Abstract
The epididymis serves a critical function of preparing the male germ cells for fertilization. In order for the epididymis to carry out this role it must undergo a highly coordinated succession of molecular and morphogenic events during development. These events begin with the formation of the Wolffian or nephric duct, the embryonic precursor of the male reproductive system, and end with the three-dimensional coiled postnatal epididymis that is comprised of several distinctly functional segments. How the duct changes from a simple straight tube to a highly convoluted structure will be the focus of this article. In reviewing the literature's current understanding of epididymal morphogenesis, we will highlight some of the classic morphological studies and discuss some of the more recent genetic models that have all served to contribute to our understanding of this system. Where published information is scarce we will provide potential hypotheses that warrant further investigation and may open up new directions of exploration using the epididymis as a model for tubular morphogenesis.
Keywords: Epididymis, Morphogenesis, Mesonephric tubules, Efferent ducts, Mesonephric duct, Wolffian duct, tubulogenesis
Introduction
Perhaps one the least studied epithelial tubes, from a morphogenic perspective, is the epididymis. Derived from the anterior Wolffian (or mesonephric) duct, the adult epididymis reaches over one meter in the mouse, three meters in the rat, an admirable six meters in the human and an extraordinary 15-18 m in the stallion (Jiang et al., 1994; Maneely, 1959; Stoltenberg et al., 1998; Takano et al., 1981; Turner et al., 1990; Von and Neuhaeuser, 1964). In the mouse, the Wolffian duct is approximately 1mm at embryonic day 14 (E14), which means it must grow over 1000 times its length within a defined space (Figure 1). However, this is not simply an elongation event, the epididymal tube is folded into a highly organized structure comprised of many lobules/segments, each with distinct morphology and function, that can be grouped into roughly five gross anatomical regions: initial segment, caput, corpus, cauda and vas deferens. The division of the epididymis into different regions or segments has been the topic of considerable discussion and the reader is referred to several articles (Hinton and Turner, 2003; Robaire and Hinton, 2002; Robaire et al., 2006).
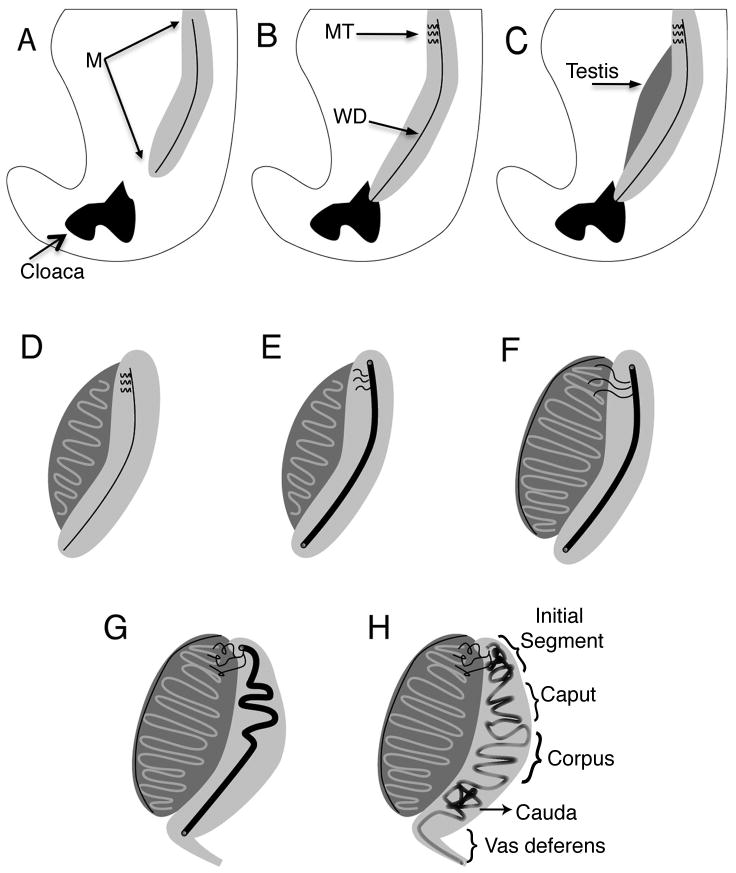
Figure 1. Stages of Urogenital Development.
(A) E9, the mesonephros is visible and the pronephric duct exists as a solid structure. (B) E10, the pronephric duct has grown caudally through the urogenital ridge and contacts the cloaca. At this point the duct is now known as the mesonephric or Wolffian duct. Cranial S-shaped mesonephric tubules meet and adjoin with the Wolffian duct. (C) E11, a distinct genital ridge is present, in XY individuals this is the precursor to the testes. (D) By E12, the sexual differentiation of the gonadal ridge has begun and distinct testis cords are visible. (E) By E13, lumenization of the Wolffian duct has occurred. The diameter of the duct is approximately 20 cells or 45 μm; epithelial cell height increases to 18 μm. (F) E14, the testes become rounder in shape and testes cords are more developed. The coelomic vessel is prominent on the surface of the testes and efferent ducts appear to connect the testes to the Wolffian duct. (G) E16-18, the efferent ducts are coiled and the caput epididymis begins bending and coiling. At this time the posterior epididymis, cauda, remains a straight tube. (H) E19-P5, the entire epididymal tube is compacting and there is an increase in 3-dimensional coiling patterns that begin in the more anterior regions and progresses to the posterior regions, distal to the testis. M=mesonephros, MT=mesonephric tubules (future efferent ducts), WD=Wolffian duct, E=embryonic day, P5=postnatal day 5 (Dyche, 1979; Hogan et al., 1994; Smith and Mackay, 1991; Theiler, 1989; Upadhyay and Zamboni, 1981).
It is clear that tubular morphogenesis is a fundamental process during the genesis of many important organs including the brain, heart, kidney, lungs and intestine of many species. The epididymis is certainly no exception in that the survival of the mammalian species depends upon this organ being fully functional. Spermatozoa leave the testis neither fully motile nor with the ability to recognize or fertilize an egg but they acquire these abilities after passing through the epididymis (Bedford, 1967; Orgebin-Crist, 1968; Robaire et al., 2006). This change in spermatozoa is referred to as sperm maturation and can take up to two weeks to complete. Hence, the elongation and morphogenesis of the Wolffian duct must be highly coordinated with its specialized function of providing an appropriate environment for sperm maturation.
