Abstract
Atrial fibrillation (AF) causes substantial morbidity and mortality. It may be triggered and sustained by either reentrant or nonreentrant electrical activity. Human atrial cellular refractory period is shortened in chronic AF, likely aiding reentry. The ionic and molecular mechanisms are not fully understood and may include increased inward rectifier K+ current and altered Ca2+ handling. Heart failure, a major cause of AF, may involve arrhythmogenic atrial electrical remodeling, but the pattern is unclear in humans. Beta-blocker therapy prolongs atrial cell refractory period; a potentially antiarrhythmic influence, but the ionic and molecular mechanisms are unclear. The search for drugs to suppress AF without causing ventricular arrhythmias has been aided by basic studies of cellular mechanisms of AF. It remains to be seen whether such drugs will improve patient treatment.
Keywords: Arrhythmias (mechanisms), Atrial fibrillation, Beta-blocker, Electrical remodeling, Heart failure, Ion current, Refractory period, Transmembrane action potential
Electrophysiological mechanisms of human atrial fibrillation and their study in single atrial cells
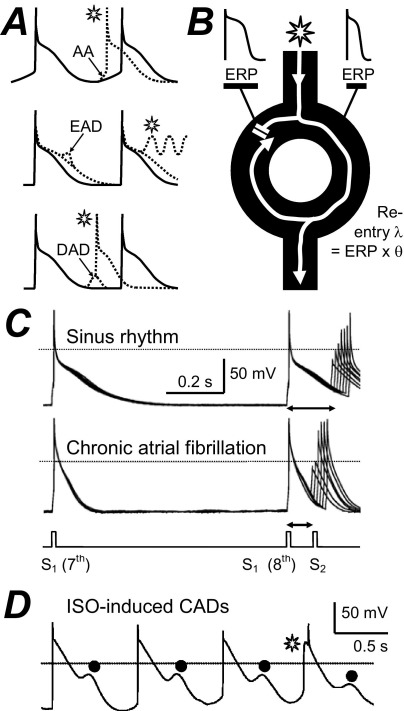
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. It causes substantial morbidity and mortality. The majority of atrial premature beats that initiate AF originate from focal ectopic electrical activity in the pulmonary veins (PVs). AF is sustained by single- or multiple- circuit intra-atrial reentry and/or focal ectopy, and the latter may be reentrant or nonreentrant.1 Nonreentrant mechanisms include abnormal automaticity (AA) and triggered activity. AA is the premature firing of action potentials (APs) because of abnormal diastolic membrane depolarisation (Figure 1A) and is favored by, for example, β-adrenergic stimulation or decreased vagal activity. Triggered activity is premature firing due to after-depolarizations. These may be early (EADs), occurring during repolarization and favored by AP prolongation, or delayed (DADs), occurring after an AP and favored by intracellular Ca2+ overload (Figure 1A). Reentry is rapid circuitous activation caused by unidirectional conduction block and favored by premature impulses, heterogeneity and shortening of the effective refractory period (ERP), and slowing of conduction velocity, θ (Figure 1B). Several electrophysiological parameters that may affect AF genesis and maintenance have been measured in human atrial isolated cells. The cellular ERP2 and AP maximum upstroke velocity, Vmax (Figure 1C), contribute to myocardial ERP and θ, respectively, so their reduction could promote reentry by shortening its wavelength, λ (Figure 1B). Cellular arrhythmic depolarizations (CADs3; Figure 1D) may represent AA, EADs, or DADs, with potential involvement in nonreentrant mechanisms.
Figure 1.
Electrophysiological mechanisms of arrhythmias and their study in human atrial cells. A: Representation of premature APs (*) from AA, EADs, or DADs. B: Premature impulse divides at functional or anatomical obstacle, blocks at tissue with normal (left side), but conducts with short (right) ERP and reenters previously inexcitable zone. λ = wavelength; θ = conduction velocity. C: Original APs stimulated in an atrial cell from a patient in SR or in AF by conditioning pulses (S1) and premature test pulses (S2). ERP (↔) = longest S1-S2 failing to produce S2 response of amplitude >80% of S1. D: Original APs stimulated by a pulse train in the presence of 0.05 μM isoproterenol (ISO), producing CADs (•). The * may represent AA. Panels C and D are based on data in references 2 and 3 with permission from Elsevier.
