Abstract
In the hippocampal formation (HF), the enkephalin opioids and estrogen are each known to modulate learning and cognitive performance relevant to drug abuse. Within the HF, leu-enkephalin (LENK) is most prominent in the mossy fiber (MF) pathway formed by the axons of dentate gyrus (DG) granule cells. To examine the influence of ovarian steroids on MF pathway LENK levels, we used quantitative light microscopic immunocytochemistry to evaluate LENK levels in normal cycling rats and in estrogen-treated ovariectomized rats. Rats in estrus had increased levels of LENK-immunoreactivity (ir) in the DG hilus compared to rats in diestrus or proestrus. Rats in estrus and proestrus had higher levels of LENK-ir in CA3a-c compared to rats in diestrus. Ovariectomized (OVX) rats 24 hrs (but not 6 or 72 hrs) after estradiol benzoate (EB; 10 µg) administration had increased LENK-ir in the DG hilus and CA3c. Electron microscopy showed a larger proportion of LENK-labeled small terminals and axons in the DGthe DG hilus compared to CA3 which may have contributed to region-specific changes in LENK-ir densities. Next we evaluated the subcellular relationships of estrogen receptor (ER) α, ERβ and progestin receptor (PR) with LENK-labeled MF pathway profiles using dual-labeling electron microscopy. ERβ-ir colocalized in some LENK-labeled MF terminals and smaller terminals while PR-ir was mostly in CA3 axons, some of which also showed colocalization with LENK. ERα-ir was in dendritic spines, but no colocalization with LENK-labeled profiles was observed. The present studies indicate that estrogen can modulate LENK in subregions of the MF pathway in a dose- and time- dependent manner. These effects might be triggered by direct activation of ERβ or PR in LENK-containing terminals.
Keywords: dentate gyrus, CA3, estrogen, progestin, estrous cycle, opioid, immunoreactivity, rat
1.0 INTRODUCTION
Addiction and drug taking behavior involve associative learning processes that require, among other structures, the hippocampal formation (HF) (Berke and Hyman, 2000;Hyman and Malenka, 2001). Hippocampal associative learning as well as addictive processes and relapse differ between males and females (Desmond and Levy, 1997;Lynch et al., 2002). In addition, drugs of abuse, such as cocaine, can alter endogenous opiates in the brain in a sex-dependent manner (Torres-Reveron et al., 2007a). Drugs of abuse as well as ovarian steroids can influence hippocampal activity and cognitive processes (McEwen and Alves, 1999;Verdejo-Garcia et al., 2007).
The HF contains two well known families of endogenous opiates: enkephalins and dynorphins (Chavkin et al., 1985;Gall et al., 1981). Morphine and other addictive opiates can produce their addictive actions by activating µ opioid receptors (Matthes et al., 1996). Since opiates remains one of the most abused drugs categories in the United States (NIDA, 2005) and enkephalin is the main endogenous ligand for the µ opioid receptor (Janecka et al., 2004), this study focuses on enkephalin and modulation of its expression by ovarian steroids within the mossy fiber pathway.
Many reports have shown that ovarian steroids modulate endogenous opiates in particular brain areas, although relatively little is known about such interactions in the HF. In the rat striatum and nucleus accumbens, chronic administration of estrogen or agonists for the α or β subtypes of estrogen receptor (ER) increases proenkephalin mRNA levels as measured by in situ hybridization (Le Saux and Di Paolo, 2005). In the ventromedial nucleus of the hypothalamus, estradiol treatment can increase proenkephalin mRNA in female rats (Lauber et al., 1990;Priest et al., 1995). In male rats, estradiol administration prevents the gonadectomy-induced decrease in proenkephalin mRNA in the ventromedial hypothalamus, suggesting that estrogen maintains proenkephalin mRNA levels (Hammer, Jr. et al., 1993). Estrogen, moreover, can regulate the number of hypothalamic µ and δ opioid receptors (which preferentially bind enkephalin) as assessed by laser-scanning confocal microscopy (Eckersell et al., 1998;Sinchak and Micevych, 2001). On the other hand, the administration of estradiol to ovariectomized rats increases µ opioid receptor binding affinity in the HF; and this effect is attenuated by co-administration of progesterone (Piva et al., 1995). While recent pharmacological studies in the HF demonstrate that estrogen can modulate leu-enkephalin (LENK), regional specificity was not determined (Roman et al., 2006). These data suggest that direct interactions between ovarian steroids and enkephalin occur in several brain areas with perhaps more prominent effects in the hypothalamus and hippocampal formation.
In the HF, high-resolution anatomical studies have shown that ERα and ERβ are in extranuclear portions of neurons, including terminals and synapses (Adams et al., 2002;Milner et al., 2001;Milner et al., 2005). The extranuclear location of ERs suggests that these receptors can modulate neuronal activity locally and on membranes (Woolley, 2007). ERs and LENK immunoreactivity (ir) have each been found in several of the same hippocampal regions, including the mossy fiber pathway in the hilus of the dentate gyrus (DG) and CA3 region (Commons and Milner, 1995;Milner et al., 1999;Milner et al., 2005;Shughrue et al., 1997). In addition, progestin receptors (PRs) have been identified in bundles of presumed mossy fiber axons and in axon terminals in the CA3 region of the hippocampus (Waters et al., 2008). This overlapping distribution supports the hypothesis that estrogen and progestin receptors are positioned to directly affect the processing and/or release of enkephalin peptides.
Ovarian hormones and enkephalin can both separately influence hippocampal excitability. Fluctuating levels of ovarian hormones in the rat estrous cycle and human menstrual cycle influence seizure susceptibility (Scharfman and MacLusky, 2006;Woolley and Schwartzkroin, 1998). This may in part be due to cyclic estradiol increase since estradiol administration can facilitate kainic acid-induced seizure activity (Woolley, 2000). Long-term potentiation (LTP) also is facilitated during the proestrus stage (high estrogen) of the rat estrous cycle (Warren et al., 1995). Enkephalin has a similar effect on hippocampal excitability: it can increase excitability and promote seizures, and conversely epileptic activity increases enkephalin levels in the DG (Mazarati et al., 1999). Since estrogen facilitates excitability and seizure activity can increase enkephalin levels in the DG, one might expect that estrogen levels may influence the expression of LENK under normal physiological conditions, through perhaps increased general excitability, direct genomic regulation, or region-specific targeting of enkephalinergic neurons.
In the present study, light and electron microscopic techniques were used to test the hypothesis that estrogen can modulate enkephalin peptide expression in a region-specific fashion. This study focuses on the dorsal hippocampus because it is closely associated with the development and persistence of addictive behaviors (Degoulet et al., 2007) and is the site of the most consistently reported estrogen-induced morphological changes (Cooke and Woolley, 2005). First, it was determined whether changes in ovarian steroids affect LENK peptide levels in the mossy fiber pathway in the dorsal DG and CA3. Normal cycling female rats at various stages of the estrous cycle and ovariectomized rats at three time points after receiving estradiol were examined. Second, to determine if observed changes in LENK-ir levels correlated with the types of LENK-immunoreactive profiles, a semi-quantitative electron microscopic analysis of LENK-labeled profiles within subregions of the DG hilus and CA3 stratum lucidum was conducted. Third, to establish whether there is an anatomical foundation for potential direct effects of ovarian steroids on LENK-containing neurons, the subcellular relations of LENK-containing MF profiles and ERα, ERβ and PR were examined using dual immunoelectron microscopy. Some of the data presented here have been previously published in an abstract (Torres-Reveron et al., 2006).