Determining how an epithelial tube will elongate, expand, taper or fold is a fascinating developmental conundrum that has attracted much interest in recent years. From a developmental standpoint the creation of the kidney and epididymis are interrelated and so the unveiling of molecular events involved in kidney formation has contributed to our understanding of processes involved in epididymal organogenesis. While there are many differences in how these organs are created, the most obvious being the lack of branching morphogenesis in the epididymis, it is quite feasible that the elongation and patterning of folding share common processes and regulatory pathways in these two organs.
The events leading to the initiation of Wolffian duct formation will only be briefly discussed since there are a number of reviews that tackle this topic in considerable depth (Brandli, 1999; Herzlinger, 1995; Schultheiss et al., 2003; Vize et al., 1997). Due to the paucity of information on Wolffian duct morphogenesis, much of what will be discussed will be based on a mixture of classical morphological studies, unpublished observations and sheer speculation. Furthermore, this review will focus primarily on the mouse since the majority of published studies have used this animal model.
Epididymal Tubulogenesis
The epididymal duct precursor, known as the Wolffian duct, arises within the urogenital ridge during embryogenesis. Within the urogenital ridge there are three overlapping units known from anterior to posterior as the pronephros, mesonephros, and metanephros. The Wolffian duct first appears as short transient segments within the most anterior region of the pronephros and then as a continuous tube that runs the length of the urogenital ridge and terminates in the cloaca at the most posterior end of the embryo. Although, there are several ways by which biological tubes can form (Hogan and Kolodziej, 2002; Lubarsky and Krasnow, 2003; Nelson, 2003; Neumann and Affolter, 2006; Rosario and Birchmeier, 2003) it appears that the Wolffian duct is a result of the rearrangement of mesenchymal cells presumably involving convergent extension (Keller et al., 1985) and not elongation by cell proliferation (see discussion on this topic by (Schultheiss et al., 2003).
Epithelial Formation
The processes that underlie the conversion of mesenchymal cells to ductal epithelia remain a topic of continuing research. There are several transcription factors that are known to be expressed before epithelial duct formation and are generally regarded as key regulatory signals during Wolffian duct establishment (Table 1). These include the paired domain transcription factors Pax2 and Pax8, the homeodomain transcription factor Lim1, and Emx2 a mammalian homologue of a Drosophila head gap gene empty spiracles (ems). In the early embryo, all of these genes have been localized to the mesenchymal condensations that form the ductal component and later in development can be found expressed in the ductal epithelium (Dressler et al., 1990; Fujii et al., 1994; Miyamoto et al., 1997; Plachov et al., 1990). These observations suggest involvement of these genes in the initial mesenchyme-epithelium transition. The generation and analysis of knockout animals has been imperative in our understanding of the function of these genes in Wolffian duct genesis. Mutant mice born without Pax2 lack several urogenital structures including kidneys, ureters and both male and female genital tracts, which is due to partial development and degeneration of the Wolffian duct (Torres et al., 1995). While absence of Pax8 alone does not cause urogenital abnormalities, embryos lacking both Pax2 and Pax8 fail to undergo the mesenchyme to epithelium transition necessary for tube formation therefore leading to a complete absence of the Wolffian duct (Bouchard et al., 2002). In the absence of Lim1, which is also thought to act as an early epithelial differentiator downstream of the Pax genes, there is a similar lack of Wolffian derivatives (Kobayashi et al., 2004). In Emx2 null mice, although there is an initial formation of a Wolffian duct its structure degenerates very early in development and these animals also lack kidneys, ureters and gonads (Miyamoto et al., 1997).
Table 1.
Mutant Phenotypes of Transcription Factors Involved in Wolffian Duct and Mesonephric Tubule Development
| Gene | Knockout Phenotype | Reference |
|---|---|---|
| Pax2 | Degeneration of Wolffian duct; lack of kidneys and ureters | Torres et al., 1995 |
| Pax8 | No urogenital abnormalities | Bouchard et al., 2002 |
| Pax2/8 | No epithelial differentiation; No Wolffian duct development | Bouchard et al., 2002 |
| Lim1 | No epithelial differentiation; No Wolffian duct development | Kobayashi et al., 2004 |
| Emx2 | Initial Wolffian duct formation and subsequent degeneration; Lack of kidneys, ureters and gonads | Miyamoto et al., 1997 |
| WT1 | Wolffian duct present; Posterior mesonephric tubules are absent | Sainio et al., 1997 |
| Six1 | Wolffian duct present; Posterior mesonephric tubules are absent | Kobayashi et al., 2007 |
| Wnt9b | Wolffian duct present; Anterior and posterior mesonephric tubules are | Carroll et al., 2005 |
| Foxc1 | Ectopic expression of excess mesonephric tubules | Kume et al., 2000 |
All of these transcription factors that are expressed before epithelial duct formation respond to signals from adjacent mesoderm and overlying ectoderm. In fact, the surface ectoderm has been shown to be essential in Wolffian duct tubulogenesis and in its absence there is a decrease in Pax2 levels (Obara-Ishihara et al., 1999). Interestingly, BMP-4, which is regulated by the surface ectoderm, can functionally substitute for the ectoderm's presence and restore Wolffian duct initiation. It is probable that opposing factors originating from both the surface ectoderm and mesoderm are responsible for restricting the expression of these transcription factors to a specific domain of the intermediate mesoderm from which the duct arises. It appears that each of these transcription factors plays an important role in the cellular differentiation necessary for tubulogenesis. However, the exact mechanisms, redundancies and interactions between these factors and others remain unknown.
Mesonephric Tubule Formation
Early in mesonephric development mesenchymal condensations form along the anterior/posterior (A/P) axis of the Wolffian duct. These condensations undergo a rearrangement of their cell positioning to form a series of tubules known as mesonephric tubules. Those mesonephric tubules that are bound to the Wolffian duct in the anterior region (closest to the testis) are believed to be precursors of the efferent ducts.
The growth of mesonephric tubules along the Wolffian duct axis may be locally controlled by several factors (Table 1). For instance, the zinc-finger transcription factor Wt1 and the homeobox gene Six1, both expressed in the mesenchyme surrounding the duct, specifically regulate formation of the more posterior mesonephric tubules by interfering with the mesenchyme-to-epithelial transition and subsequent rearrangement of cells (Kobayashi et al., 2007; Sainio et al., 1997). Wnt9b is expressed in the inductive epithelia of the duct and is thought to act as a paracrine regulator of the mesenchyme-to-epithelial transition. In animals devoid of Wnt9b there is an absence of all mesonephric tubules despite the normal formation of the Wolffian duct itself (Carroll et al., 2005). Conversely, the forkhead transcription factor, Foxc1, provides a suppressive effect on mesonephric tubule formation (Kume et al., 2000), likely by up regulating key cell death genes along the length of the duct. These data emphasize the importance of the mesonephric mesenchyme, which acts as both a structural and molecular scaffold for tubule outgrowth. In this cellular compartment two or more factors ultimately restrict mesonephric tubule growth by establishing a survival gradient within a defined region of the A/P axis. This survival gradient would be the region in which there is both an increased expression of growth promoters and a decreased presence of apoptosis regulators. The mesenchyme also provides a physical foundation with space constraints for tubule growth.