Atrial cellular electrical remodeling in AF
Atrial myocardial electrical and mechanical activity and structure adapt, or remodel, in response to a variety of diseases and other stimuli. For example, congestive heart failure (CHF) may involve electrical remodeling, atrial dilation, and interstitial fibrosis, each potentially predisposing to AF. Once AF occurs, the rapid atrial rate causes atrial electrical remodeling that promotes AF, so AF is autoperpetuating. In goats, induced AF progressively shortened the atrial ERP and AF interval over 24 hours, which reduced the reentry λ and increased AF vulnerability.4 Maximal ERP shortening may precede maximal AF duration, but the ERP shortening contributes to the AF substrate. In our laboratory, a similar ERP shortening was found in atrial cells isolated from patients with chronic AF (Figure 1C). This was associated with impaired ERP rate adaptation, shortening and triangulation of the AP and no change in Vmax.2 The shortened AP permitted full repolarization at the fast rates typically encountered in AF and thus prevented the depolarization of the maximum diastolic potential (MDP), that was observed in sinus rhythm (SR).2 This effect on MDP might limit Ca2+ overload in the remodeled atrium, but the ERP changes favor reentry. The ERP is largely determined by the AP duration (APD), which depends on a delicate balance of inward and outward ion currents flowing through a variety of membrane protein channels, pumps, and exchangers. Therefore, an understanding of the mechanisms of human AF-induced atrial electrical remodeling requires knowledge about precise changes in each of these currents, and their contributions to the AP, in AF.
Potential ionic mechanisms of electrical remodeling in AF
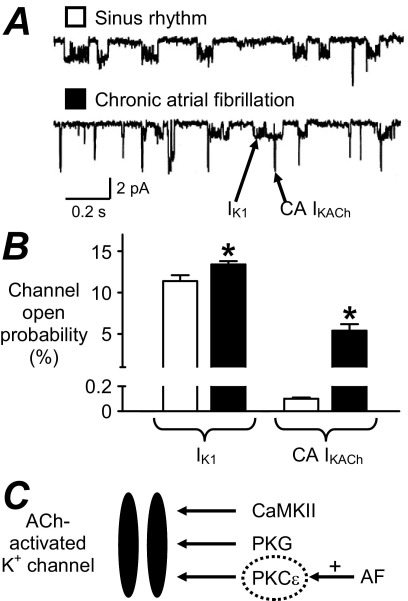
Many human atrial ion currents have so far been studied in AF; they are shown in Table 1. The inward rectifier K+ current (IK1) is the main determinant of the resting potential (Vm). Other currents contribute, including acetylcholine-activated K+ current (IKACh), Na+, K+ pump current (Ip), and possibly ATP-sensitive K+ current (IKATP). IK1 and IKACh also contribute to terminal repolarization. There is consensus that chronic AF is associated with increased density of IK1 (Table 1). Furthermore, despite decreased parasympathetic-regulated IKACh, a constitutively active (CA) IKACh, not requiring its endogenous agonist, is induced in AF (Figure 2). A single study on Ip, from our laboratory, showed no change in AF, and changes in IKATP are variable. The reported increases in IK1 and CA IKACh were most prominent (with enhanced inward current) at voltages more negative than the AP voltage range. However, enhanced outward IK1 has also been reported, within the AP voltage range,5,6,8 which may contribute to the APD and ERP shortening in AF. Increased IK1 should also hyperpolarize Vm; while difficult to ascertain in human atrial isolated cells since the “chunk” isolation method may depolarize them, this has been reported in atrial trabeculae.6 The AP fires when depolarization sufficient to drive Vm to threshold activates inward Na+ current (INa), causing the regenerative and rapid AP upstroke; the larger INa, the faster Vmax. A single study reported no change in INa density in AF, consistent with Vmax (Table 1), although its inactivation voltage dependency was altered. Partial, or early, repolarization follows the AP upstroke, via activation of a transient outward K+ current (ITO) and the ultrarapid delayed rectifier K+ current (IKur). AF consistently and markedly reduced ITO, but data for IKur are equivocal (Table 1). The ITO reduction may contribute to AP triangulation in AF, as shown by blocking ITO with 4-aminopyridine (4-AP).2 However, its contribution to the APD90 and ERP is unclear since 4-AP also blocks IKur, although mathematical modeling suggested a negligible role.34 The AP plateau is maintained by inward, L-type Ca2+, current (ICaL), which is consistently and markedly reduced in chronic AF (Table 1), despite increased single-channel open probability.35 Such ICaL reduction depresses the AP plateau, consistent with acute effects of nifedipine2 or simulated ICaL reduction,34 although its contribution alone to the shortening of the APD902,34 or ERP2 may be small. Mid/late repolarization results from activation of IKur as well as from the rapid (IKr) and slow (IKS) delayed rectifiers, which are balanced by inward Na+-Ca2+ exchange current (INa/Ca) following the [Ca2+]i transient. INa/Ca also underlies the transient inward current responsible for DADs. However, any role for these currents in human AF remodeling is presently unclear, since data are either equivocal or unavailable (Table 1). AA results from decreased outward and/or increased inward diastolic currents, including INa/Ca and the “funny” current (If). However, data on If are also lacking.
