Abstract
Vascular smooth muscle cell (SMC) proliferation plays an important role in the pathogenesis of atherosclerosis and post-angioplasty restenosis. Berberine is a well-known component of the Chinese herb medicine Huanglian (Coptis chinensis), and is capable of inhibiting SMC contraction and proliferation, yet the exact mechanism is unknown. We therefore investigated the effect of berberine on SMC growth after mechanic injury in vitro. DNA synthesis and cell proliferation assay were performed to show that berberine inhibited serum-stimulated rat aortic SMC growth in a concentration-dependent manner. Mechanical injury with sterile pipette tip stimulated the regrowth of SMCs. Treatment with berberine prevented the regrowth and migration of SMCs into the denuded trauma zone. Western blot analysis showed that activation of the MEK1/2 (mitogen-activated protein kinase kinase 1/2), extracellular signal-regulated kinase (ERK), and up-regulation of early growth response gene (Egr-1), c-Fos and Cyclin D1 were observed sequentially after mechanic injury in vitro. Semi-quantitative reverse-transcription PCR assay further confirmed the increase of Egr-1, c-Fos, platelet-derived growth factor (PDGF) and Cyclin D1 expression in a transcriptional level. However, berberine significantly attenuated MEK/ERK activation and downstream target (Egr-1, c-Fos, Cyclin D1 and PDGF-A) expression after mechanic injury in vitro. Our study showed that berberine blocked injury-induced SMC regrowth by inactivation of ERK/Egr-1 signaling pathway thereby preventing early signaling induced by injury in vitro. The anti-proliferative properties of berberine may be useful in treating disorders due to inappropriate SMC growth.
Keywords: Berberine, Early growth response gene (Egr-1), Extracellular signal-regulated kinase (ERK), MEK1/2 (mitogen-activated protein kinase kinase 1/2), Platelet-derived growth factor (PDGF), Vascular smooth muscle cell
1. Introduction
Percutaneous coronary intervention has made remarkable progress in the past 20 years and has become an important treatment for coronary artery disease. However, restenosis after angioplasty is still a major limitation [1–3]. Restenosis occurs in approximately 30–40% of patients after balloon angioplasty and in between 20 and 30% of patients following coronary stenting [4,5]. A dominant cellular event in the re-narrowing of the lumen after angioplasty is vascular smooth muscle cell (SMC) proliferation. Injury induces medial and then intima cell cycle reentry, following a wave of immediate early gene expression [1,6]. Cells undergo either cell proliferation, migration or both, with subsequent synthesis of extracellular matrix and collagen resulting in neointimal formation [1–3].
Early growth response factor 1 (Egr-1), an 80–82-kDa zinc finger transcription factor, has a low basal level in the normal vessel wall, but has been shown to rapidly and transiently express in SMCs and endothelial cells in response to arterial injury [7,8]. Once activated, Egr-1 regulates the expression of several genes, including monocyte chemotactic protein-1 (MCP-1), platelet-derived growth factor (PDGF) A and B [7,9,10], and Cyclin D1 [11,12]. Previous studies showed that mechanical injury-induced up-regulation of Egr-1 is through activation of extracellular signal-regulated kinase (ERK), a key transducer of extracellular signals that promote cell growth and movement, which are critical for the initiation and progression of vascular lesions [13]. Activation of ERK is among the earliest of biochemical changes detected after arterial injury [14]. Activation of ERK has also been observed in atherosclerotic lesions from cholesterol-fed rabbits [15]. ERK transmits mitogenic signals by translocation to the nucleus where many of its substrates, including transcription factors, Egr-1, c-Fos and Elk-1 are activated [16–18]. Since Egr-1 induces the expression of multiple genes implicated in pathogenesis of atherosclerosis and restenosis after angioplasty [11,12,19], indicating that blocking of ERK/Egr-1 signaling pathway might be an important therapeutic strategy in reducing the incidence of restenosis after angioplasty.
The inability to limit neointimal development in humans likely relates to its complex nature, which involves inflammatory cells and their mediators, angiogenesis, and SMC growth and migration. Therefore, more interventional approaches must be considered for the management of human neointimal formation. Berberine is a well-known component of the Chinese herb medicine Huanglian (Coptis chinensis), and has a varied pharmacological properties, including antibiotic, anti-tumor, and anti-motility [20,21]. Extracts of berberine-containing plants have been used for many centuries in the treatment of diarrhea, probably through inhibition of mucosal chloride secretion. Recently, berberine was shown to be a promising new lipid-lowering drug that effectively reduced serum low-density lipoprotein (LDL) cholesterol levels in both hamsters and human patients [20]. A report from Ko et al. demonstrated that berberine has vasorelaxant and anti-proliferative effects on vascular SMCs [21]. In addition, Jantova et al. had disclosed that berberine could cause G1/0 arrest in cancer cell lines [22]. Recently, Lee et al. reported that berberine could inhibit SMC growth and inhibit Akt activation after angiotensin II stimulation [23]. However, activation of ERK is among the earliest of biochemical changes detected after arterial injury and is strongly related with subsequent early response transcription factors and growth factors expression [14,24]. Up to date, little is known about the cellular and molecular mechanisms of berberine-mediated anti-proliferative effects in vascular cells after mechanical injury and no work has been done to investigate the effect of berberine on the MEK/ERK pathway and the subsequent transcription factor and growth factor changes. In this study, we attempted to explore the possible anti-proliferative effect of berberine on vascular SMCs after mechanical injury and its molecular events. Herein, we show that berberine potently inhibited serum-stimulated SMC proliferation and prevented the regrowth of SMCs after mechanic injury in vitro. Such regrowth inhibition was accompanied by reduced transcriptional activation of Egr-1 and c-Fos through an MEK/ERK-dependent pathway. Our study clearly establishes a role for berberine in limiting vascular SMC regrowth in vitro and provides a basis for further studies of this bioactive agent in vivo.
2. Methods
2.1. Materials
The berberine (Fig. 1), DAPI (4′,6-diamindino-2-phenylindole), type I elastase and collagenase were purchased from Sigma (St. Louis, MO). Anti-Egr-1, anti-c-Fos, anti-phospho-ERK, and anti-α-actin were purchased from Santa Cruz (Santa Cruz, CA). Anti-ERK antibody was obtained from CalBiochem (San Diego, CA). Anti-Cyclin D1 antibody was purchased from BD Pharmingen (Franklin Lakes, NJ). U0126, anti-MEK1/2 (mitogen-activated protein kinase kinase), and anti-phospho-MEK1/2 antibody were obtained from cell signaling (Beverly, MA).
Fig. 1.
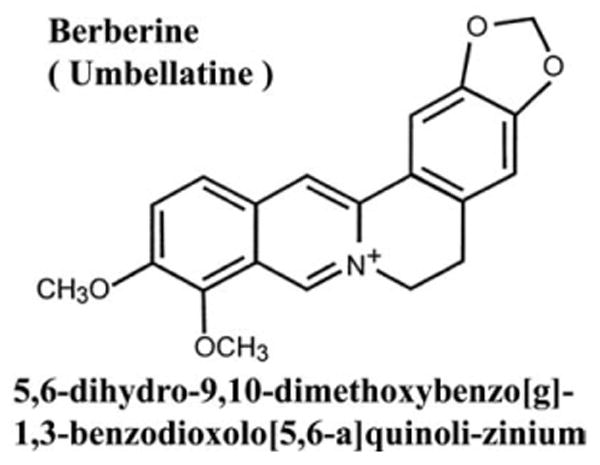
The chemical structure of berberine.
