar]
- 18.van der Velden PA, Sandkuijl LA, Bergman W, et al. Melanocortin-1 receptor variant R151C modifies melanoma risk in Dutch families with melanoma. Am J Hum Genet. 2001;69:774–779. doi: 10.1086/323411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho E, Rosner BA, Feskanich D, Colditz GA. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol. 2005;23:2669–2675. doi: 10.1200/JCO.2005.11.108. [DOI] [PubMed] [Google Scholar]
- 20.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 21.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 22.Gao X, Chen H, Choi HK, et al. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol. 2008;167:831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86:1486–1494. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Chen H, Schwarzschild MA, et al. Erectile function and risk of Parkinson's disease. Am J Epidemiol. 2007;166:1446–1450. doi: 10.1093/aje/kwm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Zecca L, Tampellini D, Gerlach M, et al. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol Pathol. 2001;54:414–418. [PMC free article] [PubMed] [Google Scholar]
- 27.Fedorow H, Tribl F, Halliday G, et al. Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease. Prog Neurobiol. 2005;75:109–124. doi: 10.1016/j.pneurobio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Lerner MR, Goldman RS. Skin colour, MPTP, and Parkinson's disease. Lancet. 1987;2:212. doi: 10.1016/s0140-6736(87)90786-0. [DOI] [PubMed] [Google Scholar]
- 29.Conti DV, Cortessis V, Molitor J, Thomas DC. Bayesian modeling of complex metabolic pathways. Hum Hered. 2003;56:83–93. doi: 10.1159/000073736. [DOI] [PubMed] [Google Scholar]
- 30.Schaffer JV, Bolognia JL. The melanocortin-1 receptor: red hair and beyond. Arch Dermatol. 2001;137:1477–1485. doi: 10.1001/archderm.137.11.1477. [DOI] [PubMed] [Google Scholar]
- 31.Leonard JH, Marks LH, Chen W, et al. Screening of human primary melanocytes of defined melanocortin-1 receptor genotype: pigmentation marker, ultrastructural and UV-survival studies. Pigment Cell Res. 2003;16:198–207. doi: 10.1034/j.1600-0749.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZX, Roman GC. Worldwide occurrence of Parkinson's disease: an updated review. Neuroepidemiology. 1993;12:195–208. doi: 10.1159/000110318. [DOI] [PubMed] [Google Scholar]
- 33.McInerney-Leo A, Gwinn-Hardy K, Nussbaum RL. Prevalence of Parkinson's disease in populations of African ancestry: a review. J Natl Med Assoc. 2004;96:974–979. [PMC free article] [PubMed] [Google Scholar]
- 34.Okubadejo NU, Bower JH, Rocca WA, Maraganore DM. Parkinson's disease in Africa: A systematic review of epidemiologic and genetic studies. Mov Disord. 2006;21:2150–2156. doi: 10.1002/mds.21153. [DOI] [PubMed] [Google Scholar]
- 35.Tan LC, Venketasubramanian N, Jamora RD, Heng D. Incidence of Parkinson's disease in Singapore. Parkinsonism Relat Disord. 2007;13:40–43. doi: 10.1016/j.parkreldis.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Zhang ZX, Roman GC, Hong Z, et al. Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet. 2005;365:595–597. doi: 10.1016/S0140-6736(05)17909-4. [DOI] [PubMed] [Google Scholar]
- 37.Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 38.Mayeux R, Marder K, Cote LJ, et al. The frequency of idiopathic Parkinson's disease by age, ethnic group, and sex in northern Manhattan, 1988-1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 39.Tanner CM, Nelson LM, Van Den Eeden SK, et al. Skin Pigmentation and Risk of Parkinson's disease (abstract) Neurology. 1999;52:S540. [Google Scholar]
- 40.Maaser C, Kannengiesser K, Kucharzik T. Role of the melanocortin system in inflammation. Ann N Y Acad Sci. 2006;1072:123–134. doi: 10.1196/annals.1326.016. [DOI] [PubMed] [Google Scholar]
- 41.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Dikshit M. Apoptotic neuronal death in Parkinson's disease: involvement of nitric oxide. Brain Res Rev. 2007;54:233–250. doi: 10.1016/j.brainresrev.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Dawson VL, Dawson TM. Role of nitric oxide in Parkinson's disease. Pharmacol Ther. 2006;109:33–41. doi: 10.1016/j.pharmthera.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, O'Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol. 2008;167:90–95. doi: 10.1093/aje/kwm260. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, Wikberg JE, Chhajlani V. Expression of melanocortin 1 receptor in periaqueductal gray matter. Neuroreport. 1995;6:2193–2196. doi: 10.1097/00001756-199511000-00022. [DOI] [PubMed] [Google Scholar]
- 46.Partridge JM, Weatherby SJ, Woolmore JA, et al. Susceptibility and outcome in MS: associations with polymorphisms in pigmentation-related genes. Neurology. 2004;62:2323–2325. doi: 10.1212/wnl.62.12.2323. [DOI] [PubMed] [Google Scholar]
- 47.Forslin Aronsson A, Spulber S, Oprica M, et al. Alpha-MSH rescues neurons from excitotoxic cell death. J Mol Neurosci. 2007;33:239–251. doi: 10.1007/s12031-007-0019-2. [DOI] [PubMed] [Google Scholar]
- 48.Forslin Aronsson S, Spulber S, Popescu LM, et al. alpha-Melanocyte-stimulating hormone is neuroprotective in rat global cerebral ischemia. Neuropeptides. 2006;40:65–75. doi: 10.1016/j.npep.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Huang Q, Tatro JB. Alpha-melanocyte stimulating hormone suppresses intracerebral tumor necrosis factor-alpha and interleukin-1beta gene expression following transient cerebral ischemia in mice. Neurosci Lett. 2002;334:186–190. doi: 10.1016/s0304-3940(02)01088-1. [DOI] [PubMed] [Google Scholar]
- 50.Gasser T. Genetics of Parkinson's disease. J Neurol. 2001;248:833–840. doi: 10.1007/s004150170066. [DOI] [PubMed] [Google Scholar]
- 51.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson's disease. Neurology. 2001;57:1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]