Table 1.
Human atrial cellular electrophysiological changes associated with AF, ventricular dysfunction, and drug therapy
| Human atrial cell electrophysiological measurement | Chronic AF | Post-CS AF | CHF, LVSD, and/or atrial dilation | Chronic β1-blocker therapy |
|---|---|---|---|---|
| ERP | ↓2 | ↔22 | NDA | ↑3,22,31 |
| APD90 | ↓2,5–7 | ↔22 | ↑23,24 ↔25,26 | ↑22,31 |
| APD50 | ↔2,6 ↑7 | ↔22 | ↔23,24 ↓26 | ↔3,31 |
| AP Vmax | ↔2 | ↔22 | NDA | ↔31 |
| IK1 | ↑2,5,6,8–12 | ↔9,22 | ↓23 ↔⁎27 | ↓32 |
| IKACh | ↑5 ↓6,9,10,12 | ↔9 | ↓23 | NDA |
| CA IKACh | ↑10,12 | NDA | NDA | NDA |
| Ip | ↔13 | NDA | NDA | NDA |
| IKATP | ↓14 ↑15 | NDA | ↔24 | NDA |
| INa | ↔5 | NDA | NDA | NDA |
| ITO | ↓2,5,8,16,17 | ↔22 | ↑26 ↓⁎27,28 | ↓31,32 |
| IKur (ISUS) | ↓8,16 ↔2,5,17 | ↔22 | ↔25,26 ↔⁎28 ↓⁎27 | ↔31,32 |
| ICaL | ↓2,5,7,11,18–21 | ↑18 ↔22 | ↓29 ↔25,30 ↓⁎27 | ↔3,7,22,31,33 |
| ICaL %↑ by β stimulation | ↑18–21 | ↔22 | ↓29,30 | ↔3,22 |
| ICaL %↑ by 5-HT | ↓7 | NDA | ↓29 | ↑7,33 |
| INa/Ca, INa/H, IKr, IKs, ICl(swell), ICaB, If | NDA | NDA | NDA | NDA |
Arrows show direction of change relative to “controls.”
= atrial dilation only; NDA = no data available. See text for definitions.
Figure 2.
Induction of constitutively active acetylcholine-activated K+ current, CA IKACh, in human AF. Single-channel IK1 and CA IKACh currents (A) and mean (±standard error) open probabilities (B), recorded at −120 mV in atrial cells from patients in SR (open symbols) and AF (solid symbols). *P <.05 versus SR. C: Potential signaling mechanism of increased CA IKACh in AF. In SR, IKACh may require channel phosphorylation by calmodulin-dependent PK II (CaMKII), PKG, and PKC.12 In AF, CA IKACh may result from upregulation of PKCϵ.12 Panels A and B are based on data in reference 10 with permission from Lippincott Williams & Wilkins.