2.2. Vascular smooth muscle cell culture
Rat aortic SMCs were isolated from thoracic aortas of 2–3-month-old Sprague–Dawley rats as described elsewhere [25]. The identification of SMCs was confirmed by their morphology and by detecting their immunoreactivity for smooth muscle cell α-actin (Fig. 2). In addition, negative control with endothelium CD31 staining was used to assure the purity of the SMC culture in this study. In some of the experiments, cells in about 80% confluency were rendered quiescent with 1% FBS in DMEM for 24 h during pretreatment with berberine before mechanical injury stimulation.
Fig. 2.
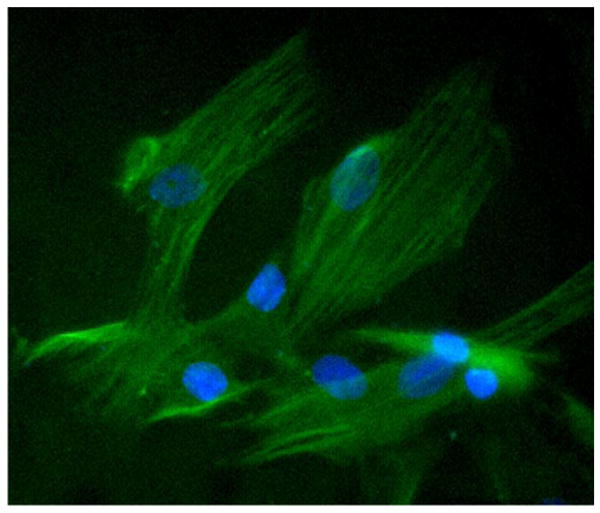
Identification of rat aortic SMCs. Rat aortic SMCs were isolated and stained with a specific anti-SMC α-actin primary antibody, and then followed by fluorescence conjugated secondary antibody. Magnification: 400×.
2.3. DAPI staining
Cells were incubated without or with berberine (100, 200 and 300 μM) for 48 h. After treatment, cells were rinsed twice with PBS and fixed in 2% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100/PBS for another 30 min at room temperature, and then washed with PBS and incubated with DAPI (1 μg/ml) at 37 °C for 20 min. Stained cells were visualized using a fluorescence microscope.
2.4. LDH Assay
Lactate dehydrogenase (LDH) activity was assayed by an enzymatic method (Roche Molecular Biochemicals, Basel, Switzerland) in the culture medium and in cell lysates after treatment with 1% Triton to assess the cytotoxic effect of various concentrations of berberine (0, 50, 100, 200, 300, and 400 μM). Results are expressed as the percentage of LDH leakage, which was calculated as LDH in the media divided by LDH in the media plus cell-contained LDH [26].
2.5. Cell proliferation assay
Cell proliferation was determined by direct cell counting and up-take of [3H]thymidine. SMCs were cultured in 12-well plates at a density of 1 × 105 cells/well for 24 h and then incubated with various concentrations of berberine (25, 50, 100, 200, 300 μM) for indicated time periods. Cells were trypsinized and cell numbers were determined by trypan blue dye exclusion method using hemocytometer. For [3H]thymidine incorporation assay, after drug treatment, SMCs were incubated in medium containing [3H]thymidine (1 μCi/well) for 6 h before harvest. The cells were washed three times with cold phosphate-buffered saline (PBS) to remove unincorporated label. The cells were then fixed with cold 5% trichloroacetic acid and washed with cold absolute ethanol. Cells were lysed with 0.1N NaOH at 37 °C for 10 min. [3H]thymidine incorporation into the DNA of SMCs was counted with a β-scintillation counter (Packard). The data were obtained as counts per minute per well and the anti-proliferative effect of berberine was expressed as a percentage of the control.
2.6. Cell cycle analysis
Cells were treated without or with berberine (120 μM) for 48 h, and cell cycle distribution was analyzed using flow cytometry. Briefly, 2 × 106 cells were trypsinized, washed with PBS, and fixed in 80% ethanol. They were then washed with PBS, incubated with 100 μg/ml RNase at 37 °C for 30 min, stained with propidium iodide (50 μg/ml), and analyzed on a FACScan flow cytometer. The percentage of cells in different phases of the cell cycle was analyzed using Cell-FIT software (Becton Dickinson Instruments).
2.7. In vitro injury model
SMCs were grown to confluence in six-well plates, injury was performed with a single scratch using a sterile pipette tip, and cells were then incubated in the absence or presence of various concentrations of berberine (10, 25, 50, 100, 200 μM). The rate of wound closure was investigated and photographed over 72 h period. For simulating balloon or stenting mechanic injury in the in vitro model, cultured SMCs were injured with a sterilized fork tips scraping 64 lines in a 100-mm culture dish. Activation of cell signal molecules, transcriptional factors or their downstream targets were evaluated by collecting cell lysates at a designated time frame post-injury.
2.8. Protein preparation and Western blot analysis
Equal amounts of protein samples were separated by SDS-PAGE gel and blotted onto PVDF membrane (Osmonics). The membranes were blocked with Tris-buffered saline with 0.1% Tween-20 and 5% non-fat dry milk at room temperature for 1 h. Afterward, membranes were incubated at 4 °C with antibodies raised against MEK1/2 (1:1000), phospho-MEK1/2 (1:1000), ERK1/2 (1:1000), phospho-ERK1/2 (1:1000), Egr-1 (1:1000), c-Fos (1:1000) or Cyclin D1 (1:800). SMC α-actin (1:1000) was used as an internal loading control. Then, the blots were washed and incubated for 1 h with horseradish peroxidase-labeled secondary antibody (1:20 000) (Amersham Phamacia, Buckinghamshire, England, UK). Immunoreactive bands were visualized with a chemiluminescence kit (PerkinElmer Life Science, Wellesley, MA) and quantified by densitometry.
2.9. Reverse transcriptase polymerase chain reaction (RT-PCR)
Egr-1, c-Fos, PDGF-A, and Cyclin D1 mRNAs were analyzed by semi-quantitative RT-PCR. Subconfluent SMCs were grown in 100-mm cell culture plates and treated with berberine (120 μM) for 1 h (Egr-1 and c-Fos), or for 8 h (Cyclin D1 and PDGF-A). Total RNA was extracted by RNA-Bee™ RNA isolation kit (TEL-TEST, Friendswood, TX). Two micrograms of total RNA was reverse-transcribed using 1st-STRAND™ cDNA Synthesis Kit (Clontech, Mountain View, CA) at 42 °C for 3 h as described in the manufacturer's protocol. Cycle-based PCR was used to semi-quantitate Egr-1, c-Fos, PDGF-A, and Cyclin D1. Each PCR reaction contained 1.5 mM MgCl2, 0.2 μM of each primer, Taq DNA polymerase (Sigma), 5 mM dNTPs and 2 μl cDNA. The cDNA templates were amplified with strand-specific primers for Egr-1, c-Fos, Cyclin D1, and PDGF-A, as listed in Table 1. GAPDH was used as an internal loading control. The following PCR reaction conditions were performed: denaturing cDNA at 95 °C for 5 min and submitted to multiple cycles of amplification (1 cycle: 94 °C, 1 min; 60 °C, 1 min; and 72 °C, 2 min) followed by a final extension of 7 min at 72 °C in a Bio-Rad icycle (Bio-Rad). For each combination of primers, the kinetics of PCR amplification was studied. The number of cycles corresponding to the plateau was determined. PCR was performed at an exponential range. The amplified products were visualized under the EverGene Image System. The expected sizes of the PCR products for Egr-1, c-Fos, PDGF-A, Cyclin D1, and GAPDH were 348, 496, 301, 410, and 317 bp, respectively.