While the posterior mesonephric tubules regress, in males the anterior tubules fuse with the Wolffian duct and are believed to become the efferent ductules, which serve as a conduit between the rete testes and epididymis. The reason for the growth and subsequent regression of the posterior mesonephric tubules is unknown. However, morphological studies in the bovine have suggested that the mesonephric tubules that regress represent a primary population of tubules that form as a part of the excretory system (Wrobel, 2001). These primary tubules undergo budding, resulting in short tubular structures, the majority of which undergo immediate regression together with the primary mesonephric tubules. This observation is consistent with the presence of aberrant and blind ending ‘efferent ducts’ that have been described in several adult mammalian species (Guttroff et al., 1992) (Figure 2). Under this developmental model the definitive efferent ducts arise from a secondary set of tubules, which may correspond with the anterior tubules that connect with the Wolffian duct. It is therefore also possible that the blind ending efferent ducts are representative of those secondary mesonephric tubules that did not reach the Wolffian duct. One also cannot rule out the possibility that there exists a selection process among the original anterior mesonephric tubules, resulting in only one surviving tubule that is attached to the Wolffian duct. Potentially as this mesonephric tubule elongates toward the testis, it is able to undergo budding and branching resulting in the complex arrangement of efferent ducts that are seen in the adult. The presence of the blind ending efferent ducts found could represent terminated growth of a budded tubule.
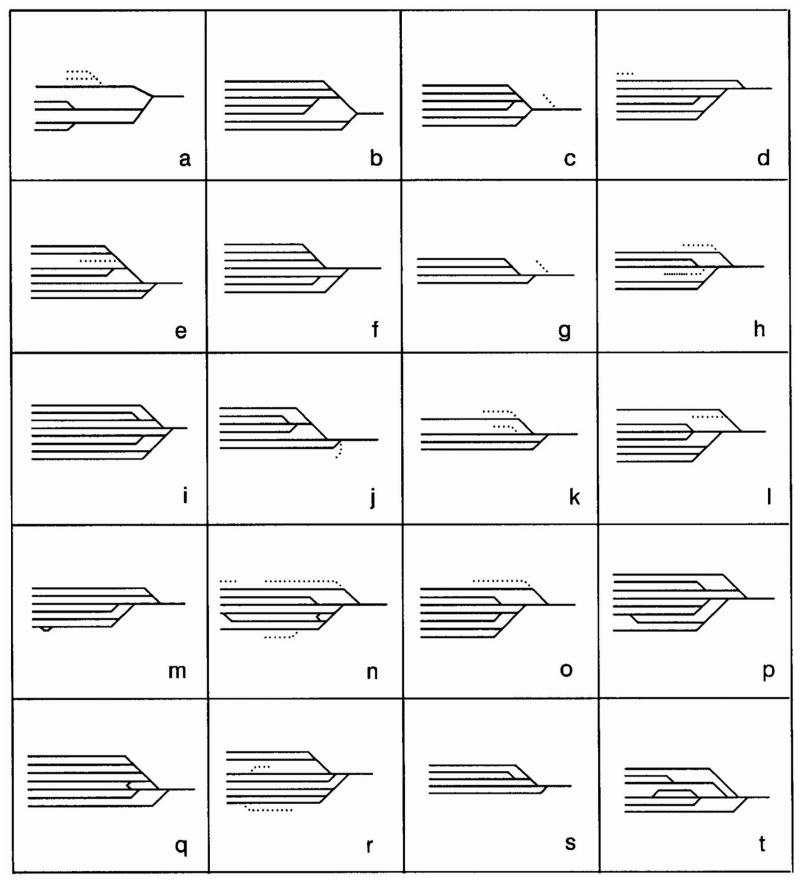
Figure 2. Patterns of microdissected efferent ducts of the rat.
Diagram represents the variations in arrangements of how the adult efferent ducts attach to the rete testes and epididymis. Patterns are drawn to represent the flow of ductal fluid from the testis to the epididymis (left to right). Each alphabetical letter represents a different animal. Blind-ended tubules are represented by dotted lines (…). Note that in the rodent all efferent ducts feed into a common duct leading into the initial segment of the epididymis with multiple points of entry into the rete testes. From Guttroff et al., 1992 and reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Depending upon the precise age of the embryo, the arrangements and the attachments of the anterior mesonephric tubules to the Wolffian duct varies (see (Sainio et al., 1997; Vazquez et al., 1998). The patterns of attachment of the mesonephric tubules may either represent variations at different stages of development and/or differences between animals (Figure 2). The latter is the most likely given the considerable variation observed in the pattern of adult efferent ducts as shown by (Guttroff et al., 1992). There is also a major difference in the number of efferent ducts that are observed between species, ranging from one in the opposum (Maruch et al., 1989) to 33 in the donkey (Mobilio and Campus, 1912). In the mouse 3 to 5 efferent ductules have been recorded in the adult, while the rat is thought to have between 2 and 8 ductules (Vazquez et al., 1998). It is unknown what regulates the number of efferent ducts in a species, or what genetic or environmental components could cause the variation observed within a species.
In most mammalian species, the efferent ductules have multiple points of entry into the rete testis. In rodents these ductules converge into a common duct that feeds into the initial segment of the epididymis while in larger mammals, including the human, parallel efferent ductules form multiple entries into the head of the epididymis. This enormous variation between species leaves many questions about the ways in which efferent duct connections to the rete testes and epididymis are made.
One hypothesis may be that a mass of cells from the mesonephros migrates into the testis forming the rete testis (Upadhyay and Zamboni, 1981). These mesonephric cells of the rete could produce factors of an attractive nature that stimulate the mesonephric tubules to elongate toward the testis and join with the rete resulting in direct connection of the lumina. In this arrangement there remains a biological border that prevents intermixing of cells originating from the genital ridge and mesonephros. While little in known regarding the mechanisms involved in mesonephric tubule connections, the expression of steroid hormone receptors in the epithelia of the early efferent ducts has been well documented. In the mouse, the efferent ducts were the first site of epithelial androgen and estrogen receptor expression in the reproductive tract (Cooke et al., 1991a; Cooke et al., 1991b), this suggests the possibility of estrogen or androgen being the simulative factor of tubule growth being produced by the testes.