Human PV isolated cell electrophysiology has not yet been studied. Chronic atrial tachypacing (AT) in dogs produced APD shortening in PV cells that was qualitatively similar to that in atrial cells and also produced similar changes in IK1, ITO, and ICaL.36 However, a current that may be analogous to human CA IKACh was increased more strongly in PV cells than in atrial cells, perhaps favoring PV reentry.37 The relative importance of reentrant versus nonreentrant activity to PV arrhythmogenesis, either before or after AF remodeling, is unknown.1
Atrial electrical activity is intricately linked with cellular and subcellular Ca2+ fluxes, particularly via INa/Ca. Intracellular Ca2+ handling is altered in AF, although human data are sparse. In canine atrial cells, acute AT, analogous to a paroxysm of AF, abruptly increases diastolic [Ca2+]i, a potential trigger of the remodeling process. Chronic AT, by contrast, markedly decreased the [Ca2+]i transient amplitude,38 perhaps reflecting protection from [Ca2+]i overload. This may result from a deficient trigger function of the markedly reduced ICaL, since sarcoplasmic reticular Ca2+ content was preserved.38 Human AF was associated with a potentially arrhythmogenic increase in the frequency of Ca2+ sparks and waves.39 This may represent sarcoplasmic reticular Ca2+ leak due to ryanodine receptor hyperphosphorylation.40
Whether the combined ionic changes so far established in human AF can account for the associated AP changes is unclear and will require the aid of mathematical models. One such model suggested that the combined IK1, ITO, and ICaL changes could explain the AP changes,34 although in the dog, concurrent [Ca2+]i changes were required.38 Another model suggested a major contribution from the IK1 increase to the stabilization of reentry.41
Potential molecular mechanisms in AF: Genetic and nongenetic
Many atrial ion current changes in human AF are accompanied by, and often considered to be caused by, altered tissue expression of the ion channel pore-forming α-subunits that carry them, for example, increased Kir2.1 (carries IK1) and decreased Kv4.3 (ITO).11 However, there are some intriguing and controversial exceptions. Protein levels of ICaL α-subunits were decreased by 40%–55% in three studies,35,42,43 in line with ICaL reduction, but were unchanged in four others.11,20,21,44 Also, despite increased CA IKACh in AF (Table 1), the Kir 3.1 protein level was decreased.43 The apparent discrepancies between changes in ion current density and protein expression suggest post-translational modification or altered channel regulation. The magnitude of ICaL is influenced by a balance between channel phosphorylation by kinases and dephosphorylation by phosphatases. Chronic AF upregulated phosphatase type-2A-C, reducing ICaL without requiring reduced channel protein.20 Similarly, induction of CA IKACh in human AF resulted from abnormal protein kinase- (PK-) C function12 (Figure 2C).
AF may be a heritable disorder: positive family history was identified in 5% of patients with AF.45 Several genetic mutations have been associated with familial AF, mainly for K+ channels. Most are gain-of-function mutations, increasing IKs, IKr, or IK1 and expected to shorten ERP and promote reentry, although an IKur loss-of-function mutation might prolong ERP.45 However, such mutations occur in other diseases, for example, dilated cardiomyopathy and long-QT, short-QT, and Brugada syndromes, some of which are comorbidities for AF. Nevertheless, it seems that genetic variants are involved in the pathogenesis of AF in a proportion of cases.
Neurohumoral involvement in AF
AF can result from a sympathetic/parasympathetic imbalance. Furthermore, neurohumoral activation in CHF, an important cause of AF, increases circulating levels of catecholamines, angiotensin, and endothelin (ET-1). Beta-adrenergic stimulation from catecholamines may promote DADs, by increasing ICaL and Ca2+-induced Ca2+ release. AF remodeling potentiated the relative increase in ICaL produced by β-stimulation (Table 1). We demonstrated that ET-1 had no direct effect on ICaL, APD, or ERP in human atrial cells. However, it abolished isoproterenol-induced increases in ICaL, APD50, and CADs (Figure 1D), with no effect on ERP.3 Thus, ET-1 might exert an antiadrenergic antiarrhythmic influence in the atria of patients with CHF. Serotonin (5-HT) is released from platelets aggregating in static blood in fibrillating atria. We demonstrated that 5-HT may be arrhythmogenic in human atrium, by increasing ICaL and producing CADs, without affecting ERP.33 Atrial remodeling by AF may protect from these effects, however, since they were attenuated in cells from patients with chronic AF7 (Table 1).
Postoperative AF: Is there a predisposing atrial cellular electrophysiological substrate?