2.0 RESULTS
2.1 Distribution of LENK in the mossy fiber pathway
In agreement with previous studies (Commons et al., 1993;Gall et al., 1981), LENK-ir was detected mainly in four areas of the hippocampal formation: 1) the temporal portion of the lateral perforant pathway which originates in the entorhinal cortex and terminates on granule cell dendrites in the outer molecular layer and on distal dendrites of CA3 and CA1 pyramidal cells; 2) the hilus of the DG; 3) the stratum lucidum of the CA3 region which includes the mossy fiber pathway; and 4) sparse labeling in the outer layer of stratum lacunosum-moleculare of CA1. For this study, analysis was conducted on the mossy fiber pathway in the hilus of the DG and stratum lucidum of CA3 (see Fig. 1A). The DG and CA3 were each divided into 3 subfields (Fig. 1A). In stratum lucidum of CA3, which contains large mossy terminals, LENK-ir was observed in large punctate varicosities (Fig. 1B). In the DG hilus, LENK-ir was abundant and observed in both small and large punctate processes (Fig. 1C).
Figure 1. Distribution of LENK immunoreactivity in the rat hippocampal mossy fiber pathway.
A: LENK-ir was found in the hilus of the dentate gyrus (DG) and stratum lucidum of the CA3 region. Three different subregions of the dorsal DG (corresponding to levels between 3.80 and 4.30 caudal to Bregma, level 32) (Swanson, 1992) were analyzed: (1) the tip, (2) body or central region and (3) dorsal blade. The CA3 region also was divided into 3 different regions: (1) CA3a, (2) CA3b and (3) CA3c based on the classical divisions of Lorente de Nó (Lorente de No, 1934). B: Many large processes with LENK labeling are visible in stratum lucidum (SL) of CA3a. SP: Stratum pyramidale. C: In the body of the hilus (Hi), LENK-immunoreactivity was seen in both small and large puncta. Some immunoreactive granule cells also were visible (arrow). GCL: Granule cell layer. Bar in A, 625 µm; bar in B, 125 µm. Pictures taken from a proestrus rat.
Semi-quantification of the number of labeled profiles with LENK was carried out in 3 randomly selected proestrus animals (Table 1). For this, the number of profiles in 4800 µm2 for each mossy fiber subregion outlined in Fig. 1A was counted in one block per animal. Consistent with prior studies (Commons and Milner, 1996;Drake et al., 2002;Pierce and Milner, 2001), three types of LENK-labeled profiles were found in the mossy fiber pathway (Fig 2). LENK-ir was localized in unmyelinated axons (Fig. 2A) and in small (0.4 to 0.6 µm in diameter) terminals that contained small, synaptic vesicles and 1–2 dense-core vesicles (Fig. 2B). LENK-ir was also found in terminals with the characteristics of mossy fibers: they were large (1.0 to 2.0 µm in diameter), contained many small synaptic vesicles and, a few dense-core vesicles and were indented by spiny dendritic processes (Fig. 2C). In all three types of profiles, LENK-ir was densest on the dense-core vesicles. Labeled axons and small terminals (Fig. 2 A and B) were most abundant in the tip of the DG-hilus as compared to all other regions. In general, the hilus contained more labeled axons and small terminals than the stratum lucidum CA3. Large mossy fiber terminals (Fig. 2C) were found in all subregions, but were most abundant in the body and dorsal blade of the DG hilus. Overall, LENK-labeled profiles were most prominent in the DG hilus, especially in the tip. All three regions of the DG hilus together had 64 % more labeled profiles than all three subregions of CA3 stratum lucidum.
Table 1.
Number of LENK labeled profiles in each subregion of the mossy fiber pathway.
| Lamina | Axons | Small terminal | MF terminals | Unidentified | Total |
|---|---|---|---|---|---|
| DG tip | 118 | 72 | 24 | 4 | 218 |
| DG body | 54 | 81 | 49 | 2 | 186 |
| DG dorsal bl. | 47 | 54 | 56 | 2 | 159 |
| CA3a | 14 | 9 | 28 | 0 | 51 |
| CA3b | 22 | 13 | 27 | 1 | 63 |
| CA3c | 24 | 26 | 35 | 0 | 85 |
Total number of LENK-labeled profiles in 4800 µm2 per subregion in one section per proestrus rat (n= 3).
Figure 2. Electron micrographs showing LENK -ir in axons and axon terminals within all subregions of the mossy fiber pathway.
A: In CA3b, a LENK labeled axon (LE-A) is found among several unlabeled axons (uA). B: In the DG tip of the hilus, a small LENK-labeled terminal containing a dense-core vesicle (dcv) and several small synaptic vesicles (ssv) is found. For comparison, a nearby unlabeled terminal (uT) and unlabeled dendrite (uD) are shown. C: In the body of the hilus, a large LENK immunoreactive mossy fiber terminal (mfT) contains numerous intensely labeled dense-core vesicles and unlabeled mitochondria (m) and contacts several unlabeled dendritic spines (uS) An unlabeled mossy fiber (u-mfT) is found nearby. Bars in A–C, 500 nm. All panels from proestrus rats.
2.2 Rats showed higher levels of LENK during estrus in selected lamina
The estrous cycle was found to influence the density of LENK-ir in the mossy fiber pathway (Fig. 3). A significant main effect of estrous stage was found in all three subregions of the DG hilus (Tip: F(2, 22)= 7.95, p< 0.01; Body: F(2, 22)= 15.56, p< 0.01; Dorsal blade: F(2, 22)= 15.56, p< 0.01). Post-hoc analysis revealed that rats in the estrus stage of the cycle showed significantly higher LENK-ir in the tip, body and dorsal blade of the DG compared to rats in diestrus or proestrus (p< 0.05 for all comparisons). Similar results also were obtained in stratum lucidum of CA3, with a significant main effect of estrous stage observed for all three lamina of CA3 (CA3a: F(2, 22)= 10.46, p< 0.01; CA3b: F(2, 22)= 9.98, p< 0.01; CA3c: F(2, 22)= 7.24, p< 0.01). Post-hoc analyses revealed that in CA3a and CA3b both proestrus and estrus had higher LENK-ir than rats in diestrus (CA3a: p< 0.01 both comparisons; CA3b: p< 0.05 pro vs. di and p< 0.01 est vs. di). In CA3c, the estrus group was different from diestrus (p< 0.01) but not from proestrus (p> 0.05). When all three DG hilus subregions were averaged together (Fig. 3A) we found a significant main effect of estrous cycle (F(2, 22)= 11.0, p< 0.01) and post-hoc analyses revealed that the estrus group showed higher LENK-ir than proestrus and diestrus groups (p< 0.01 both comparisons). Similarly, when all three subregions of CA3 were averaged together there was a significant main effect of estrous cycle (F(2, 22)= 15.23, p< 0.01) showing that estrus and proestrus groups displayed higher LENK-ir than the diestrus group (p< 0.01, both comparisons). While there was a slight elevation in LENK-ir by the estrus group over the proestrus group, no significant difference was observed (p> 0.05). In summary, animals in estrus showed higher LENK-ir in the DG hilus than the other two groups, while animals in estrus or proestrus displayed higher LENK-ir than animals in diestrus in the CA3 region.
Figure 3. LENK-ir fluctuates in normal cycling female rats.
A: Female rats in estrus (day after estrogen and progesterone peaks) showed significantly higher LENK-ir than rats in diestrus (low estrogen) or proestrus for in all hilar subfields considered separately or combined. * represents p< 0.05 compared to diestrus or proestrus on using Tukey post-hoc test. B: In stratum lucidum of CA3, the estrus group showed higher LENK-ir compared to the diestrus group but not to proestrus group. The proestrus group was higher than diestrus for the CA3a, the CA3b and all CA3 regions combined. # respresents p< 0.05 from diestrus group only. For this and all subsequent figures error bars represent SEM and number in parentheses represents number of number of animals per group.