Of particular fascination is the consistent pattern of the rodent efferent ductules to form a common collecting duct that funnels into the initial segment of the epididymis, while maintaining several points of entry into the rete. A possible hypothesis to explain this developmental phenomenon is that cells from the mesonephric tubules segregate into two cell lineages. At some point during elongation toward the testis, a portion of cells from the tubule migrate back toward the Wolffian duct and form the common efferent duct and a part of the initial segment. These cells retain their “embryonic need” for lumicrine factors (Hinton et al., 2000) secreted by the testis for their survival. This hypothesis would explain why only certain cells of the initial segment and no other epididymal cells undergo apoptosis when deprived of testicular luminal fluid factors. The remaining cells of the mesonephric tubules form the efferent ductules and develop their own cellular characteristics independent of testicular factors.
Of course these proposed hypotheses are not necessarily mutually distinct and experiments that track cell linage during development would help to elucidate the cellular mechanisms taking place that allow for the formation of the efferent ducts and head of the epididymis.
Elongation of the Wolffian duct
Transformation of the straight Wolffian duct into the complex and coiled components of the male reproductive tract requires considerable duct elongation combined with a morphogenetic program that is able to define boundaries and specify regional characteristics of the duct. This multifaceted process, which includes synchronized elongation and coiling, likely involves a combination of cell proliferation, cell shape changes, cell rearrangements, fluid secretion, apoptosis and/or possible cell division “hotspots” that contribute to coiling.
The Wolffian duct elongates stereotypically along a caudal path until it reaches the cloaca. A study by (Grote et al., 2006) has suggested that Gata3, a transcription factor present in the Wolffian duct anlage is at least partially responsible for guiding the Wolffian duct extension by controlling epithelial cell proliferation. Presumably, cell proliferation is the major contributor of Wolffian duct growth and results not only in duct lengthening but also duct thickening (Dyche, 1979) both of which are highly coupled to coiling ability. Measurements taken of the Wolffian duct during late gestation of the mouse, E13 to postnatal day 1 (P1), indicated that the number of cells per cross section increased from approximately 20 to 27, the cross sectional diameter of the duct increased from 40μm to 50 μm and the cell height increased from 18 μm to 21 μm (Dyche, 1979). These latter measurements indicate that changes in cell size would also contribute to Wolffian duct elongation.
Fluid secretion into the lumen has also been considered to be a driver of tube growth (Alcorn et al., 1977; Hooper and Harding, 1995; Lubarsky and Krasnow, 2003) and since a patent lumen is observed in the mouse Wolffian duct around day E13-14 (Dyche, 1979), it would indicate that fluid is secreted into the duct around this time. The timing is critical because at day E13-14 the duct is straight and elongation and coiling is about to begin. By utilizing a fluorescent tracer it has become evident that at this stage of development testosterone can be transported via the lumen from the testes throughout the length of the duct (Tong et al., 1996). It is well accepted that androgens can act via androgen receptors located in the mesenchymal compartment and contribute to Wolffian duct stabilization and subsequent elongation. In addition, epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), both shown to be important in Wolffian duct stabilization, have been found in high concentrations in the adult rat rete testis fluid and could act as stimulators of tube growth (Cooke et al., 1991a; Cooke et al., 1991b; Kirby et al., 2003; Lan et al., 1998).
Coiling of the Wolffian/Epididymal Duct
It is quite feasible to imagine that the mesenchyme surrounding the developing epididymis might vary in its instructive properties that effect not only cell proliferation, but also coiling and differentiation of the epithelia. A morphogenic gradient formed within the mesenchymal compartment could be a means to induce folding differentially along the length of the duct. In this case, the gradient would be produced along the basolateral surface of the Wolffian duct via interstitial fluid flow (Rutkowski and Swartz, 2007). When the morphogen(s) reached a critical concentration at a particular region, elongation and folding of that region would result. As a means to promote coiling one could also envisage cell division “hotspots”, that is, epithelial cells dividing at discrete regions along the duct at a greater rate compared to other regions. The rapid proliferation of these hotspots in the Wolffian duct epithelium combined with the relative quiescence in proliferation of the mesenchyme would result in a space constraint for the elongating epithelium resulting in bending/coiling of the duct at those points.
Studies from several investigators (Dyche, 1979; Staack et al., 2003; Tsuji et al., 1991) including our own unpublished observations (see also (Robaire et al., 2006) have shown that the coiling of the mouse Wolffian duct from E14 to P1 begins in the proximal region nearest to the testis and progresses distally over time. Early in the development of the mouse epididymis, coiling occurs in only one plane, but from about E16-E18 three-dimensional (3-D) coiling is observed in the initial segment and caput regions (Hannema and Hughes, 2007; Staack et al., 2003; Tsuji et al., 1991). Presumably, space limitations as dictated by the boundaries of the surrounding mesenchyme play a role in initiating the 3-D coiling (Figure 3). This would indicate that there is indeed active communication between epithelial cells and the surrounding mesenchyme such that the correct shape and size of that particular epididymal region is maintained. As development continues little three-dimensional coiling is observed in the corpus region but three-dimensional coiling is reinstated in the duct of the cauda region. Interestingly, coiling is not initiated in the vas deferens, which remains a straight tube originating from the cauda (Hannema and Hughes, 2007; Staack et al., 2003; Tsuji et al., 1991). Hence, it seems plausible that during embryonic development the Wolffian duct receives “coil” and “do not coil,” signals as well as “coil in a three-dimensional manner” and “do not coil in a three-dimensional manner” signals. One simple hypothesis to explain the “do not coil signal” of the vas deferens may be the contribution of the thick layers of smooth muscle cells that surround this region of the duct and physically limit its coiling.