AF is common in patients after cardiac surgery (CS). Post-CS AF is independently predicted by old age, pre-CS AF, and pre-CS P-wave changes. Therefore, pre-CS atrial cellular electrophysiology could influence the propensity for new-onset AF post-CS, which is an issue presently under debate. An early study showed an association between post-CS AF and an enhanced pre-CS ICaL,18 potentially arrhythmogenic post-CS, when catecholamines are elevated. However, we recently demonstrated, by contrast, that pre-CS ICaL, AP parameters, or ERP were not predictive of post-CS AF.22 Furthermore, no other ion current that was measured, nor the ICaL response to β-stimulation, was different between patients with and without post-CS AF (Table 1). Some currents remain to be studied, but it appears that the electrically remodeled state caused by chronic AF (Table 1) is not present pre-CS in the atrial cells of patients who develop new-onset post-CS AF.
Heart failure–induced atrial remodeling
AF is common in patients with CHF, and left ventricular systolic dysfunction (LVSD) substantially increases the risk of AF. It is unclear whether atrial cellular electrical remodeling, in patients in SR, contributes to this predisposition to AF. The available human data are scarce and inconsistent (Table 1) and are compounded by inevitable variability in the patients' disease states and drug treatments. Atrial cellular electrical remodeling has been demonstrated in canine models of chronic ventricular tachypacing (VTP)-induced CHF. AF was invariably promoted, but the remodeling pattern differed from AF: atrial ERP was unchanged or increased, IK1 was not increased, both ITO and IKS were decreased, ICaL was only moderately decreased, and INa/Ca was increased.46,47 The increased INa/Ca might favor a triggered origin of AF in this model. CHF also caused atrial fibrosis, and while the ionic remodeling reversed after ceasing VTP, the fibrosis and AF persistence did not.46 Thus, atrial electrical remodeling may contribute to AF genesis but was not necessary for its maintenance in this model. Human CHF or LVSD were associated with variable changes in APD, and cellular ERP has not been studied (Table 1). Human atrial ionic changes in CHF or LVSD may be expected to differ from those in chronic AF, with decreased IK1 and increased ITO, decreased or unchanged ICaL, and a decreased ICaL response to β-stimulation so far reported (Table 1). The pattern may depend on the degree of atrial dilation, which itself may cause ionic remodeling (Table 1). Moreover, CHF- and AF-induced atrial remodeling interact. In dogs, this interaction was complex, not cumulative: chronic AT, imposed on a CHF-remodeled atrium, caused moderate ERP shortening, IK1 increase, and ICaL decrease but did not further remodel ITO, IKS, or INa/Ca.47 No comparative human atrial data could be found.
Atrial remodeling by chronic drug therapy
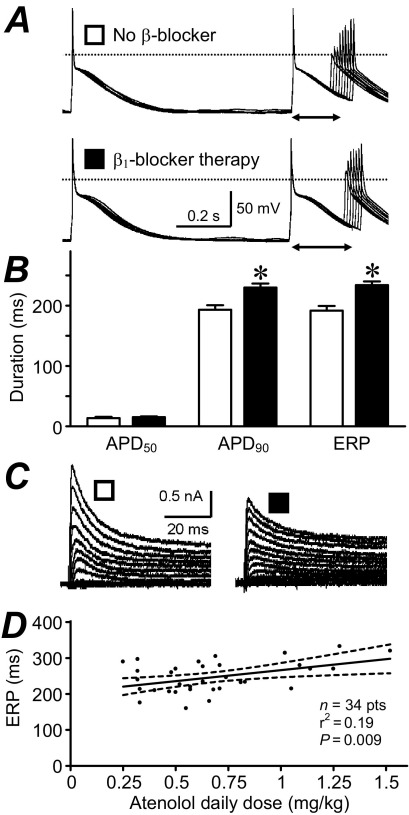
Atrial electrophysiology remodels in response not only to diseases and aging but also to long-term drug treatments; so-called pharmacological remodeling.31 This was originally demonstrated in rabbits: treatment with the β1-blocker metoprolol caused an adaptational prolongation of the atrial APD, maximally after 6 days.48 Beta-blockers are increasingly used to treat AF and HF. We demonstrated that in patients in SR, β1-blocker treatment for ≥7 days was independently associated with prolonged atrial cell APD90 and ERP22,31 (Figure 3A, 3B), and not with ICaL.22 The ITO was reduced (Figure 3C), and ISUS was unchanged (Table 1). Preliminary data from our group suggest that the ITO reduction does not involve altered voltage dependency or kinetics32 or altered ion channel expression49 and that IK1 is also reduced.32 Recent subgroup analysis revealed a significant correlation between ERP and atenolol dose (Figure 3D), suggesting that the ERP prolongation is at least partly caused, directly or indirectly, by the atenolol treatment. Such ERP prolongation might contribute to the antiarrhythmic effects of beta-blockers, although a potentiation by chronic β-blockade of effects of 5-HT on ICaL (Table 1) and CADs33 could also oppose them.