2.3 LENK levels were influenced by estradiol in ovariectomized animals
Since our findings in the normal cycling rats suggested that ovarian steroids modulate LENK levels, the effect of estradiol alone on the levels of hippocampal LENK-ir was examined. Because the effects of estrogen can vary depending on time after exposure (Smith and McMahon, 2005;Tanapat et al., 2005), four different groups of rats receiving either vehicle or estradiol at 6, 24 or 72 hrs pre-sacrifice were compared (Fig. 4). Analysis showed a significant main effect of estradiol treatment for the body and dorsal blade of the DG (Body: F(3, 19)= 3.87, p< 0.05; Dorsal blade: F(3, 19)= 10.30, p< 0.01), while no differences were found at the tip of the DG (F(3, 19)= 1.25, p> 0.05). Post-hoc analysis of the body and dorsal blade subregions revealed that LENK-ir was higher in animals assessed 24 hrs following a single injection of estradiol. Specifically, in the DG-body, LENK-ir in the 24-hr group was significantly higher than in the control and 72-hr group (p< 0.05 both comparisons). In the DG-dorsal blade, LENK-ir in the 24-hr group was higher than the other three groups (p≤ 0.05 all three comparisons). When all three DG hilus subregions were averaged together (see Fig. 4A) a significant main effect of estrous cycle (F(3, 19)= 8.64, p< 0.01) was found. Post-hoc analysis confirmed that LENK-ir in the 24-hr group was higher than all three other groups (p< 0.05 all comparisons). In the stratum lucidum of CA3 we also found a significant main effect of estradiol treatment only for the CA3c subregion (F(3, 19)= 3.97, p< 0.05). Post-hoc analysis revealed that the 24- hr group was only significantly different from the 72-hr group (p< 0.05). However when we averaged all three subregions of the CA3 together we found a significant main effect of estradiol treatment (F(3, 19)= 7.85, p< 0.01) and post-hoc analysis revealed that the 24-hr group showed significantly higher LENK-ir than all other groups (p< 0.05 all three comparisons), similar to the results observed for the hilus of the DG. In summary, 24 hrs of estradiol treatment produced an increase in LENK-ir in both the DG and CA3 regions of the dorsal hippocampus.
Figure 4. Quantitative light microscopic analysis of DG and region CA3 in ovariectomized rats that received estrogen at different time points before perfusion.
A: Twenty four hours following administration of estrogen LENK-ir was significantly increased over control group in the body and dorsal blade of the DG. When all three regions were analyzed together, the 24-hr group was significantly higher than all other groups. # represents p< 0.05 from control and 6-hr group. * represents p< 0.05 from all other three groups. B: While the 24-hr group showed only a slightly increased LENK-ir in the CA3c region, when all three subregions were analyzed together, the 24-hr group showed a small but significant increase in LENK-ir over the other three groups. Groups examined at 6 or 72 hrs after estrogen administration did not show any changes in LENK-ir compared to control groups (p > 0.5). * represents p< 0.05 from all other three groups. × represents significantly different from 72 hrs group only.
2.4 By electron microscopy, leu-enkephalin colocalizes with ERβ and PR but not with ERα
Quantitative light microscopic results suggested that ovarian steroids have complex effects on LENK levels in the mossy fiber pathway. To establish whether there are anatomical substrates for direct extranuclear modulation of LENK by ovarian steroids, the subcellular relationship of LENK-labeled profiles in the mossy fiber pathway with ERα, ERβ and PR was evaluated using dual label immunocytochemical electron microscopy (EM). Consistent with our immunoperoxidase data, LENK-ir identified using immunogold was found in axons, small terminals and in large mossy fiber terminals (Fig. 5).
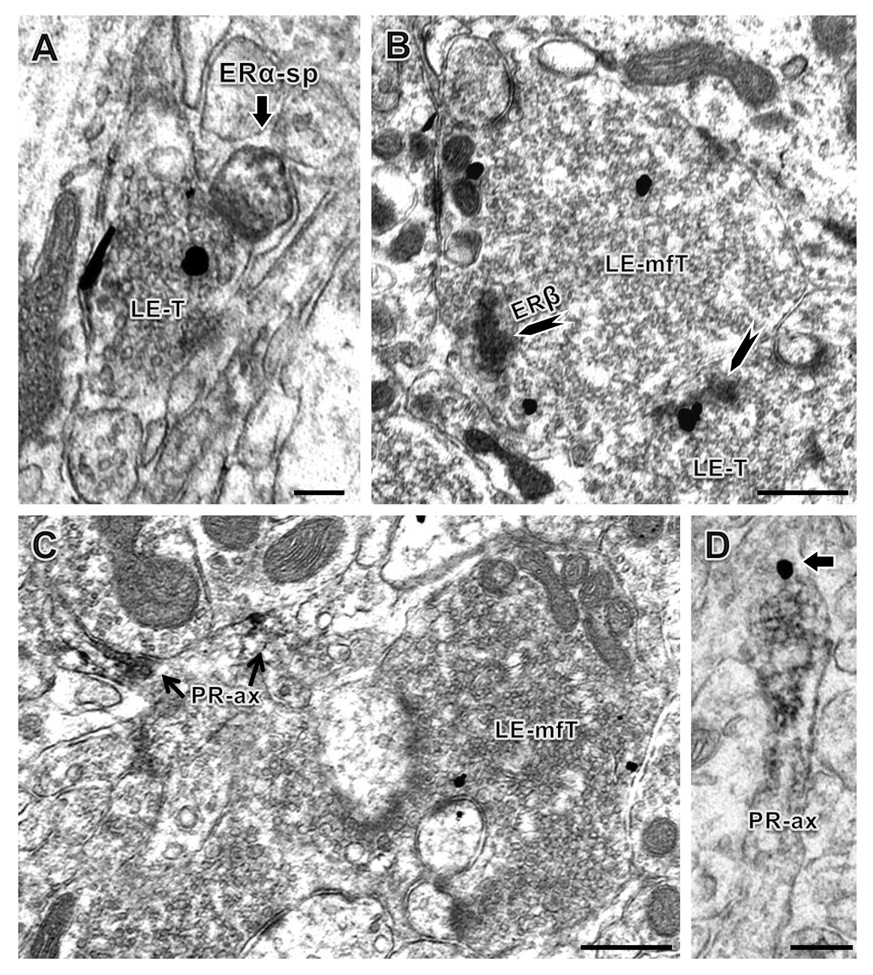
Figure 5. Electron microscopic localization of LENK relative to ERs or PR in the mossy fiber pathway.
A: A small terminal (LE-T) with ENK-immunogold (black particles) contacts an ERα-immunoperoxidase labeled spine (sp). B: A large LENK-immunoreactive mossy fiber terminal (LE-mfT) and a smaller LENK-labeled terminal (LE-T) also contain patches of ERβ-ir (arrowhead) affiliated with a cluster of small synaptic vesicles. C: A large LENK-labeled mossy fiber terminal (LE-T) is in close proximity to PR labeled axons (PR-ax). D: PR-ir and LENK-ir (arrow) colocalize in a small axon. A and B: central hilus; C and D: stratum lucidum of CA3. Bars A, C: 500 nm, B: 100 nm, D: 250 nm. Panels A, C and D from proestrus rats, panel B from a diestrus rat.
Extranuclear ERα-ir was found in dendrites, spines and in presynaptic profiles that included axons and axon terminals, consistent with prior observations (Milner et al., 2001;Towart et al., 2003). ERα-ir was also observed in small terminals and glia. ERα–ir was not observed in profiles containing LENK-ir. In some instances, LENK-labeled terminals closely apposed ERα-labeled profiles (Fig. 5A). Even though profiles were labeled with ERα or LENK in the same field (within 0.42 ± 0.06 µm), no dual labeling was seen.
ERβ-ir was found in dendritic shafts approximately 1 – 2 µm in diameter and was localized to small terminals ranging from 0.5 µm to 0.8 µm in diameter, as reported previously (Milner et al., 2005). Notably, ERβ-ir was observed in large (about 1.0 µm in diameter) LENK-labeled terminals in the central hilus (body sub-region) of the DG. Within these terminals, ERβ-ir was associated with clusters of small synaptic vesicles and was close to the plasma membrane (Fig. 5B). In addition, some LENK-labeled terminals formed asymmetric synapses on ERβ-labeled dendritic spines (< 0.7 µm; not shown). Finally, some ERβ-labeled profiles lacked LENK immunoreactivity although immunoreageants were accessible as demonstrated by the presence of LENK-labeled profiles nearby (within 0.51 ± 0.1 µm).