Table 1. Primers for RT-PCR.
| Accession no. | Forward primer | Reverse primer | |
|---|---|---|---|
| Egr-1 | NM007913 | 5′-GCA CCC AAC AGT GGC AAC-3′ | 5′-GGG ATC ATG GGA ACC TGG-3′ |
| c-Fos | NM022197 | 5′-TAG CAA CAT GGA GCT GAA GGC TGA-3′ | 5′-GCT TGG TGT GTT TCA CGC ACA GAT-3′ |
| Cyclin D1 | NM053056 | 5′-CAT CCG CAA ACA TGC ACA GAC CTT-3′ | 5′-TTT CTC TTC GCG GAT GCC ACT ACT-3′ |
| PDGF-A | L06894 | 5′-TTG GAG ACA AAC CTG AGA GCC CAT-3′ | 5′-TGA CAT ACT CCA CTT TGG CCA CCT-3′ |
| GAPDH | X02231 | 5′-TAT GAC AAC TCC CTC AAG AT-3′ | 5′-AGA TCC ACA ACG GAT ACA TT-3′ |
2.10. Statistical analysis
All data are expressed as mean ± S.D. Student's unpaired t-test was used to compare differences between two groups. ANOVA was performed when more than two groups were compared. The mean values of two groups were considered significantly different if *p < 0.05, **p < 0.01, ***p < 0.001. Figures were obtained from at least three independent experiments with similar patterns.
3. Results
3.1. Inhibition of rat aortic SMC growth and DNA synthesis by berberine
Rat aortic SMCs were grown in 10% FBS containing medium in the absence or presence of various concentrations of berberine (10–400 μM) for 48 h. As shown in Fig. 3A, treatment of SMCs with high concentrations of berberine (400 μM) resulted in rounding and detaching due to cellular toxicity. SMCs treated with as low as 300 μM berberine never displayed any signs of toxicity or apoptosis by morphological investigation (Fig. 3A), nuclear DAPI staining (Fig. 3B), and LDH assay (Fig. 3C). Thus, the highest concentration of berberine was set at 300 μM. The effects of berberine on SMC DNA synthesis and cell growth were investigated. As shown in Fig. 3D, berberine significantly inhibited the serum-stimulated [3H]thymidine incorporation in a concentration-dependent manner. In addition, the effect of berberine on cell proliferation was measured by direct cell counting after treatment with various concentrations of berberine for 48 h. As depicted in Fig. 3E, administration of berberine caused a concentration-dependent inhibitory effect on serum-stimulated SMC growth. The 50% growth inhibition concentration (IC50) is105 μM. Moreover, the inhibition of SMC growth by berberine was related with a G1-phase arrest by cell cycle analysis as revealed by flow cytometry in Fig. 3F.
Fig. 3.
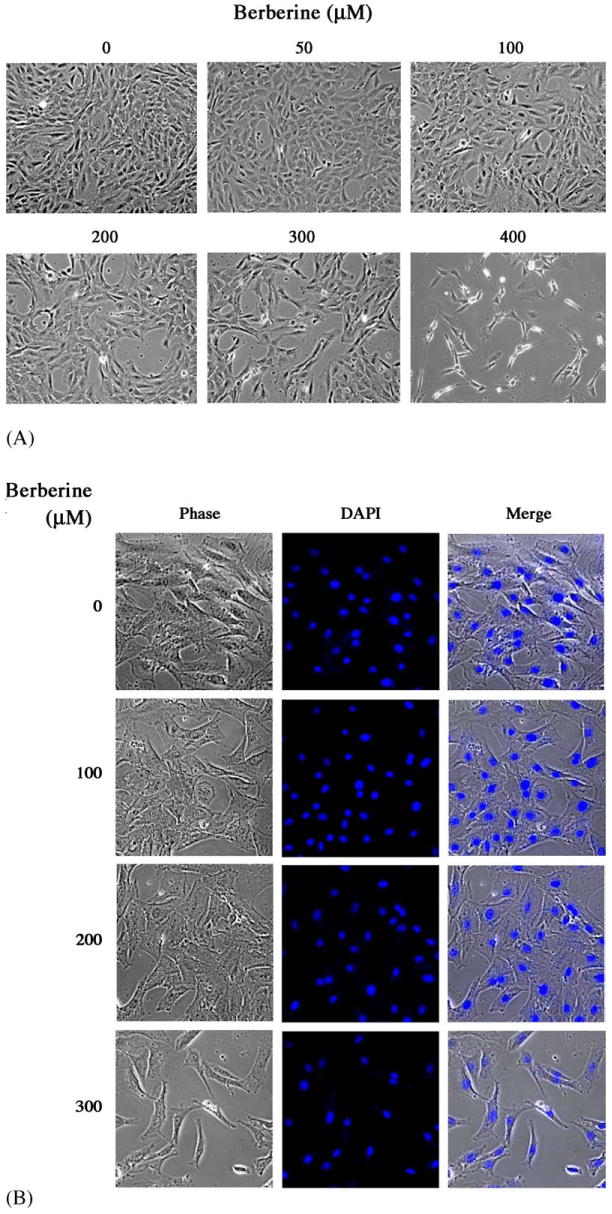
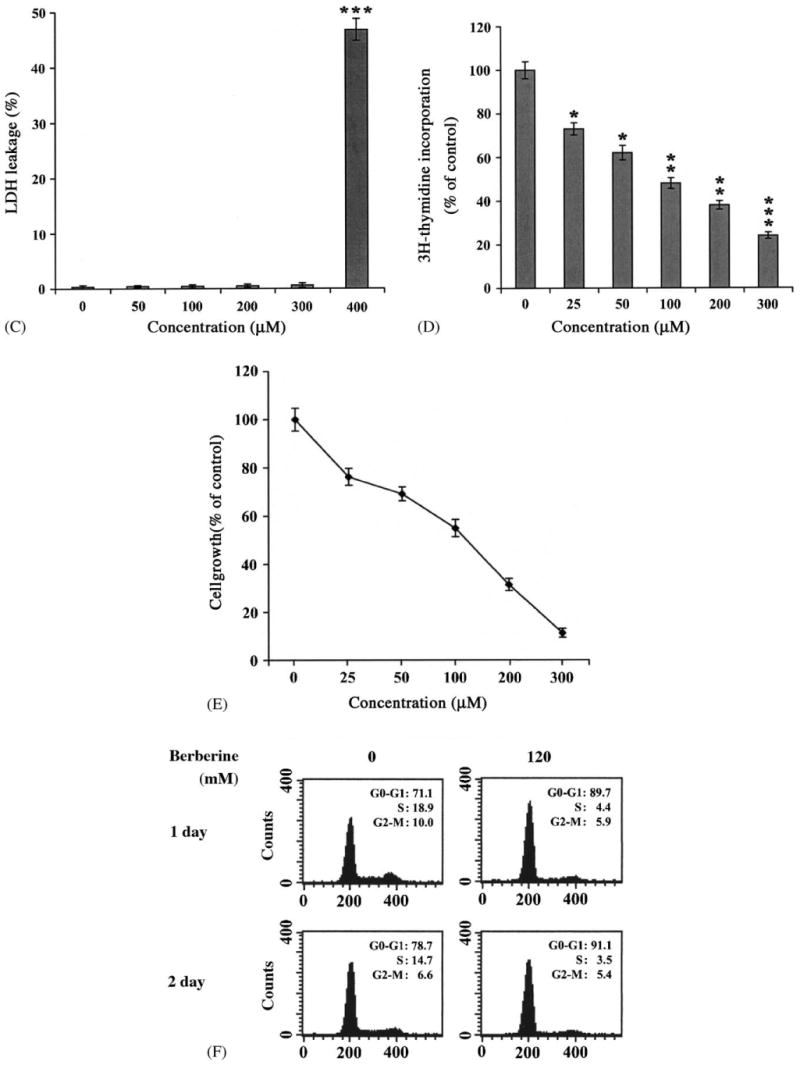
Berberine inhibited rat aortic SMC growth. Rat aortic SMCs were grown in 5% FBS, DMEM medium with various concentrations of berberine for 2 days: (A) Cell morphology was microphotographed using an Olympus IX70 phase-contrast microscope. Magnification: 200×. (B) DAPI staining and (C) LDH assay were performed as described in Section 2. (D) DNA synthesis was analyzed by [3H]thymidine incorporation method. (E) Cell number was directly counted by trypan blue dye exclusion method. Data shown in (C), (D) and (E), are presented as mean ± S.D. of at least nine replicates from three separate experiments. (F) Distribution of cell cycle with or without berberine treatment by flow cytometry.