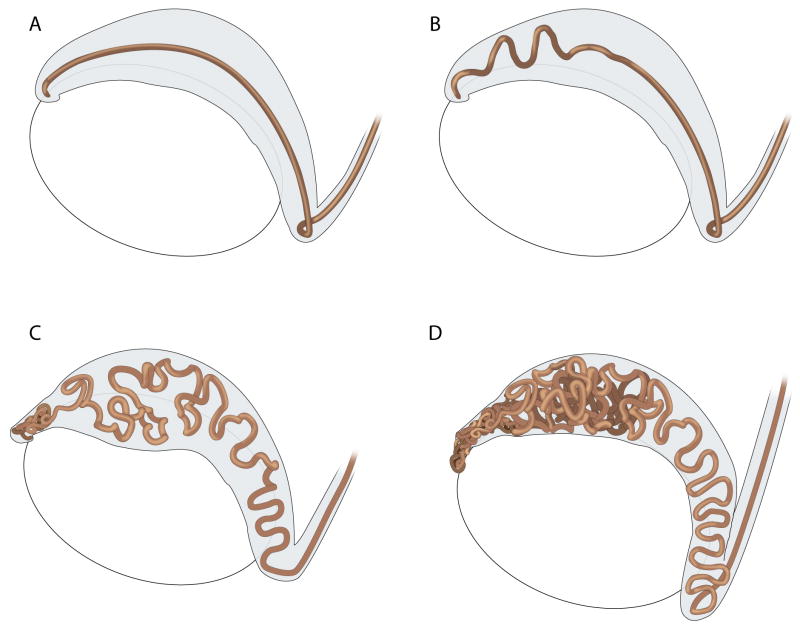
Figure 3. Coiling of the Wolffian/Epididymal Duct from E14-P1.
(A) At E14 the epididymal duct is a straight tube that extends along the length of the organ, (B) Just 2 days later, at E16, the rapid growth of the duct causes the tube to curve and bend beginning in the anterior region, distal to the testis. (C) By E 18, the duct has planar bends along its length, and has begun the process of 3-dimensional coiling. Again, beginning in the most anterior region, the duct changes direction in the plane and twists into another plane, folding onto itself. The duct never completes a 360-degree turn, and therefore does not spiral. The coiling has been purposely opened in this figure to show the manner in which coiling occurs. (D) As the tube continues to elongate, constraints of the surrounding tissue presumably force the duct to compact even more, causing dense coiling in the anterior regions, which can be observed by P1. The more distal regions continue to lengthen and exhibit planar coiling. Note that the vas deferens, located immediately posterior to the epididymis, never coils and instead remains a straight tube that forms a continuation of the epididymal duct. Efferent ducts have been omitted from this diagram. E=embryonic day, P1=postnatal day 1 (unpublished observations).
Regionalization of the Epididymal Duct
Region specific expression of several homeobox genes is important for the differentiation of the Wolffian duct into its morphologically and functionally distinct segments. The vertebrate Hox genes are the homologues of Drosophila homeotic genes, which are evolutionarily conserved transcriptional regulators that determine patterning (McGinnis and Krumlauf, 1992). In the Wolffian duct, Hoxa and Hoxd genes, particularly 10, 11, and 13, exhibit unique patterns and functional roles along the anterior/posterior axis of the duct. Hoxa10 and Hoxa11 appear to work redundantly to define the boundary between the epididymis and vas deferens. Loss of Hoxa10 or Hoxa11 or both resulted in various degrees of transformation of the vas deferens into the coiled epididymal structure (Benson et al., 1996; Branford et al., 2000; Davis et al., 1995; Hsieh-Li et al., 1995; Podlasek et al., 1999). On the other hand, Hoxa13 and Hoxd13 seem to have a gene dosage-dependent effect on formation of the mouse seminal vesicle and prostate, derivatives of the posterior Wolffian duct and urogenital sinus, respectively (Podlasek et al., 1999). These genetic studies implicate that Hox genes serve as intrinsic programmers that establish the A/P pattern of the Wolffian duct. However, it remains unknown how these transcription regulators induce regional transformation of cell morphology and phenotype variation in different parts of the Wolffian duct.
One proposed mechanism is that these Hox genes control the expression of morphogens. It is reasonable to assume that cells in the Wolffian duct could acquire different phenotypes based on their distance from the source of the morphogen or morphogenetic field that is established along the A/P axis of the Wolffian duct. Differentiation of the anterior Wolffian duct into the coiled epididymis is a perfect model to study this possibility. At the time of birth, the anterior Wolffian duct is completely transformed into the caput, corpus, and caudal regions of the epididymis along the A/P axis. The vas deferentia are an extension of the cauda epididymis, positioned most distal from the testis, but vary from the cauda in cellular morphology, function and lack of coiling. We have recently found that inhibin beta A (Inhba), a component of inhibins and activins, is highly expressed in the mesenchyme of the anterior Wolffian duct (distal to the testis) and diminishes posteriorly before the onset of Wolffian duct morphogenesis (Tomaszewski et al., 2007). The gradient of Inhba expression coincides with the degree of coiling in the epididymis (Figure 4). When the Inhba gene was inactivated in mouse embryos, the coiling of the caput epididymis was completely abolished (Tomaszewski et al., 2007). It appears that Inhba acts as a proliferation-stimulating signal to the Wolffian duct epithelium as proliferation of the epithelium in Inhba knockout epididymis was significantly decreased and a loss of caput-characteristic coiling was observed.
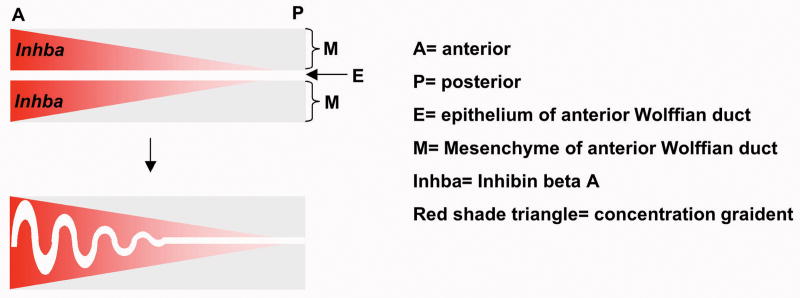
Figure 4. Morphogenetic gradient established along the anterior-posterior axis of the epididymis.
Cells of the epididymal duct may acquire different phenotypes based on their distance from the highest concentration of a morphogen. In the case of Inhibin Beta A (Inhba), which is highly expressed in the anterior mesenchymal cell compartment, coiling of the epididymal duct epithelium is induced in the area of highest Inhba expression.
Another example of a gene mutant showing a regional epididymal phenotype is the c-Ros knockout. This gene encodes an orphan tyrosine kinase receptor that is highly expressed in the initial segment of the mouse epididymis (Sonnenberg-Riethmacher et al., 1996). The intriguing phenotype of the c-Ros null animal is the total absence of the regional characteristics that define the initial segment of the epididymis. While there are no apparent abnormalities in the remaining epididymal regions, the loss of initial segment causes male infertility that is at least partly due to the failure of the spermatozoa to regulate its volume once it enters the higher osmotic environment of the female reproductive tract (Yeung et al., 1999). From a developmental point of view, the proximal region of the epididymis in the c-Ros null animal lacks the predominant vasculature that is characteristic of the initial segment in the normal mouse epididymis (Suzuki, 1982). This lack of vascularization is also observed in the genetically female (XX) mice that carry the sex reversal factor from the Y chromosome known as XXSxr. XXSxr mice develop testes and male secondary sexual structures, however the initial segment region of the epididymis fails to develop (Le Barr and Blecher, 1987). Therefore, it would be very interesting to understand the relationship between the development of the epithelium of this special epididymal region and how it relates to the development of blood vessels.