The subcellular relationships of LENK-labeling with PR-labeled profiles were examined in the CA3 field only, since PR labeling in the DG is scarce (Waters et al., 2008). PR-ir was mostly observed in bundles of axons in stratum lucidum that were consistent with mossy fiber axons (Tabori et al., 2004). LENK-labeled terminals were found in the same field as PR labeled axons (Fig. 5C) and in some instances PR-labeled axons contained LENK-ir (Fig. 5D).
2.5 Uterine weights and ovarian steroids levels
Vaginal smear cytology was the main method used to determine estrous cycle stage. However we also determined uterine weights and ovarian steroids levels as a way of corroborating the cytology. Since our main objective for cycling animals was to compare LENK at different estrous cycle stages rather than at particular ovarian steroid levels, we show the results as a percent change, in addition to absolute values. Average uterine weights were: proestrus: 0.80 ± 0.07 g; diestrus: 0.63 ± 0.07 g and estrus: 0.54 ± 0.05 g. Uterine weights were on average 21.2 % and 32.5% heavier in the proestrus group compared to the diestrus and estrus groups respectively. One-way ANOVA revealed a significant difference in uterine weights between groups (F(2,14)= 5.36, p< 0.05) for which post hoc analyses revealed that the uterine weights of proestrus group was higher than the estrus (p< 0.05) but not the diestrus group.
Plasma estradiol and progesterone levels were measured by radioimmunoassay. Average blood levels for estradiol were: proestrus: 33.9 ± 1.7 pg/ml; diestrus: 24.35 ± 1.2 pg/ml and estrus: 11.9 ± 0.6 pg/ml. Average blood levels for estradiol were 62.1 % and 28.2 % higher in proestrus compared to estrus and diestrus respectively. ANOVA showed a main effect of estrous cycle stage (F(2,15)= 87.89, p< 0.001). Post hoc analyses showed that estradiol in proestrus group was significantly higher than estrus (p< 0.01) and diestrus groups (p< 0.01). Average blood levels for progesterone were: proestrus: 10.14 ± 1.25 ng/ml; diestrus: 33.42 ± 5.12 ng/ml and estrus: 7.13 ± 1.22 ng/ml. Average blood levels for progesterone were 78.6 % and 69.6 % higher in diestrus compared to estrus and proestrus respectively. Similar to estradiol, ANOVA revealed a main effect of estrous cycle stage (F(2,13)= 25.59, p< 0.001). Post hoc analyses showed that progesterone in diestrus group was significantly higher than estrus (p< 0.01) and proestrus groups (p< 0.01). Ovarian steroids values in cycling animals were all within previously reported ranges for normal cycling female rats (Belanger et al., 1981;Smith et al., 1975).
In ovariectomized animals, unfortunately uterine weights were not collected; however, absence of ovaries was verified following perfusion. Blood levels of estradiol for treated animals were as follows: 6 hrs group: 88.13 ± 14.5 pg/ml; 24 hrs group: 32.4 ± 3.7 pg/ml; 72 hrs group: 29.7 ± 2.5 pg/ml. Blood estradiol levels in the control group were very low and fell under the detectable capacity of the radioimmunoassay kit used. ANOVA analysis for the 6, 24 and 72 hrs groups only revealed significant differences between groups (F(2,17)= 14.25, p< 0.01). Post hoc analyses showed that estradiol levels in the 6 hrs group were significantly higher than the other two groups (p< 0.05, both comparisons), but the 24 and 72 hrs groups were not different from each other (p> 0.05).
3.0 DISCUSSION
This study demonstrates that ovarian steroids, particularly estradiol, modulate LENK peptide levels in the rat mossy fiber pathway in a regional and time-dependent manner. The regional differences in LENK-ir may be linked to the observed region-specific distribution of the diverse types of LENK-containing profiles. Additionally, this study provides anatomical evidence that activation of extranuclear ERβs and PRs may directly modulate processing or release of LENK.
3.1 Methodological considerations
We first used the “gold standard” model of intact, cycling animals to examine if there were changes in LENK in relationship to ovarian hormone fluctuations and subsequently used an established ovariectomy (OVX) model to elucidate the specific role of estradiol in LENK modulation. Steroid hormone replacement of ovariectomized animals can only approximate a perfect model of the normal cycling animal, given that OVX may also alter the ratio of receptors and the replacement will not result in the same pattern of hormone changes present in the intact animal. Estradiol effects, moreover, may be difficult to observe as they depend strongly on hormone dose, time examined after steroid administration, and interval following OVX (Tanapat et al., 2005). In addition, the roles of estradiol and progesterone have been studied using many different OVX models (for example: Cyr et al., 2001;McEwen, 2001). While each model has different strengths and weaknesses, future studies similar to or extending the present study could make good use of the steroid administration paradigm using 24-hr estradiol-replaced animals.
To date, four different splice variants of the ERβ receptor have been identified (Weiser et al., 2007), of which the most abundant in the HF is ERβ1δ4 (Price, Jr. et al., 2000). ERβ1δ4 lacks exon 4 and, therefore, cannot localize to the nucleus and has an altered ligand-binding domain (Price, Jr. et al., 2000). The ERβ antibody used in these studies has been shown to most likely recognize the ERβ1δ4 splice variant and, consistent with a previous report (Milner et al., 2005) most of the observed localization was extranuclear. Although the ERβ1δ4 variant may be most abundant, other variants such as the classic ERβ1 are present in the hippocampus and may produce the observed effects of estrogen. Importantly, ERβ1δ4 can heterodimerize with the ERβ1 splice variant and restore its capacity for ligand activation (Leung et al., 2006). Estrogen-induced changes in enkephalin reported for other brain areas such as the striatum (Le Saux and Di Paolo, 2005) that differ from results reported here in the HF could be explained by differences in the assortment of ERβ splice variants expressed in the two regions. That is, estrogen would be expected to differentially regulate enkephalin levels depending on the types and ratios of ERβ splice variants present in a particular brain region.
3.2 Ovarian hormones modulate LENK levels in the mossy fiber pathway
In normal cycling female rats, we found that LENK-ir is higher during estrus, which is the day following the peak in estrogen and progesterone that occurs during proestrus. Functionally this may lead to cyclic fluctuations in LENKs normal actions in the HF, which are thought to include the modulation of, among others, learning processes associated with drug abuse (Stevens et al., 1991), adult neurogenesis (Eisch et al., 2000) and the actions of ovarian hormones (Slamberová et al., 2003). For example, µ opioid receptors in the CA3 modulate acquisition and retrieval of spatial memory (Meilandt et al., 2004), which can also be affected by ovarian hormones since rats in proestrus or estrus show a significantly better performance in an object placement task than rats in diestrus (Frye et al., 2007). Thus it is conceivable that normal cyclic elevations in ovarian hormones lead to increased hippocampal LENK, which can activate µ opioid receptors and subsequently affect learning processes.