3.2. Berberine prevented SMC regrowth and migration after mechanical injury
To determine whether berberine could inhibit SMC repair after mechanical injury, we created a linear injury line at the center of culture dish and treated them with various concentrations of berberine. After 72 h of incubation to allow cell proliferation and migration, the injured monolayers were fixed, and phase contrast images were digitized for analysis. The relative area of the wound, covered by proliferating and migrating cells, is shown in Fig. 4A. Control cultures almost completely regrew the entire denuded zone within 3 days. However, SMC regrowth and migration from the wound edge was significantly inhibited by berberine in a concentration-dependent manner (Figs. 4A and B). Treatment with high concentration (200 μM) berberine prevented additional growth even after 6 days (data not shown). Thus, as well as suppressing the serum-induced cell proliferation, berberine could also inhibit the repair response to injury in vitro.
Fig. 4.
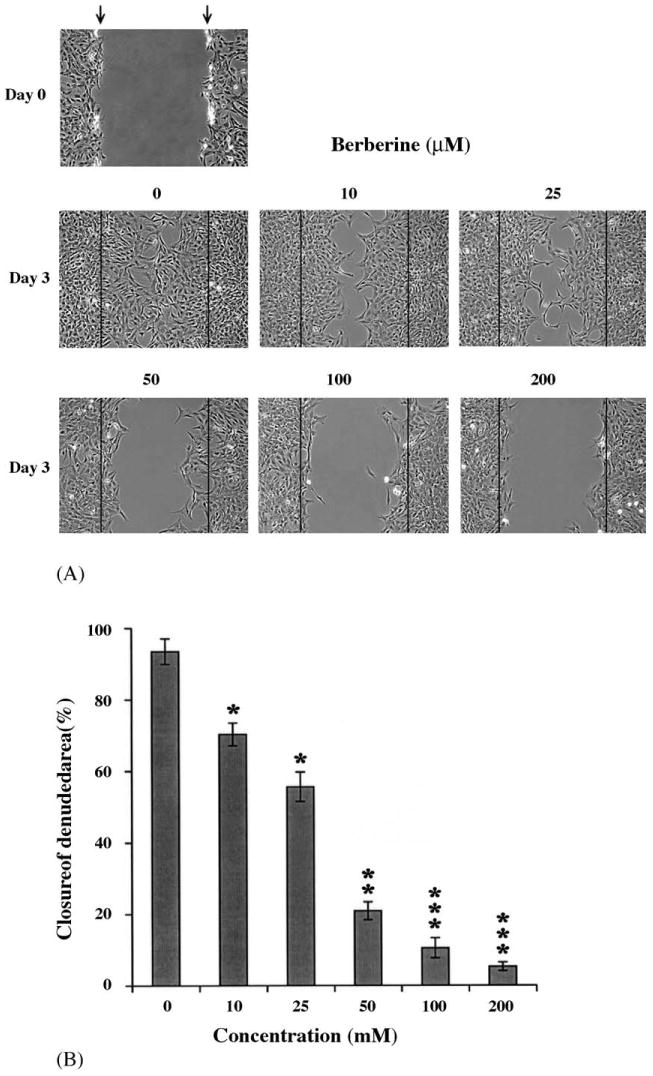
Berberine inhibited SMC regrowth and migration after mechanical injury. SMCs were grown to 80% confluency. We created a linear injury line at center of cultured monolayer by scraping with a sterile pipette tip. The cells were then maintained in 1% FBS-DMEM with various concentrations of berberine for 3 days. (A) Cell morphology was microphotographed. Magnification: 100×. (B) The closure of denuded area was calculated as described in Section 2.
3.3. Mechanical injury-induced Egr-1 and c-Fos up-regulation was MEK/ERK dependent
Previous reports indicated that transcription factors Egr-1 and c-Fos play a key regulatory role in SMC proliferation and repair following injury [27]. As shown in Fig. 5A, mechanical injured SMCs caused a time-dependent induction of Egr-1 and c-Fos proteins, being evident at 60 min, peaking at 90 min, and declining thereafter. Determination of Egr-1 and c-Fos protein induction was done by quantification of optical densities from autoradiograms of three experiments. Results showed that exposure of SMCs to mechanical injury produced seven- and three-fold changes in Egr-1 and c-Fos protein levels at 90 min post-injury, respectively.
Fig. 5.