As previously mentioned the testicular fluid is a rich source of growth factors. In addition to the role of bFGF in stimulating cell proliferation, it also appears particularly important in establishing cellular characteristics and function of the initial segment (Tomsig and Turner, 2006). bFGF has been found to act directly on receptors located in the adult rat initial segment (Kirby et al., 2003). It is known that growth factors can differentially regulate gene expression, eliciting a variety of responses. In this case, these genes may function as signals for ductal elongation, coiling and/or cellular differentiation.
So the question here is, how can a single tube receive signals, that are often contradictory from one region to another, without one adjacent piece of duct being influenced by its neighbor ? A possible clue is the formation of individual septa between the segments of the epididymis. Studies by Turner and colleagues (Turner et al., 2003) have suggested a potential role of septa in the regulation of gene expression and segmentation in the epididymis. Although these studies were performed in the adult epididymis, we would like to extend this hypothesis by suggesting that the septa would play a role during the process of regionalization in the acquisition of segmental identity of the duct during embryonic and postnatal development. Our unpublished studies have shown that septa have fully formed by postnatal days 3-5 in the mouse epididymis. Having individual septa between different regions of the duct would allow for the secretion and concentration of morphogens /stimulatory factors at discrete sites and therefore influence the cellular characteristics of that particular region. Interstitial fluid flow between segments may possibly affect the concentration of individual substances within each segmental region (Rutkowski and Swartz, 2007). However, if it assumed that flow is very slow, as observed for other organs and tissues, then presumably this would not dramatically affect the final concentration of that substance being secreted (Rutkowski and Swartz, 2007). The whole idea of septa playing a role in early development of the epididymis and in regulating gene expression in individual segments of the epididymis is very attractive. However, the expression of several genes is not restricted to one segment, but can overlap with other segments (see (Robaire et al., 2006). Hence, several segments appear to have similar gene expression patterns. Obviously, further studies are needed to clarify the role of these septa and to address whether they play a role during early development and/or in the adult epididymis.
Size and shape of the Wolffian duct
The enormous length of the epididymis reflects the important nature of this organ. The male gametes leave the testis as polarized cells that are functionally immature and that require differentiation in the epididymis to become motile and to acquire fertilizing capacity. This crucial developmental process requires sperm to interact with a progressively changing luminal environment regulated by region specific secretory and absorptive activities of the epididymal epithelium (Hinton and Palladino, 1995). This process of sperm maturation involves extensive biochemical and physical remodeling of the sperm plasma membrane as well as structural changes in several intracellular organelles. In order for the epididymis to accomplish all of this in the two weeks it takes for sperm passage in the mouse, the organ has delineated the physiological processes that occur within each region and thus the size and shape of these regions reflects their function.
Although the vas deferens develops in close proximity to the epididymis, and is often considered an extension of the epididymal duct, it is distinguished by its function, cell morphology, and gene/protein expression. The vas deferens originates as a straight tube from the coiled cauda epididymis and ends as the ejaculatory duct, which joins with the urethra as an exit from the body. The vas deferens is surrounded by layers of smooth muscle innervated by sympathetic nerves, whose stimulation propels sperm through the ejaculatory duct. Because humans do not have the bulbous cauda epididymis, characteristic in rodents, the proximal vas deferens serves as the storage area for sperm in man (Turner, 1991).
For many years investigators have tried to understand how size and shape of particular organs is determined. Many of these studies have focused on using Drosophila as the model organism, for example, understanding the mechanisms that regulate wing shape and size. Through these studies, the hippo kinase pathway was discovered and is essentially a growth suppressive pathway that functions by simultaneously inhibiting cell proliferation while promoting apoptosis (see recent reviews by (Buttitta and Edgar, 2007; Dong et al., 2007; Harvey and Tapon, 2007; Lecuit and Le Goff, 2007; Pan, 2007; Saucedo and Edgar, 2007). The mRNAs of several members of the pathway are highly expressed in the adult rat and mouse epididymis (Johnston et al., 2005) and therefore, it would be interesting to examine the role of this pathway during Wolffian duct development.
The size and shape of the epididymal duct may also be influenced by its attachments within the abdomen of the growing animal. The head of the developing epididymis is attached to the kidney by a ligament and the cauda region is attached to the lower abdominal wall by the gubernaculum. In the absence of insulin-like peptide 3 (INSL3) or its receptor Lgr8/Rxfp2, the gubernaculum fails to differentiate resulting in cryptorchidism, or a failure of testicular descent (Agoulnik, 2007). Epididymal abnormalities are very commonly found in conjunction with cryptorchidism, including the failure of proper epididymal elongation and reduction in epididymal size (De Miguel et al., 2001; Kocak et al., 2001; Koff and Scaletscky, 1990). Hence, as the animal grows and the testis and epididymis begin their descent, torsion forces are presumably generated across the entire organ, which may influence the growth and shape of the epididymis. Obviously, more studies are necessary to understand how the shape and size of the epididymis is determined.
Summary
The epididymis is formed from the anterior portion of the Wolffian duct and must undergo elongation, expansion, coiling and segmentation to morph from a straight tube to an elaborately convoluted organ. The complex interplay between the epithelial and mesenchymal cell compartments, the regional expression of genes, the establishment of morphogen gradients, and the exposure to testicular fluids makes the epididymis a challenging and incredibly fascinating model of morphogenesis. In this review we have summarized the current state of understanding in regards to epididymal tubulogenesis and morphogenesis, and where the literature was limited, we have speculated on ways in which the organ may undergo parts of this process. We hope this review has brought attention to the many unanswered questions and illuminated avenues of research that may contribute to a broader understanding of this organ.