One of the well-known mechanisms by which LENK can increase hippocampal excitability is by rapid inhibition of GABAergic transmission (Lupica and Dunwiddie, 1991;Madison and Nicoll, 1988). LENK may also enhance hippocampal excitation on a slower time frame through interactions with brain-derived neurotrophic factor (BDNF). Similar to LENK, BDNF is highly concentrated in the mossy fiber pathway in the rat (Conner et al., 1997). BDNF mRNA in the dorsal HF is up-regulated by LENK through a δ and µ opioid receptor-dependent mechanism (Zhang et al., 2006). BDNF also appears to be regulated by estradiol; mossy fiber BDNF-ir is high during proestrus (Scharfman et al., 2003) and can increase as soon as 2 hrs after estradiol administration to ovariectomized rats (Scharfman et al., 2007) Also, estradiol replacement in young ovariectomized rats restores hippocampal BDNF expression (Sohrabji and Lewis, 2006). These effects may well involve ERs since both ERα and ERβ are found in hippocampal CA3 pyramidal cells that also contain BDNF (Milner et al., 2005;Solum and Handa, 2002) and the BDNF gene has an estrogen response element (ERE) homologous sequence (Sohrabji et al., 1995). Physiological evidence also shows that estrogen effects on hippocampal excitability are likely mediated by interaction with BDNF (Scharfman and MacLusky, 2005). The specific mechanism of how BDNF and LENK may interact in the mossy fiber pathway to increase hippocampal excitation is not yet characterized. However, given their anatomical overlap and the upregulation of both by estrogen, it is likely that estrogen may enhance hippocampal excitability through the combined actions on both LENK and BDNF.
In ovariectomized animals, the induction of LENK-ir by estradiol has a critical time window and also depends on the dosing regimen. Too short a period (6 hr) following estradiol treatment is insufficient for increasing LENK-ir. Similarly, LENK-ir assessed after too long a period (72 hr) was not different from controls, perhaps because LENK has already been released from mossy terminals. Interestingly, we found the largest increase in LENK in intact rats was during estrus, which has an onset of approximately 24 hrs after the surge in estradiol and progesterone has occurred. Thus, data from the hormone replacement model confirms that the increase in LENK-ir observed in the mossy fiber pathway is due in part to the effects of increased estradiol. Our observations agree with electrophysiological data showing that, in ovariectomized rats, LTP is increased in rats that received estradiol 24 or 48 hours before assessment, but not 72 hrs (Smith and McMahon, 2005). Thus, a period of approximately 24 hrs following estradiol administration is critical to observe an increase in LENK levels in the hilus and CA3 mossy fibers. This finding is consistent with reports that the effects of estradiol require time to activate gene transcription (Vasudevan and Pfaff, 2007) and subsequently induce LENK expression.
While the classic mechanism of estrogen action involves estrogen binding to an ERE in DNA and subsequent modulation of gene transcription (Tsai and O'Malley, 1994) non-genomic mechanisms might also be involved (Belcher and Zsarnovszky, 2001;Simoncini and Genazzani, 2003). We observed ERβ immunoreactivity colocalized with LENK in axon terminals. Such close proximity provides an anatomical substrate for estrogen to induce localized non-genomic changes in excitability or signal transduction cascades in the terminal (Yakovleva et al., 2006), which may in turn modulate the final processing of LENK peptides or LENK release. Evidence for non-genomic effects on excitability include ERβ-selective inhibition of Ca++ currents in C1 neurons of the rostral ventrolateral medulla (Wang et al., 2006). Given the anatomical evidence presented here, it is possible that a similar mechanism of ERβ-selective non-genomic modulation can also operate in the HF.
The effects of ovarian steroids within the mossy fiber pathway were not homogeneous. In ovariectomized rats 24 hours after estradiol administration, LENK-ir increased more in the body and dorsal blade of the DG. Moreover, CA3 stratum lucidum showed a minimal increase in LENK immunoreactivity that was only apparent when all three (CA3 a, b and c) sub-regions where averaged together. Regional differences could be due to several factors. One possibility is the different organization of the two regions. The size, dense-core vesicle content and synaptic connections vary for LENK terminals in the hilus and CA3 (Commons and Milner, 1995). Semi-quantification of LENK distribution for each subregion of the DG and CA3 confirmed that indeed, there is a higher concentration of LENK-labeled small axons and terminals in the DG hilus compared to the CA3 stratum lucidum. It is conceivable that the small terminals and axons in the DG hilus, are more likely to undergo increases in LENK-ir than larger mossy fiber terminals in the CA3. Second, estradiol administration to ovariectomized rats can increase the cytoplasmic distribution of µ-opioid receptors in larger diameter dendrites and terminals more than in smaller terminals of parvalbumin interneurons (Torres-Reveron et al., 2007b). This suggests compartment and size-specific targeting of opioid receptors between dendrites and axons/terminals. Third, the distribution of axon collaterals that arise from granule cells differs depending on the location of the cell body: axons that arise from granule cells in the suprapyramidal blade are more confined to the CA3c while those that arise from granule cells near the crest and infrapyramidal blade ramify extensively through the hilus (Claiborne et al., 1986). The heterogeneity of LENK distribution between the hilus and CA3 mossy fibers subfields may underlie the observed localized effects exerted by ovarian steroids due to anatomical distribution in each subfield and compartment-selective effects of estradiol in the DG hilus and mossy fibers.
3.3 LENK profiles colocalized ERβ and PR
Our previous electron microscopic studies showed that profiles containing nuclear and extranuclear ERs are found in regions of the DG and CA3 known to contain LENK-labeled mossy fibers (Commons and Milner, 1995;Milner et al., 1999;Milner et al., 2005;Shughrue et al., 1997). The present study confirms and extends these findings. Notably, non-nuclear ERβ-ir was colocalized with LENK in mossy fiber terminals. Moreover, PR-ir was found in small, unmyelinated axons, some of which contained LENK. No colocalization of LENK-ir was found with extranuclear ERα-ir, but direct appositions between LENK- and ERα-labeled profiles were observed.
While the EM data suggest that ERβ mediates its effects through a non-genomic mechanism, our quantitative densitometry data are consistent with estradiol acting through genomic mechanisms to increase LENK levels since the effect was observed 24 hrs after estradiol exposure in both normal cycling and ovariectomized animals. It is important to remember that the antibody against ERβ used in the present study recognizes mostly the extranuclear receptors (see Methodological Considerations), but it does not rule out the possibility that nuclear ERβ are involved. Nuclear ERβ has been shown to be present in the CA3 pyramidal cell layer (Milner et al., 2005). Experimental evidence in the hypothalamus suggest that estrogen might affect LENK through direct interaction with its gene since the preproenkephalin gene is induced by an ER mechanism (Vasudevan et al., 2001b;Vasudevan et al., 2001a). Also, the promoter of the preproenkephalin gene contains two ERE-like sequences that display specific binding activity (Zhu et al., 2001;Zhu and Pfaff, 1995). Although the same mechanism has not been shown in the HF, it is likely that a similar method might be operating to increase LENK in the hilus and stratum lucidum. However, the presence of ERβ and PR in conjunction with LENK in axons and terminals suggests that both estrogen and progesterone may rapidly affect LENK processing and/or release through non-genomic actions. ERα was in dendritic spines and may affect LENK-ir, but would require an indirect mechanism beyond the scope of the present study to discover. Taken together, our EM and other findings on different ER mechanisms of action suggest that estradiol and progesterone may directly modulate LENK levels through both genomic and non-genomic mechanisms by activating ERβ and PRs while leaving open the possibility that ERα activation which alters pyramidal cell excitability, may indirectly produce effects on LENK levels.
3.4 Clinical implications
Estrogen has been shown to modulate neuronal activity. It is well documented that seizure frequency changes during the menstrual cycle, a phenomenon known as catamenial epilepsy (Backstrom, 1976;Herzog et al., 2004). In rats, seizure susceptibility is increased during proestrus (Terasawa and Timiras, 1968), and administration of estradiol to ovariectomized rats reduces the threshold for achieving seizure activity (Buterbaugh and Hudson, 1991). However, estrogen may not always increase seizure susceptibility due to differences in steroid doses and experimental preparations (Scharfman and MacLusky, 2006). The DG projection to CA3 pyramidal neurons through the mossy fiber pathway is a putative high resistance gateway for seizure propagation (Nadler, 2003). The effect of estradiol in increasing seizure susceptibility is attributed to an increase in excitability at the CA3-CA1 projections, while LENK release from the mossy fiber pathway can increase excitability by decreasing GABAergic activity, contributing to the propagation and maintenance of status epilepticus (Madison and Nicoll, 1988;Neumaier et al., 1988). The increase in LENK-ir in CA3 startum lucidum observed during proestrus in this study suggests that LENK may be a factor in the increased susceptibility to seizures at this stage, and supports other findings that the normal cyclic changes in estrogen during the estrous cycle result in changes in hippocampal excitability.