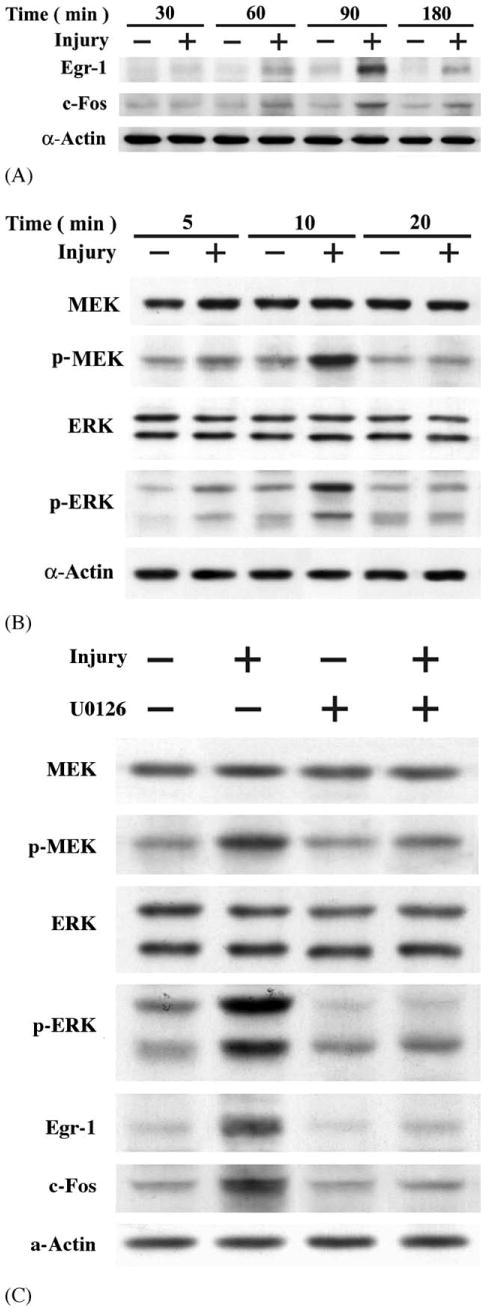
Mechanical injury-stimulated Egr-1 and c-Fos expression was MEK/ERK-dependent. SMCs were diffusely injured with 64 lines of scraping by sterile fork tips. Cell lysates were isolated: (A) the levels of Egr-1 and c-Fos protein, and (B) the levels of ERK, phosphorylated ERK, MEK, and phospho-MEK were detected by Western blot analysis. (C) U0126 blocked injury-induced MEK/ERK activation as well as Egr-1 and c-Fos expression. SMCs were treated with a specific MEK inhibitor, U0126 (20 μM) 30 min prior to mechanical injury. Cell lysates were isolated; the levels of MEK/ERK, phosphor-MEK/ERK, Egr-1 and c-Fos were measured by Western blot analysis.
Several studies reported that ERK-dependent expression of Egr-1 and c-Fos in response to mechanical injury were observed in both in vivo and in vitro cultured endothelial cells and SMCs [24,27–30]. We therefore tested the effect of mechanical injury on ERK phosphorylation and activation in SMCs. ERK activation was measured using a phosphospecific anti-ERK antibody, which specifically recognizes ERK after catalytic activation by phosphorylation (Tyr204). As shown in Fig. 5B, monolayer injury resulted in detectable increase in phosphor-ERK by 5 min, peaking at 10 min, and returning nearly to baseline by 20 min. To define upstream signaling events, we tested the possibility that MEK1/2 mediates the activation of ERK by injury. Activation of MEK1/2 was detected by immunoblotting using anti-phosphospecific MEK1/2 antibody. Activation of MEK1/2 was observed at 5 min after mechanical injury (Fig. 5B). For further confirmation that MEK and ERK participated in the regulation of Egr-1 and c-Fos after injury, pretreatment of rat aortic SMCs with U0126, a specific cell-permeable inhibitor of MEK1/2, potently inhibited the wound-induced MEK/ERK activation, as well as the expression of Egr-1 and c-Fos (Fig. 5C). Neither wounding nor treatment with U0126 resulted in any change in the level of total ERK (Fig. 5B and C), suggesting that ERK activity in our model was regulated by catalytic phosphorylation by MEK, rather than by changes in total ERK protein expression. Pretreatment with SB203580, a specific inhibitor of p38MAP kinase, did not affect Egr-1 and c-Fos protein levels after injury (data not shown). Consistent with previous reports from several other groups, we confirmed that rapid activation and phosphorylation of ERK mediated mechanical injury-induced Egr-1 and c-Fos up-regulation in SMC.
3.4. Berberine inhibited the phosphorylated activation of MEK/ERK and sequentially reduced the expression of Egr-1, c-fos, Cyclin D1, and PDGF-A after mechanical injury
After identification that injury leads to rapid activation of MEK/ERK-dependent Egr-1 and c-Fos expression pathway, we undertook experiments to examine the effect of berberine on endogenous expression of Egr-1 and c-Fos proteins, as well as the activation of ERK; we exposed growth-quiescent SMCs to berberine before injuring them with tip. Treatment with 120 μM berberine resulted in a significant reduction in MEK and ERK activation at 10 min post-injury in SMCs (Fig. 6A). Likewise, the expression of Egr-1 and c-Fos protein in SMCs was markedly reduced in the presence of berberine at 90 min post-injury (Fig. 6B). However, another early response gene Ets-1 was not activated after injury and treatment with berberine had no effect on its expression, either. Densitometric analysis showed that treatment with 120 μM berberine caused a significant inhibition of injury-inducible Egr-1 and c-Fos protein levels by 85 and 65%, respectively. Cyclin D1 is a downstream molecule regulated by Egr-1 and is a key molecule that controls the cell cycle entry from G1 to S phase. As indicated in Fig. 7A, the level of Cyclin D1 protein increased significantly 16 h after injury. Treatment of SMCs with 120 μM berberine could significantly reduce the injury-induced Cyclin D1 protein level (Fig. 7A). For further confirming our finding, we did RT-PCR to check the mRNA expression. As depicted in Fig. 7B, treatment with 120 μM berberine significantly abolished the injury-stimulated Egr-1 and c-Fos mRNA expression. We next determined whether berberine could affect the mRNA expression of Egr-1-dependent genes, Cyclin D1 and PDGF-A. As shown in Fig. 7C, the expression levels of Cyclin D1 and PDGF-A mRNA increased in SMCs 4 h after mechanical injury. However, the injury-induced Cyclin D1 and PDGF-A up-regulation was significantly inhibited by berberine in SMCs.
Fig. 6.
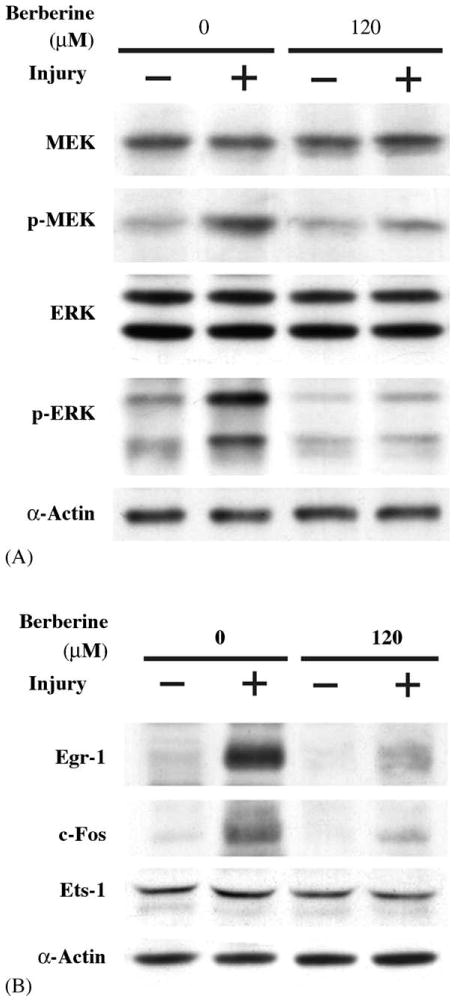
Berberine blocked MEK/ERK activation, as well as Egr-1 and c-Fos expression induced by injury. SMCs were injured in the absence or in the presence of berberine (120 μM): (A) 10 min post-injury, the levels of phosphorylated MEK and ERK were determined; (B) 90 min post-injury, the levels of Egr-1 and c-Fos proteins were measured.