Acknowledgments
The authors would like to thank the National Institutes of Health, NICHD HD 052035 (BTH) and NICHD HD 054330 (A.J) for their financial support. The authors acknowledge the help of Stanton Fernald for the drawing of Figure 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoulnik AI. Relaxin and related peptides in male reproduction. Adv Exp Med Biol. 2007;612:49–64. doi: 10.1007/978-0-387-74672-2_5. [DOI] [PubMed] [Google Scholar]
- Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977;123:649–60. [PMC free article] [PubMed] [Google Scholar]
- Bedford JM. Effects of duct ligation on the fertilizing ability of spermatozoa from different regions of the rabbit epididymis. J Exp Zool. 1967;166:271–81. doi: 10.1002/jez.1401660210. [DOI] [PubMed] [Google Scholar]
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–96. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16:2958–70. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandli AW. Towards a molecular anatomy of the Xenopus pronephric kidney. Int J Dev Biol. 1999;43:381–95. [PubMed] [Google Scholar]
- Branford WW, Benson GV, Ma L, Maas RL, Potter SS. Characterization of Hoxa-10/Hoxa-11 transheterozygotes reveals functional redundancy and regulatory interactions. Dev Biol. 2000;224:373–87. doi: 10.1006/dbio.2000.9809. [DOI] [PubMed] [Google Scholar]
- Buttitta LA, Edgar BA. How size is controlled: from Hippos to Yorkies. Nat Cell Biol. 2007;9:1225–7. doi: 10.1038/ncb1107-1225. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–92. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991a;128:2867–73. doi: 10.1210/endo-128-6-2867. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology. 1991b;128:2874–9. doi: 10.1210/endo-128-6-2874. [DOI] [PubMed] [Google Scholar]
- Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375:791–5. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- De Miguel MP, Marino JM, Gonzalez-Peramato P, Nistal M, Regadera J. Epididymal growth and differentiation are altered in human cryptorchidism. J Androl. 2001;22:212–25. [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–95. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Dyche WJ. A comparative study of the differentiation and involution of the Mullerian duct and Wolffian duct in the male and female fetal mouse. J Morphol. 1979;162:175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- Fujii T, Pichel JG, Taira M, Toyama R, Dawid IB, Westphal H. Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev Dyn. 1994;199:73–83. doi: 10.1002/aja.1001990108. [DOI] [PubMed] [Google Scholar]
- Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- Guttroff RF, Cooke PS, Hess RA. Blind-ending tubules and branching patterns of the rat ductuli efferentes. Anat Rec. 1992;232:423–31. doi: 10.1002/ar.1092320311. [DOI] [PubMed] [Google Scholar]
- Hannema SE, Hughes IA. Regulation of Wolffian duct development. Horm Res. 2007;67:142–51. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–91. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- Herzlinger D. Inductive interactions during kidney development. Semin Nephrol. 1995;15:255–62. [PubMed] [Google Scholar]
- Hinton BT, Lan ZJ, Lye RJ, Labus JC. Testicular regulation of epididymal function: The lumicrine hypothesis. In: Goldberg E, editor. The Testis: From Stem Cell to Sperm Function. Springer-Verlag; New York: 2000. pp. 163–173. [Google Scholar]
- Hinton BT, Palladino MA. Epididymal epithelium: its contribution to the formation of a luminal fluid microenvironment. Microsc Res Tech. 1995;30:67–81. doi: 10.1002/jemt.1070300106. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Turner TT. Third International Conference on the Epididymis; Charlottesville: Van Doren Press; 2003. [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo. Cold Spring Harbor Press; Plainview, NY: 1994. [Google Scholar]
- Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–23. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Harding R. Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung. Clin Exp Pharmacol Physiol. 1995;22:235–47. doi: 10.1111/j.1440-1681.1995.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Witte DP, Szucsik JC, Weinstein M, Li H, Potter SS. Gsh-2, a murine homeobox gene expressed in the developing brain. Mech Dev. 1995;50:177–86. doi: 10.1016/0925-4773(94)00334-j. [DOI] [PubMed] [Google Scholar]
- Jiang FX, Temple-Smith P, Wreford NG. Postnatal differentiation and development of the rat epididymis: a stereological study. Anat Rec. 1994;238:191–8. doi: 10.1002/ar.1092380205. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, Kopf GS, Turner TT. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- Keller RE, Danilchik M, Gimlich R, Shih J. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morphol. 1985;89(Suppl):185–209. [PubMed] [Google Scholar]
- Kirby JL, Yang L, Labus JC, Hinton BT. Characterization of fibroblast growth factor receptors expressed in principal cells in the initial segment of the rat epididymis. Biol Reprod. 2003;68:2314–21. doi: 10.1095/biolreprod.102.011270. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer RR. Requirement of Lim1 for female reproductive tract development. Development. 2004;131:539–49. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kawakami K, Asashima M, Nishinakamra R. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev. 2007 doi: 10.1016/j.mod.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kocak I, Dundar M, Culhaci N. Epididymal changes associated with cryptorchidism in rats. Asian J Androl. 2001;3:277–80. [PubMed] [Google Scholar]
- Koff WJ, Scaletscky R. Malformations of the epididymis in undescended testis. J Urol. 1990;143:340–3. doi: 10.1016/s0022-5347(17)39954-8. [DOI] [PubMed] [Google Scholar]
- Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–95. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- Lan ZJ, Labus JC, Hinton BT. Regulation of gamma-glutamyl transpeptidase catalytic activity and protein level in the initial segment of the rat epididymis by testicular factors: role of basic fibroblast growth factor. Biol Reprod. 1998;58:197–206. doi: 10.1095/biolreprod58.1.197. [DOI] [PubMed] [Google Scholar]
- Le Barr DK, Blecher SR. Decreased arterial vasculature of the epididymal head in XXSxr pseudomale (‘sex-reversed’) mice. Acta Anat (Basel) 1987;129:123–6. [PubMed] [Google Scholar]
- Lecuit T, Le Goff L. Orchestrating size and shape during morphogenesis. Nature. 2007;450:189–92. doi: 10.1038/nature06304. [DOI] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Maneely RB. Epididymal structure and function: a historical and critical review. Acta Zool. 1959;40:1–21. [Google Scholar]