Changes in hippocampal excitability are especially important with regard to drug abuse. Gender and estrogen influences on drug dependence, withdrawal, reward perception and relapse have been well established (Carroll et al., 2004). Since drug-seeking behavior is thought to involve associative learning (Berke and Hyman, 2000) and estrogen enhances drug-seeking behavior in all phases of drug abuse (Carroll et al., 2004), this study provides new evidence on how ovarian hormones affect hippocampal opioid systems and, by extension, associative learning.
In conclusion, this is the first region-specific analysis of the effects of ovarian hormones and estradiol on LENK-ir in the rat dorsal HF. Ovarian hormone changes during the estrous cycle affect the levels of LENK present within the DG and mossy fiber pathway. Estradiol displays a transient effect on LENK-ir in particular subfields, revealing a critical window for estradiol-induced changes in LENK and hippocampal excitability.
4.0 EXPERIMENTAL PROCEDURE
4.1 Animals and estrous cycle determination
Adult female Sprague Dawley rats (225–250 g at time of arrival; approximately 60 days old) from Charles River Laboratories (Wilmington, MA) were used. Rats were housed in pairs with ad libitum access to food and water and with 12:12 light/dark cycles (lights on 0600 – 1800). All procedures were approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. Rats were allowed to acclimate for one week after which estrous cycle stage was determined using vaginal smear cytology (Turner and Bagnara, 1971). Only rats with regular, 4-day estrous cycles for 2 weeks were included in the study. Animals in proestrus, estrus and diestrus 2 stages of the estrous cycle were analyzed. Rats in diestrus 1 (i.e., metestrus) were not used. Diestrus 2 rather than metestrus was chosen to be certain that the animal was completely out of the estrus phase. For simplicity purposes, we will only use the term “diestrus”, referring specifically to diestrus 2 for the rest of the paper. While vaginal smear cytology was the main method used to determine estrous stages, stages were further verified by measuring uterine weights and levels of estradiol and progestin from blood samples collected during the perfusion procedure. Plasma serum levels for progesterone and estradiol were determined by radioimmunoassay using Coat-A-Count kits from Diagnostics Products Corporation (Los Angeles, CA) with a sensitivity of 8 pg/ml of estradiol and a sensitivity of 0.02 ng/ml of progesterone. Rats destined for ovariectomy were not cycled.
4.2 Ovariectomy and steroid replacement
Ovariectomy was either performed at Weill Cornell following modified surgical guidelines previously published for ovariectomy surgery (Eddy, 1986) or rats were bought ovariectomized (OVX) from Charles River Laboratories (Wilmington, MA). Rats were anesthetized with isoflurane (2–3%) and during surgical procedures body temperature was monitored and maintained at 37°C using a heating pad. After anesthesia reached a surgical level, the lumbar dorsum was shaved on both sides and cleaned with betadine scrub then 70% ethanol in accordance with surgical guidelines. Each ovary and fat around the ovary was identified, exposed, severed and removed. The muscle wall was closed with absorbable suture and the skin was closed using stainless steel wound clips.
The OVX model tested the effects of estradiol at different time points (6, 24, and 72 hours), since the effects of estrogen is highly time-sensitive (Smith and McMahon, 2005;Tanapat et al., 2005). In the 6 hr and 24 hr groups, OVX rats received a single s.c. injection of 10µg/ 0.2 mL of estradiol benzoate (E; Sigma, St. Louis, MO) in sesame oil 6 or 24 hrs prior to perfusion. The 72 hr group received 2 injections of E 24 hrs apart and these rats were perfused 2 days after the last injection. The control or oil (O) group received an injection of sesame oil 24 hrs before perfusion. All injections started two weeks after ovariectomy.
4.3 Antisera
A mouse monoclonal anti-LENK antibody was purchased from Sera Labs (Crawley Down, UK) (Drake et al., 2002). The specificity of this antibody has been previously characterized (Commons and Milner, 1995;Milner et al., 1989). The rabbit polyclonal antibody used for the detection of ERα was a generous gift from S. Hayashi (Yokohama City University, Yokohama, Japan). The antibody was raised against the native rat ERα and recognizes amino acids 61 through the carboxyl terminus. The specificity of this antibody has been previously tested (Alves et al., 1998;Okamura et al., 1992). The rabbit polyclonal antibody used for the detection of ERβ was obtained from Merck Research Laboratories (Rahway, NJ) and targeted the conserved sequence of N-terminus amino acids 64–82. This sequence is not present in the ERα sequence (Mitra et al., 2003). This antibody has been previously characterized by Western blot and preadsorption (Mitra et al., 2003) as well as immunolabeling of forebrain sections with no primary antibody (Milner et al., 2005). A rabbit polyclonal antibody against the DNA binding domain of the human PR was purchased from DAKO (A0098, Carpinteria, CA). It recognizes N-terminus amino acids 533–547 present in both the A and B isoforms of PR; the specificity of this antibody has been previously characterized (Kurita et al., 1998;Molenda et al., 2002;Tibbetts et al., 1999;Traish and Wotiz, 1990) and has been shown to recognize rat PR and other species by immunocytochemistry (Quadros et al., 2002).
4.4 Section preparation and light microscopy immunocytochemistry
Rats were deeply anesthetized with pentobarbital (150 mg/kg) on the morning of proestrus, diestrus or estrus (all animals perfused between 9:30 and 11:30 AM alternating animals at different stages of the cycle) or following estradiol treatment (OVX groups) and their brains fixed by aortic arch perfusion with: 1) 10 – 15 ml saline (0.9%) containing 1000 units of heparin; 2) 50 ml of 3.75% acrolein (Polysciences, Washington, PA) mixed in 2% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.6); and 3) 200 ml of 2% paraformaldehyde in PB (Milner and Veznedaroglu, 1992). Before perfusion, aortic blood was collected for estradiol and progesterone radioimmunoassay. After perfusion, the brains were removed from the skull, cut into 5 mm coronal blocks using a brain mold (Activational Systems, Inc., Warren, MI), and post-fixed for 30 minutes in the latter fixative. The block containing the hippocampal formation was sectioned (40 µm thick) on a Vibratome (VT1000S, Leica, Wein, Austria) and collected into PB. Sections were then stored in cryoprotectant (30% sucrose and 30% ethylene glycol in PB) until immunocytochemical processing. To insure identical labeling conditions during immunocytochemistry (Pierce et al., 1999), sections of each treatment group were rinsed in PB, coded with hole punches in the cortex and pooled into single containers. Sections were then treated with 1% sodium borohydride in PB for 30 minutes to neutralize free aldehydes.
For quantitative light microscopic localization of LENK, serial dilutions of the antibody were established and a linear function of antibody concentration against labeling intensity was obtained using densitometry, as previously described (Chang et al., 2000). A dilution of 1:15000 was chosen since this concentration produces slightly less than half-maximal labeling of LENK. This labeling intensity allows for variations in LENK in either direction that might be produced by the different treatment groups examined (Chang et al., 2000). The tissue was processed according to the avidin-biotin complex (ABC) method (Hsu et al., 1981). For this, tissue sections were rinsed in PB followed by Tris-buffered saline (TS; pH 7.6) and incubated in: 1) in 0.5% bovine serum albumin (BSA) in TS, 30 min; 2) a 1:15000 dilution of LENK antisera in 0.25% Triton X100 and 0.1% BSA / TS for 18–24 hours at room temperature and 24 hours at 4°C; 3) a 1:400 dilution of biotinylated horse anti-mouse immunoglobulin (IgG) (Vector Labs, Burlingame, CA), 30 min; 4) ABC (at twice the recommended dilution; Vector), 30 min and 5) 3,3’-diaminobenzidine (DAB; Sigma, St. Louis, MO) and H2O2 in TS for 6 minutes. All incubations were separated by washes in TS. Sections were mounted on gelatin coated slides, dehydrated in ascending concentrations of alcohols, cleared in xylene and cover-slipped with D.P.X. neutral mounting medium (Sigma, St. Louis, MO).