Fig. 7.
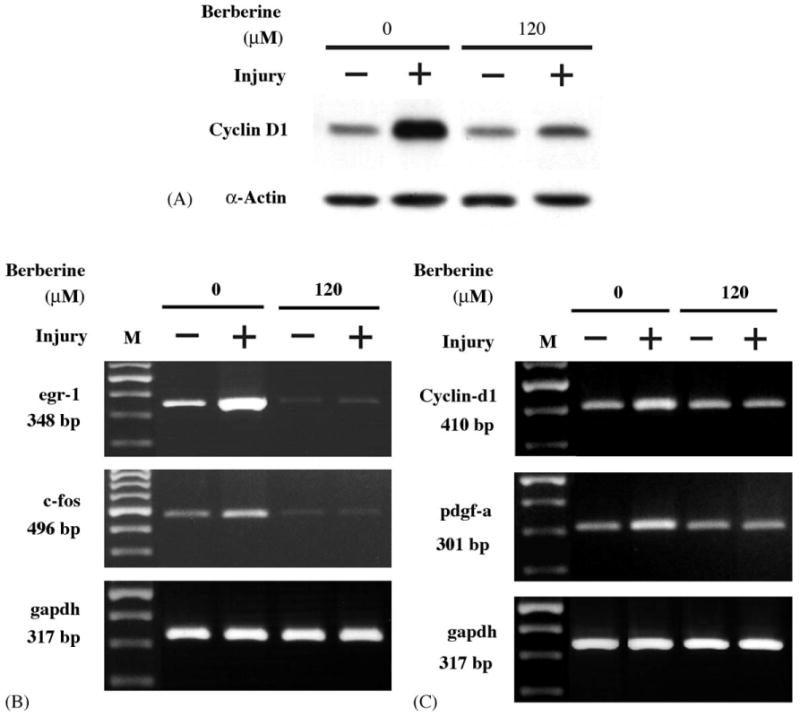
(A) Berberine inhibited the expression of Cyclin D1 protein stimulated by injury. SMCs were treated without or with mechanical injury in the absence or presence of berberine (120 μM) for 16 h. After treatment, cell lysates were isolated, the levels of Cyclin D1 protein was detected by Western blot analysis using anti-Cyclin D1 antibody. (B) Berberine blocked the up-regulation of Egr-1 and c-Fos at a transcriptional level. (C) Berberine suppressed Cyclin D1 and PDGF-A expression at a transcriptional level. Total RNA was isolated 30 min post-mechanic injury for detection of Egr-1/c-Fos or 4 h after injury for analysis of Cyclin D1 and PDGF-A. The levels of mRNA were determination by RTPCR as described in Section 2. GAPDH used as an internal loading control.
4. Discussion
Percutaneous coronary intervention (PCI) with balloon or stenting angioplasty is an important and effective treatment for symptomatic coronary artery disease. Its long-term efficacy, however, is limited by restenosis. Over the past 3 years remarkable progress has been achieved with the introduction of two kinds of drug eluting stents that were approved by American Food and Drug Administration. One is rapamycin (Sirolimus) [31] and the other is paclitaxel (Taxol) [32] based. With the polymer-based slow releasing of the therapeutic agents into the vessel wall, they shared common characteristics in inhibiting the smooth muscle overgrowth and migration after injury and could decrease neointimal formation and restenosis. The current clinical trials showed that the drug eluting stenting could achieve a 5–10% angiographic restenosis rate in comparison with the 20–25% for traditional bare metal stenting [31,32]. However, the scope of clinically proven anti-restenosis agents is extremely limited, and additional new compounds are needed. Berberine has been used extensively in Orient to treat a variety of diseases. It is reported to be effective in lowering blood glucose and low-density lipoprotein cholesterol and inhibiting aldose reductase [20,33,34], as well as preventing SMC proliferation [21]. However, the precise mechanism involved in berberine-mediated anti-proliferative effect in vascular cells has not been well addressed. In this study, we found that 3H-thymidine incorporation and direct cell number counting in SMCs were reduced by berberine treatment. In addition, migration was also reduced by berberine in this in vitro mechanical injury study. We have provided the first evidence that berberine directly inhibited MEK/ERK activation in vascular SMCs within minutes of injury, and blocked injury-induced Egr-1, c-Fos, Cyclin D1 and PDGF-A expression, that are crucial in the regulation of cell proliferation in SMCs. These results imply that the initial signaling pathways leading to such gene activation are compromised by berberine.
Cell proliferation and migration require de novo gene transcription from transducing extracellular signals to the nucleus, where multiple genes are regulated to participate in lesion formation. It has been reported that mechanical injury induces the activation of ERK, which transmits signals from the cytosol to the nucleus. Potential nucleus targets of ERK include the transcription factors ELK-1, Egr-1 and c-Fos, which are known to regulate cellular growth, differentiation, and migration [35–38]. Here we found that treatment of SMCs with berberine reduced the levels of phosphorylated ERK stimulated by injury, in the same concentration range that inhibited DNA synthesis and regrowth. Recently, Kong et al. demonstrated that berberine could rapidly and persistently activate ERK in human hepatoma HepG2 cells, and this berberine-mediated ERK activation was required for the stabilizing of LDL receptor mRNA [20,39]. In mouse macrophage, berberine induced IL-12 production and activation of p38MAPK [40]. The apparent discrepancy between these studies (the effects of berberine on ERK and p38MAPK activation) may be a cell type-specific effect (SMCs versus hepatoma cells or macrophages, rat versus human or mouse). In addition, different experimental parameters and/or conditions used in these studies could also explain the discrepancy in results.
Egr-1 and c-Fos are transcription factors belonging to a class of immediate-early genes that are expressed in response to growth and differentiation signals in a variety of cells and species, including vascular SMCs. It has been reported that Egr-1 or c-Fos gene expression is mediated through different subgroups of mitogen-activated protein kinase (MAPKs), including ERK, JNK, and p38MAPK pathways [41]. In our study, treatment with U0126 (a MEK inhibitor) selectively blocked ERK phosphorylated activation and inhibited the expression of Egr-1 and c-Fos induced by mechanical injury, indicating that injury-mediated Erg-1 and c-Fos up-regulation in rat aortic SMCs was via a MEK/ERK-dependent pathway.
Egr-1 is expressed at low or undetectable levels in the normal rat artery wall [30]. However, it is dramatically induced following balloon dilatation of the carotid artery [8] or on denudation of arterial endothelium [7,42]. In injured vascular cells, Egr-1 is rapidly activated and found to be involved in profound chemotactic and mitogenic effects, which may contribute to the structural remodeling that typically occurs in the pathogenesis of vascular diseases [7,43]. Antisense oligonucleotides or DNA enzymes targeting Egr-1 inhibited SMC proliferation after mechanic injury in vitro [6,27,30,44]. These DNA enzymes also block Egr-1 induction and neointima formation in a rat carotid angioplasty model [30] and after stenting pig coronary arteries in vivo [6]. These findings indicate that Egr-1 as a potential target gene for a therapeutic approach to inhibit neointima formation after angioplasty. In the current study, we showed that berberine could drastically inhibit vascular SMC regrowth after injury and its suppressive effect on Egr-1 and c-Fos expression could bring to light a new potential compound that could help regulate the vascular SMC over-growth after injury.