Figure 3.
Pharmacological remodeling of human atrial cell electrophysiology by β1-blocker therapy. A: APs and ERP (↔); B: mean (±standard error) APD at 50% and 90% repolarization (APD50 and APD90) and ERP; C: transient outward K+ currents, recorded in single atrial myocytes from patients in SR, treated with a β1-blocker ≥7 days (solid symbols) versus those in SR who were not treated with a β-blocker (open symbols). *P <.05 versus non β-blocker. D: Correlation between atrial cell ERP and patient's atenolol dose/body weight. Heart rate ≤75 bpm. Dashed lines indicate the 95% confidence interval. Panels A and C are based on data in reference 31 with permission from Elsevier.
How research on cellular bases for human AF is driving new therapeutic strategies
Traditional ERP-prolonging drugs, which are used to inhibit reentry, act by blocking IKr. This is problematic because IKr exists in the ventricle as well as atrium, which risks ventricular EADs and fibrillation. IKur and IKACh are considered to be atrium specific, so their block might prolong ERP in atrium only, depending on secondary ionic effects. However, targeting ion channel regulation may be preferable to ion channel block. Altering the PKC pathway that induces CA IKACh in chronic AF12 might avoid undesirable effects of inhibiting parasympathetic-regulated IKACh on sinoatrial node and bladder. Blocking the phosphatase-induced ICaL decrease caused by AF20 is another possibility. Moreover, “de-remodeling,” in theory, might be better than such “anti-remodeling” since blocking potentially protective adaptations may be risky. Pharmacological targeting of nonreentrant mechanisms of AF also may be considered.
AF is a highly complex, multifactorial, and dynamic disorder with differing characteristics and etiologies among individuals. As such, it presents an enormous challenge for the development of drugs for its effective and safe treatment. Current basic research is driving the search for new drugs. Several drugs, including IKur and IKACh blockers, are entering clinical trials. It remains to be seen whether they will improve patient treatment.
Footnotes
Antony J. Workman received British Heart Foundation Basic Science Lectureship award BS/06/003.
References
- 1.Wit A.L., Boyden P.A. Triggered activity and atrial fibrillation. Heart Rhythm. 2007;4:S17–S23. doi: 10.1016/j.hrthm.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workman A.J., Kane K.A., Rankin A.C. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 3.Redpath C.J., Rankin A.C., Kane K.A. Anti-adrenergic effects of endothelin on human atrial action potentials are potentially anti-arrhythmic. J Mol Cell Cardiol. 2006;40:717–724. doi: 10.1016/j.yjmcc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Wijffels M.C.E.F., Kirchhof C.J.H.J., Dorland R. Atrial fibrillation begets atrial fibrillation: A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 5.Bosch R.F., Zeng X., Grammer J.B. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 6.Dobrev D., Graf E., Wettwer E. Molecular basis of downregulation of G-protein-coupled inward rectifying K+ current (IK,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced IK,ACh and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- 7.Pau D., Workman A.J., Kane K.A. Electrophysiological and arrhythmogenic effects of 5-hydroxytryptamine on human atrial cells are reduced in atrial fibrillation. J Mol Cell Cardiol. 2007;42:54–62. doi: 10.1016/j.yjmcc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Wagoner D.R., Pond A.L., McCarthy P.M. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 9.Dobrev D., Wettwer E., Kortner A. Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res. 2002;54:397–404. doi: 10.1016/s0008-6363(01)00555-7. [DOI] [PubMed] [Google Scholar]