4.5 Electron microscopy immunocytochemistry
For single localization of LENK, sections were processed for the ABC procedure (above) except that the LENK antibody was diluted 1:100 in 0.025% Triton-X 100. For electron microscopic localization of LENK and ERs or PR, sections from proestrus rats were dually labeled for ERα, ERβ or PR using immunoperoxidase and LENK using immunogold by methods described previously (Towart et al., 2002). Since incubation requirements are different for the receptors antibodies compared to the LENK antibody, optimal labeling conditions were first established for an ERβ and LENK combination. For the ERβ-LENK dual labeling experiment, 6 rats in proestrus and 6 rats in diestrus were analyzed. From these animals, a total of 14 blocks were cut and a minimum of 3 ultra thin sections from each block were photographed. For ERα-LENK and PR-LENK combinations, 2 rats in proestrus and 2 rats in diestrus were analyzed for each combination. A minimum of 2 blocks from each rat and 3 ultra thin sections from each block were photographed and analyzed. Sections were incubated first in either ERα (1:3000 dilution), ERβ (1:2500 dilution) or PR (1:1000 dilution) antisera in 0.1% BSA / TS for 4 days at 4°C, followed by an incubation in LENK antisera (1:50 dilution) for 24 hr at 4°C in 0.1% BSA / TS with 0.025% Triton. ER- or PR-labeling was visualized using biotinylated goat anti-rabbit IgG (Vector) and the ABC immunoperoxidase technique described above. Following the DAB reaction step, sections were processed for LENK labeling using the silver-enhanced immunogold technique (Chan et al., 1990). For this, sections were rinsed in TS and incubated in a 1:50 dilution of donkey anti-mouse IgG conjugated to 1-nm gold particles (Electron Microscopy Sciences, EMS, Washington, PA) in 0.001% gelatin and 0.08% BSA in PBS overnight at 4°C. Sections were rinsed in PBS, postfixed in 1.25% glutaraldehyde in PBS for 10 min, rinsed again in PBS followed by 1.2% sodium citrate buffer, pH 7.4. The conjugated gold particles were enhanced by incubation in silver solution (IntenSE; Amersham) for 5–7 min. Sections were fixed 1 hr in 2% osmium tetroxide, dehydrated in ascending concentrations of ethanols and propylene oxide, and embedded in EMBed 812 (EMS) between two sheets of Aclar plastic (Honeywell, Pottsville, PA). Ultrathin sections (70–72 nm thick) through the midseptotemporal dentate gyrus (Swanson level 32 or 33; Swanson, 1992) were cut on a Leica UCT ultratome. Sections were counterstained with Reynold’s lead citrate and uranyl acetate and examined with a FEI Tecnai Biotwin electron microscope equipped with an Advanced Microscopy Techniques digital camera (software version 3.2; Danvers, MA).
For electron microscopic semi-quantification of LENK labeled profiles in each subregion of the mossy fiber pathway, three randomly selected animals in proestrus were used. Ultrathin sections through the mid-septotemporal DG or CA3 were cut (Swanson level 32–33). From each animal, two different hippocampal sections and two grid squares (2400 µm2 each) per subregion in each section were quantified.
4.6 Analysis
4.6.1 Light microscopic densitometry
For quantitative densitometry, images of regions of interest (R.O.I.) were captured using a Dage MTI CCD-72 camera and NIH Image 1.50 software on a Nikon Eclipse 80i microscope. R.O.I. in the dentate gyrus and CA3 were outlined as previously described (Pierce et al., 1999), and the mean gray value of pixel density (across a range of 256 gray levels) was determined for each R.O.I. Previously we showed that light microscopic pixel density linearly correlated with the density of dynorphin labeled dense core vesicles (average r= 0.92) (Pierce et al., 1999). Most neuropeptides like dynorphin and LENK are stored within dense core vesicles (Thureson-Klein and Klein, 1990). In addition, pixel density obtained at the light microscopy level from the hilus linearly correlated with the transmittance obtained from neutral density filters with defined transmittance [Pearson correlation, r= 0.998 (Pierce et al., 1999)]. Tissue from control and experimental animals were processed together in the same crucibles. For each animal, one hippocampal section was analyzed. R.O.I. were outlined using Image J software. To compensate for background staining and to control for variations in illumination level between images, the average pixel density for 3 small regions within the middle molecular layer, that has been shown to lack enkephalin labeling (Gall et al., 1981) was subtracted. To make it easier to visualize relative changes in immunocytochemistry between groups, net optical density values obtained after subtracting background values were converted to a percentage scale of 256 preset gray values ranging from 0 to 100% using Image J. For statistical analyses, percentages were transformed by calculating the inverse sine of the proportion (Eisenhart and Hastay, 1947). Both, normal cycling and ovariectomized animals were analyzed using a One Way ANOVA followed by Tukey post hoc analyses using SPSS v. 11.0 for Windows.
4.6.2 Electron microscopy
LENK labeled profiles for each subregion of the DG hilus and CA3 stratum lucidum were semi-quantified to elucidate possible differences in immunocytochemistry between regions that might arise as a result of different morphological distributions of labeled profiles. The subcellular relations of LENK profiles with ERs and PR in the mossy fiber pathway were also analyzed. Most of the analysis concentrated on proestrus animals since it has been shown previously that ERα concentration is higher at the proestrus stage of the cycle (Romeo et al., 2005). Labeled profiles were classified according to the existing nomenclature (Peters et al., 1991). Briefly, dendrite profiles were commonly postsynaptic to axon terminals and usually contained microtubular arrays. Unmyelinated axons had a small diameter (less than 0.2 µm) with few small synaptic vesicles and usually absent synaptic junctions in the section. Terminal profiles contained numerous small synaptic vesicles, frequently had contacts with other neuronal profiles and had minimal diameters greater than 0.2 µm. Astrocytic profiles had no microtubules, contained glial filaments and usually conformed to the boundaries of surrounding profiles.
Graphs were prepared with Graph Pad Prism 4.01 (Graph Pad Software, Inc., San Diego CA). For construction of figures, immunoperoxidase labeled sections were photographed with a Nikon Eclipse 80i microscope equipped with bright-field and DIC optics and a Micropublisher digital camera (Q-imaging, Barnaby, British Columbia). Figure 1, Figure 2 and Figure 5 were prepared by adjusting levels, brightness and contrast in Adobe Photoshop 7.0.1 on a Dell computer. Final figures were assembled in Microsoft Power Point.