Egr-1 binding elements have been identified in the promoter of several important genes, including MCP-1, PDGF-A and B [7,9,10], and Cyclin D1 [11,12]. Although all these factors may play a role in driving the early cellular events leading to restenosis, it is generally accepted that PDGF, appears to play an important role in neointimal proliferation and development of restenosis [45]. The association between PDGF and vascular SMC proliferation has been demonstrated in animal experiments in which the increasing levels of PDGF after arterial injury correlated with neointimal cellular proliferation [45]. Besides stimulating cell growth, PDGF also can induce migration in SMCs, as PDGF is the strongest reported chemoattractant for vascular SMCs [46]. In this study, we found that mechanical injury stimulates PDGF-A expression in SMCs via a MEK/ERK-dependent Egr-1 pathway. Treatment with berberine drastically abolished this injury-stimulated PDGF-A gene expression in SMCs. Moreover, exposure to berberine also reduced the levels of Cyclin D1, which is a positive regulator of cell cycle G1 phase.
Recently, Lee et al. reported similar observation that berberine could inhibit vascular smooth muscle cell growth and migration after angiotensin II or heparin binding epidermal growth factor stimulation [23]. They showed the inhibitory effect of berberine was via decreasing Akt phosphorylation in vitro; however, the study did not confirm the Akt activation or the inhibition of Akt by the berberine in the balloon injury model. Nor did they explain the contradictory late re-activation of Akt in the berberine pretreated SMC experiment. Our study did disclose the rapid MEK/ERK activation and the inhibitory effect of berberine on this pathway in the in vitro injury model. Further animal studies to confirm this finding is indicated in the future.
Concerning the strong inhibitory effect of berberine on SMC growth, this effect related to the high concentrations of berberine treatment (>10 μM). This raises the question of whether the observed strong anti-proliferative effect of berberine at higher concentrations is exact and of value in vivo. Further animal and clinical studies may help to elucidate this question. A previous study also demonstrated that berberine significantly inhibited proliferation of cultured rat aortic smooth muscle with concentrations between 10 and 100 μM [21]. In our study, we observed that berberine inhibited regrowth and migration after injury at a lower dosage than just suppressing growth without a preceded injury. The possible explanation is that berberine not only inhibited the transcription factors that are related with growth but also suppressed other pathways that are related with migration. However, this part needs further study. The cellular and molecular mechanisms of anti-proliferative effect of berberine in SMC are still large unknown, but its elucidation will most probably pave the way for treatment of restenosis using this drug. In the current study, we have postulated that at least one mechanism by which berberine inhibits vascular SMC regrowth following mechanical injury is through the inactivation of MEK/ERK and inhibition of Egr-1 and c-Fos expression. Other possible mechanisms, including the down-regulation of ERK-independent cell cycle molecules or the induction of apoptosis, were not examined in this study. However, we found that berberine could induce apoptosis in SMC at the concentration higher than 300 μM.
In conclusion, our findings have provided the first evidence that a pure compound berberine from traditional Chinese herbal medicine, Huanglian, could have inhibitory effect on SMC regrowth in an in vitro injury model. The suppressive effect may be explained by the observed decrease in the expression levels of MEK/ERK-dependent transcription factors Egr-1 and c-Fos, which are related with growth factor activation and cell cycle re-entry. We then confirmed its inhibitory effect on Cyclin D1 and PDGF-A (downstream molecules regulated by Egr-1 or c-Fos). These observations suggest that berberine may be potentially useful in therapeutic efforts to control SMC growth in the injured artery wall; however, the results reported here should be assessed further in animal studies.
Acknowledgments
This study was supported by grants TCVGH-927308C, TCVGH-944305D, and TCVGH-943102B from Taichung Veterans General Hospital, Taiwan, ROC.
References
- 1.Bennett MR. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart. 2003;89(2):218–24. doi: 10.1136/heart.89.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu MW, Roubin GS, King SB., III Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989;79(6):1374–87. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- 3.Lowe HC, Oesterle SN, Khachigian LM. Coronary in-stent restenosis: current status and future strategies. J Am Coll Cardiol. 2002;39(2):183–93. doi: 10.1016/s0735-1097(01)01742-9. [DOI] [PubMed] [Google Scholar]
- 4.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331(8):496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331(8):489–95. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 6.Lowe HC, Fahmy RG, Kavurma MM, Baker A, Chesterman CN, Khachigian LM. Catalytic oligodeoxynucleotides define a key regulatory role for early growth response factor-1 in the porcine model of coronary in-stent restenosis. Circ Res. 2001;89(8):670–7. doi: 10.1161/hh2001.097867. [DOI] [PubMed] [Google Scholar]
- 7.Khachigian LM, Lindner V, Williams AJ, Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996;271(5254):1427–31. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Kawamura M, Wanibuchi H, Ohta K, Hamaguchi A, Omura T, et al. Angiotensin II type 1 receptor blockade inhibits the expression of immediate-early genes and fibronectin in rat injured artery. Circulation. 1995;92(1):88–95. doi: 10.1161/01.cir.92.1.88. [DOI] [PubMed] [Google Scholar]
- 9.Khachigian LM, Williams AJ, Collins T. Interplay of Sp1 and Egr-1 in the proximal platelet-derived growth factor A-chain promoter in cultured vascular endothelial cells. J Biol Chem. 1995;270(46):27679–86. doi: 10.1074/jbc.270.46.27679. [DOI] [PubMed] [Google Scholar]
- 10.Silverman ES, Khachigian LM, Lindner V, Williams AJ, Collins T. Inducible PDGF A-chain transcription in smooth muscle cells is mediated by Egr-1 displacement of Sp1 and Sp3. Am J Physiol. 1997;273(3 Pt 2):H1415–26. doi: 10.1152/ajpheart.1997.273.3.H1415. [DOI] [PubMed] [Google Scholar]
- 11.Yan YX, Nakagawa H, Lee MH, Rustgi AK. Transforming growth factor-alpha enhances cyclin D1 transcription through the binding of early growth response protein to a cis-regulatory element in the cyclin D1 promoter. J Biol Chem. 1997;272(52):33181–90. doi: 10.1074/jbc.272.52.33181. [DOI] [PubMed] [Google Scholar]
- 12.Guillemot L, Levy A, Raymondjean M, Rothhut B. Angiotensin II-induced transcriptional activation of the cyclin D1 gene is mediated by Egr-1 in CHO-AT(1A) cells. J Biol Chem. 2001;276(42):39394–403. doi: 10.1074/jbc.M103862200. [DOI] [PubMed] [Google Scholar]