- 10.Dobrev D., Friedrich A., Voigt N. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 11.Gaborit N., Steenman M., Lamirault G. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 12.Voigt N., Friedrich A., Bock M. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Workman A.J., Kane K.A., Rankin A.C. Characterisation of the Na, K pump current in atrial cells from patients with and without chronic atrial fibrillation. Cardiovasc Res. 2003;59:593–602. doi: 10.1016/s0008-6363(03)00466-8. [DOI] [PubMed] [Google Scholar]
- 14.Balana B., Dobrev D., Wettwer E. Decreased ATP-sensitive K+ current density during chronic human atrial fibrillation. J Mol Cell Cardiol. 2003;35:1399–1405. doi: 10.1016/s0022-2828(03)00246-3. [DOI] [PubMed] [Google Scholar]
- 15.Wu G., Huang C.X., Tang Y.H. Changes of IK,ATP current density and allosteric modulation during chronic atrial fibrillation. Chin Med J. 2005;118:1161–1166. [PubMed] [Google Scholar]
- 16.Brandt M.C., Priebe L., Bohle T. The ultrarapid and the transient outward K+ current in human atrial fibrillation: Their possible role in postoperative atrial fibrillation. J Mol Cell Cardiol. 2000;32:1885–1896. doi: 10.1006/jmcc.2000.1221. [DOI] [PubMed] [Google Scholar]
- 17.Grammer J.B., Bosch R.F., Kuhlkamp V. Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation. J Cardiovasc Electrophysiol. 2000;11:626–633. doi: 10.1111/j.1540-8167.2000.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 18.Van Wagoner D.R., Pond A.L., Lamorgese M. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 19.Skasa M., Jungling E., Picht E. L-type calcium currents in atrial myocytes from patients with persistent and non-persistent atrial fibrillation. Basic Res Cardiol. 2001;96:151–159. doi: 10.1007/s003950170065. [DOI] [PubMed] [Google Scholar]
- 20.Christ T., Boknik P., Wohrl S. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 21.Greiser M., Halaszovich C.R., Frechen D. Pharmacological evidence for altered src kinase regulation of ICa,L in patients with chronic atrial fibrillation. Naunyn-Schmiedeberg's Arch Pharmacol. 2007;375:383–392. doi: 10.1007/s00210-007-0174-6. [DOI] [PubMed] [Google Scholar]
- 22.Workman A.J., Pau D., Redpath C.J. Post-operative atrial fibrillation is influenced by beta-blocker therapy but not by pre-operative atrial cellular electrophysiology. J Cardiovasc Electrophysiol. 2006;17:1230–1238. doi: 10.1111/j.1540-8167.2006.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koumi S., Arentzen C.E., Backer C.L. Alterations in muscarinic K+ channel response to acetylcholine and to G protein-mediated activation in atrial myocytes isolated from failing human hearts. Circulation. 1994;90:2213–2224. doi: 10.1161/01.cir.90.5.2213. [DOI] [PubMed] [Google Scholar]
- 24.Koumi S., Martin R.L., Sato R. Alterations in ATP-sensitive potassium channel sensitivity to ATP in failing human hearts. Am J Physiol. 1997;272:H1656–H1665. doi: 10.1152/ajpheart.1997.272.4.H1656. [DOI] [PubMed] [Google Scholar]
- 25.Schreieck J., Wang Y.G., Kalra B. Differential rate dependence of action potentials, calcium inward and transient outward current in atrial myocytes of patients with and without heart failure. Circulation. 1998;98:611. (abstract) [Google Scholar]
- 26.Schreieck J., Wang Y., Overbeck M. Altered transient outward current in human atrial myocytes of patients with reduced left ventricular function. J Cardiovasc Electrophysiol. 2000;11:180–192. doi: 10.1111/j.1540-8167.2000.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 27.Le Grand B., Hatem S., Deroubaix E. Depressed transient outward and calcium currents in dilated human atria. Cardiovasc Res. 1994;28:548–556. doi: 10.1093/cvr/28.4.548. [DOI] [PubMed] [Google Scholar]
- 28.Mansourati J., Le Grand B. Transient outward current in young and adult diseased human atria. Am J Physiol. 1993;265:H1466–H1470. doi: 10.1152/ajpheart.1993.265.4.H1466. [DOI] [PubMed] [Google Scholar]
- 29.Ouadid H., Albat B., Nargeot J. Calcium currents in diseased human cardiac cells. J Cardiovasc Pharmacol. 1995;25:282–291. doi: 10.1097/00005344-199502000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Cheng T.H., Lee F.Y., Wei J. Comparison of calcium-current in isolated atrial myocytes from failing and nonfailing human hearts. Mol Cell Biochem. 1996;157:157–162. doi: 10.1007/BF00227894. [DOI] [PubMed] [Google Scholar]