- Maruch SM, Alves HJ, Machado CR. Sympathetic innervation of the reproductive organs of the male opossum, Didelphis albiventris (Lund, 1841) Acta Anat (Basel) 1989;134:257–62. doi: 10.1159/000146697. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–64. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Mobilio C, Campus A. Osservazioni sull ‘epididimo dei nostri animali domestici’. Ann N Y Acad Sci. 1912;55:629. [Google Scholar]
- Nelson WJ. Tube morphogenesis: closure, but many openings remain. Trends Cell Biol. 2003;13:615–21. doi: 10.1016/j.tcb.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Affolter M. Remodeling epithelial tubes through cell rearrangements: from cells to molecules. EMBO reports. 2006;7:36–40. doi: 10.1038/sj.embor.7400597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara-Ishihara T, Kuhlman J, Niswander L, Herzlinger D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development. 1999;126:1103–8. doi: 10.1242/dev.126.6.1103. [DOI] [PubMed] [Google Scholar]
- Orgebin-Crist MC. Maturation of spermatozoa in the rabbit epididymis: delayed fertilization in does inseminated with epididymal spermatozoa. J Reprod Fertil. 1968;16:29–33. doi: 10.1530/jrf.0.0160029. [DOI] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–97. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–51. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Seo RM, Clemens JQ, Ma L, Maas RL, Bushman W. Hoxa-10 deficient male mice exhibit abnormal development of the accessory sex organs. Dev Dyn. 1999;214:1–12. doi: 10.1002/(SICI)1097-0177(199901)214:1<1::AID-DVDY1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Robaire B, Hinton BT, editors. The Epididymis. From Molecules to Clinical Practice. Kluwer Academic/Plenum Publishers; New York: 2002. [Google Scholar]
- Robaire B, Hinton BT, Orgebin-Crist MC. The Epididymis. In: Neill JD, editor. Physiology of Reproduction, Vol Third Edition. Elsevier; New York: 2006. pp. 1071–1148. [Google Scholar]
- Rosario M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13:328–35. doi: 10.1016/s0962-8924(03)00104-1. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Sainio K, Hellstedt P, Kreidberg JA, Saxen L, Sariola H. Differential regulation of two sets of mesonephric tubules by WT-1. Development. 1997;124:1293–9. doi: 10.1242/dev.124.7.1293. [DOI] [PubMed] [Google Scholar]
- Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–21. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, James RG, Listopadova A, Herzlinger D. Formation of the nephric duct. In: Vize PD, Woolf AS, Bard JBL, editors. The Kidney. From Normal Development to Congenital Disease. Academic Press; New York: 2003. pp. 51–60. [Google Scholar]
- Smith C, Mackay S. Morphological development and fate of the mouse mesonephros. J Anat. 1991;174:171–84. [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Godecke S, Birchmeier C. The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev. 1996;10:1184–93. doi: 10.1101/gad.10.10.1184. [DOI] [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation. 2003;71:402–13. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- Stoltenberg M, Therkildsen P, Andreasen A, Jensen KB, Juhl S, Ernst E, Danscher G. Computer-assisted visualization of the rat epididymis: a methodological study based on paraffin sections autometallographically stained for zinc ions. Histochem J. 1998;30:237–44. doi: 10.1023/a:1003255705503. [DOI] [PubMed] [Google Scholar]
- Suzuki F. Microvasculature of the mouse testis and excurrent duct system. Am J Anat. 1982;163:309–25. doi: 10.1002/aja.1001630404. [DOI] [PubMed] [Google Scholar]
- Takano H, Abe K, Ito T. Changes in the mouse epididymis after ligation of the ductuli efferentes or proximal epididymal duct: qualitative and quantitative histological studies (author's transl) Kaibogaku Zasshi. 1981;56:79–90. [PubMed] [Google Scholar]
- Theiler K. The House Mouse: Atlas of Embryonic Development. Springer-Verlag; New York, NY: 1989. [Google Scholar]
- Tomaszewski J, Joseph A, Archambeault D, Yao HH. Essential roles of inhibin beta A in mouse epididymal coiling. Proc Natl Acad Sci U S A. 2007;104:11322–7. doi: 10.1073/pnas.0703445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsig JL, Turner TT. Growth factors and the epididymis. J Androl. 2006;27:348–57. doi: 10.2164/jandrol.05182. [DOI] [PubMed] [Google Scholar]
- Tong SY, Hutson JM, Watts LM. Does testosterone diffuse down the wolffian duct during sexual differentiation? J Urol. 1996;155:2057–9. [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–65. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Shima H, Cunha GR. In vitro androgen-induced growth and morphogenesis of the Wolffian duct within urogenital ridge. Endocrinology. 1991;128:1805–11. doi: 10.1210/endo-128-4-1805. [DOI] [PubMed] [Google Scholar]
- Turner TT. Spermatozoa are exposed to a complex microenvironment as they traverse the epididymis. Ann N Y Acad Sci. 1991;637:364–83. doi: 10.1111/j.1749-6632.1991.tb27323.x. [DOI] [PubMed] [Google Scholar]
- Turner TT, Bomgardner D, Jacobs JP, Nguyen QA. Association of segmentation of the epididymal interstitium with segmented tubule function in rats and mice. Reproduction. 2003;125:871–8. doi: 10.1530/rep.0.1250871. [DOI] [PubMed] [Google Scholar]
- Turner TT, Gleavy JL, Harris JM. Fluid movement in the lumen of the rat epididymis: effect of vasectomy and subsequent vasovasostomy. J Androl. 1990;11:422–8. [PubMed] [Google Scholar]
- Upadhyay S, Zamboni L. The Role of the Mesonephros in the Development of the Mouse Testis and Its Excurrent Pathways. In: Byskov AG, Peters H, editors. Vth Workshop on the Development and Function of the Reproductive Organs; Copenhagen: Excerpta Medica; 1981. pp. 18–30. [Google Scholar]
- Vazquez MD, Bouchet P, Mallet JL, Foliguet B, Gerard H, LeHeup B. 3D reconstruction of the mouse's mesonephros. Anat Histol Embryol. 1998;27:283–7. doi: 10.1111/j.1439-0264.1998.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Vize PD, Seufert DW, Carroll TJ, Wallingford JB. Model systems for the study of kidney development: use of the pronephros in the analysis of organ induction and patterning. Dev Biol. 1997;188:189–204. doi: 10.1006/dbio.1997.8629. [DOI] [PubMed] [Google Scholar]
- Von L, Neuhaeuser G. Morphometric Analysis of the Human Epididymis. Z Anat Entwicklungsgesch. 1964;124:126–52. [PubMed] [Google Scholar]
- Wrobel KH. Morphogenesis of the bovine rete testis: extratesticular rete, mesonephros and establishment of the definitive urogenital junction. Anat Embryol (Berl) 2001;203:293–307. doi: 10.1007/s004290100162. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Sonnenberg-Riethmacher E, Cooper TG. Infertile spermatozoa of c-ros tyrosine kinase receptor knockout mice show flagellar angulation and maturational defects in cell volume regulatory mechanisms. Biol Reprod. 1999;61:1062–9. doi: 10.1095/biolreprod61.4.1062. [DOI] [PubMed] [Google Scholar]