- 13.Goetze S, Xi XP, Kawano Y, Kawano H, Fleck E, Hsueh WA, et al. TNF-alpha-induced migration of vascular smooth muscle cells is MAPK dependent. Hypertension. 1999;33(1 Pt 2):183–9. doi: 10.1161/01.hyp.33.1.183. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Izumi Y, Yano M, Hamaguchi A, Miura K, Yamanaka S, et al. Angiotensin blockade inhibits activation of mitogen-activated protein kinases in rat balloon-injured artery. Circulation. 1998;97(17):1731–7. doi: 10.1161/01.cir.97.17.1731. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Dietrich H, Metzler B, Wick G, Xu Q. Hyperexpression and activation of extracellular signal-regulated kinases (ERK1/2) in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 2000;20(1):18–26. doi: 10.1161/01.atv.20.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, et al. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. Embo J. 1995;14(5):951–62. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gille H, Kortenjann M, Strahl T, Shaw PE. Phosphorylation-dependent formation of a quaternary complex at the c-fos SRE. Mol Cell Biol. 1996;16(3):1094–102. doi: 10.1128/mcb.16.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetze S, Xi XP, Graf K, Fleck E, Hsueh WA, Law RE. Troglitazone inhibits angiotensin II-induced extracellular signal-regulated kinase 1/2 nuclear translocation and activation in vascular smooth muscle cells. FEBS Lett. 1999;452(3):277–82. doi: 10.1016/s0014-5793(99)00624-9. [DOI] [PubMed] [Google Scholar]
- 19.Khachigian LM, Collins T. Inducible expression of Egr-1-dependent genes. A paradigm of transcriptional activation in vascular endothelium. Circ Res. 1997;81(4):457–61. doi: 10.1161/01.res.81.4.457. [DOI] [PubMed] [Google Scholar]
- 20.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 21.Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, et al. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol. 2000;399(2–3):187–96. doi: 10.1016/s0014-2999(00)00339-3. [DOI] [PubMed] [Google Scholar]
- 22.Jantova S, Cipak L, Cernakova M, Kost'alova D. Effect of berberine on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol. 2003;55(8):1143–9. doi: 10.1211/002235703322277186. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Lim HJ, Park HY, Lee KS, Park JH, Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo Berberine improves neointima formation in a rat model. Atherosclerosis. 2005 doi: 10.1016/j.atherosclerosis.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Koyama H, Olson NE, Dastvan FF, Reidy MA. Cell replication in the arterial wall: activation of signaling pathway following in vivo injury. Circ Res. 1998;82(6):713–21. doi: 10.1161/01.res.82.6.713. [DOI] [PubMed] [Google Scholar]
- 25.Miano JM, Topouzis S, Majesky MW, Olson EN. Retinoid receptor expression and all-trans retinoic acid mediated growth inhibition in vascular smooth muscle cells. Circulation. 1996;93(10):1886–95. doi: 10.1161/01.cir.93.10.1886. [DOI] [PubMed] [Google Scholar]
- 26.Cabre A, Girona J, Vallve JC, Heras M, Masana L. Cytotoxic effects of the lipid peroxidation product 2,4-decadienal in vascular smooth muscle cells. Atherosclerosis. 2003;169(2):245–50. doi: 10.1016/s0021-9150(03)00196-5. [DOI] [PubMed] [Google Scholar]
- 27.Santiago FS, Atkins DG, Khachigian LM. Vascular smooth muscle cell proliferation and regrowth after mechanical injury in vitro are Egr-1/NGFI-A-dependent. Am J Pathol. 1999;155(3):897–905. doi: 10.1016/S0002-9440(10)65189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santiago FS, Lowe HC, Day FL, Chesterman CN, Khachigian LM. Early growth response factor-1 induction by injury is triggered by release and paracrine activation by fibroblast growth factor-2. Am J Pathol. 1999;154(3):937–44. doi: 10.1016/S0002-9440(10)65341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YH, Fang LH, Ku BS. Fangchinoline inhibits rat aortic vascular smooth muscle cell proliferation and cell cycle progression through inhibition of ERK1/2 activation and c-fos expression. Biochem Pharmacol. 2003;66(9):1853–60. doi: 10.1016/s0006-2952(03)00550-1. [DOI] [PubMed] [Google Scholar]
- 30.Santiago FS, Lowe HC, Kavurma MM, Chesterman CN, Baker A, Atkins DG, et al. New DNA enzyme targeting Egr-1 mRNA inhibits vascular smooth muscle proliferation and regrowth after injury. Nat Med. 1999;5(11):1264–9. doi: 10.1038/15215. [DOI] [PubMed] [Google Scholar]
- 31.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 32.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 33.Leng SH, Lu FE, Xu LJ. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin. 2004;25(4):496–502. [PubMed] [Google Scholar]
- 34.Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, et al. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51(11):1439–43. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 35.Haas TL, Stitelman D, Davis SJ, Apte SS, Madri JA. Egr-1 mediates extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. J Biol Chem. 1999;274(32):22679–85. doi: 10.1074/jbc.274.32.22679. [DOI] [PubMed] [Google Scholar]
- 36.Hultgardh-Nilsson A, Cercek B, Wang JW, Naito S, Lovdahl C, Sharifi B, et al. Regulated expression of the ets-1 transcription factor in vascular smooth muscle cells in vivo and in vitro. Circ Res. 1996;78(4):589–95. doi: 10.1161/01.res.78.4.589. [DOI] [PubMed] [Google Scholar]
- 37.Mollinedo F, Gajate C, Tugores A, Flores I, Naranjo JR. Differences in expression of transcription factor AP-1 in human promyelocytic HL-60 cells during differentiation towards macrophages versus granulocytes. Biochem J. 1993;294(Pt 1):137–44. doi: 10.1042/bj2940137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72(2):197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 39.Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005 doi: 10.1161/01.ATV.0000181761.16341.2b. [DOI] [PubMed] [Google Scholar]
- 40.Kang BY, Chung SW, Cho D, Kim TS. Involvement of p38 mitogen-activated protein kinase in the induction of interleukin-12 p40 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid. Biochem Pharmacol. 2002;63(10):1901–10. doi: 10.1016/s0006-2952(02)00982-6. [DOI] [PubMed] [Google Scholar]
- 41.Blaschke F, Bruemmer D, Law RE. Egr-1 is a major vascular pathogenic transcription factor in atherosclerosis and restenosis. Rev Endocr Metab Disord. 2004;5(3):249–54. doi: 10.1023/B:REMD.0000032413.88756.ee. [DOI] [PubMed] [Google Scholar]
- 42.Silverman ES, Khachigian LM, Lindner V, Williams AJ, Collins T. Inducible PDGF A-chain transcription in smooth muscle cells is mediated by Egr-1 displacement of Sp1 and Sp3. Am J Physiol Heart Circ Physiol. 1997;273(3):H1415–26. doi: 10.1152/ajpheart.1997.273.3.H1415. [DOI] [PubMed] [Google Scholar]
- 43.Ross R. The pathogenesis of atherosclerosis: a perspective for the. Nature. 1993;362(6423):801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 44.Fahmy RG, Khachigian LM. Antisense Egr-1 RNA driven by the CMV promoter is an inhibitor of vascular smooth muscle cell proliferation and regrowth after injury. J Cell Biochem. 2002;84(3):575–82. [PubMed] [Google Scholar]
- 45.Uchida K, Sasahara M, Morigami N, Hazama F, Kinoshita M. Expression of platelet-derived growth factor B-chain in neointimal smooth muscle cells of balloon injured rabbit femoral arteries. Atherosclerosis. 1996;124(1):9–23. doi: 10.1016/0021-9150(95)05742-0. [DOI] [PubMed] [Google Scholar]
- 46.Bornfeldt KE, Raines EW, Nakano T, Graves LM, Krebs EG, Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994;93(3):1266–74. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]