- 31.Workman A.J., Kane K.A., Russell J.A. Chronic beta-adrenoceptor blockade and human atrial cell electrophysiology: evidence of pharmacological remodelling. Cardiovasc Res. 2003;58:518–525. doi: 10.1016/s0008-6363(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 32.Marshall G., Rankin A.C., Kane K.A. Pharmacological remodelling of human atrial K+ currents by chronic beta-blockade. Eur Heart J. 2006;27:30. (abstract) [Google Scholar]
- 33.Pau D., Workman A.J., Kane K.A. Electrophysiological effects of 5-hydroxytryptamine on isolated human atrial myocytes, and the influence of chronic β-adrenoceptor blockade. Br J Pharmacol. 2003;140:1434–1441. doi: 10.1038/sj.bjp.0705553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Garratt C.J., Zhu J. Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans. Cardiovasc Res. 2005;66:493–502. doi: 10.1016/j.cardiores.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Klein G., Schroder F., Vogler D. Increased open probability of single cardiac L-type calcium channels in patients with chronic atrial fibrillation: role of phosphatase 2A. Cardiovasc Res. 2003;59:37–45. doi: 10.1016/s0008-6363(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 36.Cha T.J., Ehrlich J.R., Zhang L. Atrial tachycardia remodeling of pulmonary vein cardiomyocytes: comparison with left atrium and potential relation to arrhythmogenesis. Circulation. 2005;111:728–735. doi: 10.1161/01.CIR.0000155240.05251.D0. [DOI] [PubMed] [Google Scholar]
- 37.Ehrlich J.R., Cha T.J., Zhang L. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol. 2004;557:583–597. doi: 10.1113/jphysiol.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kneller J., Sun H., Leblanc N. Remodeling of Ca2+-handling by atrial tachycardia: evidence for a role in loss of rate-adaptation. Cardiovasc Res. 2002;54:416–426. doi: 10.1016/s0008-6363(02)00274-2. [DOI] [PubMed] [Google Scholar]
- 39.Hove-Madsen L., Llach A., Bayes-Genis A. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 40.Vest J.A., Wehrens X.H.T., Reiken S.R. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 41.Pandit S.V., Berenfeld O., Anumonwo J.M.B. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brundel B.J.J.M., Van Gelder I.C., Henning R.H. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc Res. 1999;42:443–454. doi: 10.1016/s0008-6363(99)00045-0. [DOI] [PubMed] [Google Scholar]
- 43.Brundel B.J.J.M., Van Gelder I.C., Henning R.H. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- 44.Schotten U., Haase H., Frechen D. The L-type Ca2+-channel subunits α1C and β2 are not downregulated in atrial myocardium of patients with chronic atrial fibrillation. J Mol Cell Cardiol. 2003;35:437–443. doi: 10.1016/s0022-2828(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 45.Fatkin D., Otway R., Vandenberg J.I. Genes and atrial fibrillation: a new look at an old problem. Circulation. 2007;116:782–792. doi: 10.1161/CIRCULATIONAHA.106.688889. [DOI] [PubMed] [Google Scholar]
- 46.Cha T.J., Ehrlich J.R., Zhang L. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation. 2004;109:412–418. doi: 10.1161/01.CIR.0000109501.47603.0C. [DOI] [PubMed] [Google Scholar]
- 47.Cha T.J., Ehrlich J.R., Zhang L. Atrial ionic remodeling induced by atrial tachycardia in the presence of congestive heart failure. Circulation. 2004;110:1520–1526. doi: 10.1161/01.CIR.0000142052.03565.87. [DOI] [PubMed] [Google Scholar]
- 48.Raine A.E.G., Vaughan Williams E.M. Adaptation to prolonged β-blockade of rabbit atrial, Purkinje, and ventricular potentials, and of papillary muscle contraction: Time-course of development of and recovery from adaptation. Circ Res. 1981;48:804–812. doi: 10.1161/01.res.48.6.804. [DOI] [PubMed] [Google Scholar]
- 49.Marshall G.E., Tellez J.O., Russell J.A. Reduction of human atrial ITO by chronic beta blockade is not due to changes in ion channel expression. Proc Physiol Soc. 2007;8:PC20. (abstract) [Google Scholar]