ACKNOWLEDGEMENTS
We acknowledge the support from NIH grants DA08259 (T.A.M. & C.T.D.), NS07080 (B.S.M.) and minority supplement to DA08259 (A.T.R.). We thank Ms. Nora Tabori, Ms. Katherine Mitterling, Mr. Scott Herrick and Dr. Russell Romeo (Barnard College) for technical assistance. Helpful advice from Dr. Joe Pierce is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J. Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor β (ERβ) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: An immunocytochemical study. Proc. Natl. Acad. Sci. USA. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Belanger A, Cusan L, Caron S, Barden N, Dupont A. Ovarian progestins, androgens and estrogen throughout the 4-day estrous cycle in the rat. Biol. Reprod. 1981;24:591–596. doi: 10.1095/biolreprod24.3.591. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Zsarnovszky A. Estrogenic actions in the brain: Estrogen, phytoestrogens, and rapid intracellular signalling mechanisms. J. Pharmacol. Exp. Ther. 2001;299:408–414. [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Buterbaugh GG, Hudson GM. Estradiol replacement to female rats facilitates dorsal hippocampal but not ventral hippocampal kindled seizure acquisition. Exp. Neurol. 1991;111:55–64. doi: 10.1016/0014-4886(91)90050-m. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J. Neurosci. Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Aicher SA, Drake CT. Kappa opioid receptors in rat spinal cord vary across the estrous cycle. Brain Res. 2000;861:168–172. doi: 10.1016/s0006-8993(99)02461-0. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Shoemaker WJ, McGinty JF, Bayon A, Bloom FE. Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J. Neurosci. 1985;5:808–816. doi: 10.1523/JNEUROSCI.05-03-00808.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. The light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Commons K, Pierce J, Milner TA. Ultrastructural heterogeneity of enkephalin-containing terminals in the hippocampal formation. Soc. Neurosci. Abstr. 1993;19:353. doi: 10.1002/cne.903580303. [DOI] [PubMed] [Google Scholar]
- Commons KG, Milner TA. Ultrastructural heterogeneity of enkephalin-containing neurons in the rat hippocampal formation. J. Comp. Neurol. 1995;358:324–342. doi: 10.1002/cne.903580303. [DOI] [PubMed] [Google Scholar]
- Commons KG, Milner TA. The ultrastructural relationships between leu-enkephalin and GABA containing neurons differ within the hippocampal formation. Brain Res. 1996;724:1–15. doi: 10.1016/0006-8993(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res. Brain Res. Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Degoulet M, Rouillon C, Rostain JC, David HN, Abraini JH. Modulation by the dorsal, but not the ventral, hippocampus of the expression of behavioural sensitization to amphetamine. Int J Neuropsychopharmacol. 2007:1–12. doi: 10.1017/S146114570700822X. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Ovarian steroidal control of connectivity in the female hippocampus: An overview of recent experimental findings and speculations on its functional consequences. Hippocampus. 1997;7:239–245. doi: 10.1002/(SICI)1098-1063(1997)7:2<239::AID-HIPO10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chang PC, Harris JA, Milner TA. Neurons with mu opioid receptors interact indirectly with enkephalin-containing neurons in the rat dentate gyrus. Exp. Neurol. 2002;176:254–261. doi: 10.1006/exnr.2002.7948. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of µ-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J. Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy CA. Experimental Surgery of the Genitalia System. In: Gay WI, Heavner JE, editors. Methods of Animal Experimentation. Orlando, FL: Academic Press; 1986. p. 191. [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc. Natl. Acad. Sci. USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhart C, Hastay MW. Techniques of Statistical Analysis. New York, NY: McGraw-Hill; 1947. [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol. Learn. Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall C, Brecha N, Karten HJ, Chang K-J. Localization of enkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J. Comp. Neurol. 1981;198:335–350. doi: 10.1002/cne.901980211. [DOI] [PubMed] [Google Scholar]
- Hammer RP, Jr, Bogic L, Handa RJ. Estrogenic regulation of proenkephalin mRNA expression in the ventromedial hypothalamus of the adult male rat. Brain Res. Mol. Brain Res. 1993;19:129–134. doi: 10.1016/0169-328x(93)90157-k. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann. Neurol. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981;29:557–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Janecka A, Fichna J, Janecki T. Opioid receptors and their ligands. Curr. Top. Med. Chem. 2004;4:1–17. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody JR, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Mobbs CV, Howells RD, Pfaff DW. Estradiol induction of proenkephalin messenger RNA in hypothalamus: dose-response and relation to reproductive behavior in the female rat. Brain Res. Mol. Brain Res. 1990;8:47–54. doi: 10.1016/0169-328x(90)90008-2. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Chronic estrogenic drug treatment increases preproenkephalin mRNA levels in the rat striatum and nucleus accumbens. Psychoneuroendocrinology. 2005;30:251–260. doi: 10.1016/j.psyneuen.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: a key to understanding ER-beta signaling. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Dunwiddie TV. Differential effects of mu- and delta-receptor selective opioid agonists on feedforward and feedback GABAergic inhibition in hippocampal brain slices. Synapse. 1991;8:237–248. doi: 10.1002/syn.890080402. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;213:1–28. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurons in the rat hippocampus. J. Physiol. 1988;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Liu H, Wasterlain C. Opioid peptide pharmacology and immunocytochemistry in an animal model of self-sustaining status epilepticus. Neuroscience. 1999;89:167–173. doi: 10.1016/s0306-4522(98)00320-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J. Appl. Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endo. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL., Jr Role of hippocampal CA3 micro-opioid receptors in spatial learning and memory. J. Neurosci. 2004;24:2953–2962. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Soc. Neurosci. Abstr. 1999;25 [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Milner TA, Pickel VM, Reis DJ. Tyrosine hydroxylase and enkephalin in the rostral ventrolateral medulla: Major synaptic contacts from opiod terminals on catecholaminergic neurons. In: Quiron R, Jhamandas K, Gianoulakis C, editors. The International narcotic research conference (INRC) '89. New York: Alan R. Liss, Inc.; 1989. pp. 195–198. [PubMed] [Google Scholar]
- Milner TA, Veznedaroglu E. Ultrastructural localization of neuropeptide Y-like immunoreactivity in the rat hippocampal formation. Hippocampus. 1992;2:107–126. doi: 10.1002/hipo.450020204. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Molenda HA, Griffin AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem. Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Mailheau S, Chavkin C. Opioid receptor-mediated responses in the dentate gyrus and CA1 region of the rat hippocampus. J. Pharmacol. Exp. Ther. 1988;244:564–570. [PubMed] [Google Scholar]
- NIDA. National Institute on Drug Abuse Research Report Series; Prescription Drugs, Abuse and Addiction. 2005;1:1–12.
- Okamura H, Yamamoto K, Hayashi S, Kuroiwa A, Muramatsu M. A polyclonal antibody to the rat oestrogen receptor expressed in Escherichia coli: Characterization and application to immunohistochemistry. J. Endocrinol. 1992;135:333–341. doi: 10.1677/joe.0.1350333. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The fine structure of the nervous system. 3rd ed. New York: Oxford University Press; 1991. [Google Scholar]
- Pierce JP, Kurucz O, Milner TA. The morphometry of a peptidergic transmitter system before and after seizure. I. Dynorphin B-like immunoreactivity in the hippocampal mossy fiber system. Hippocampus. 1999;9:255–276. doi: 10.1002/(SICI)1098-1063(1999)9:3<255::AID-HIPO6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Milner TA. Parallel increases in the synaptic and surface areas of mossy fiber terminals following seizure induction. Synapse. 2001;39:249–256. doi: 10.1002/1098-2396(20010301)39:3<249::AID-SYN1006>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Piva F, Limonta P, Dondi D, Pimpinelli F, Martini L, Maggi R. Effects of steroids on the brain opioid system. J. Steroid Biochem. Mol. Biol. 1995;53:343–348. doi: 10.1016/0960-0760(95)00072-8. [DOI] [PubMed] [Google Scholar]
- Price RH, Jr, Lorenzon N, Handa RJ. Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Mol. Brain Res. 2000;80:260–268. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Priest CA, Eckersell CB, Micevych PE. Estrogen regulates preproenkephalin-A mRNA levels in the rat ventromedial nucleus: temporal and cellular aspects. Brain Res. Mol. Brain Res. 1995;28:251–262. doi: 10.1016/0169-328x(94)00213-x. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, de Vries GJ, Wagner CK. Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology. 2002;143:3727–3739. doi: 10.1210/en.2002-211438. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Gustafsson L, Meyerson BJ, Nylander I. Variations in opioid peptide levels during the estrous cycle in Sprague-Dawley rats. Neuropeptides. 2006;40:195–206. doi: 10.1016/j.npep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrin. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, MacLusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur. J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue? Trends Neurosci. 2005;28:79–85. doi: 10.1016/j.tins.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J. Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Genazzani AR. Non-genomic actions of sex steroid hormones. Eur. J Endocrinol. 2003;148:281–292. doi: 10.1530/eje.0.1480281. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J. Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberová R, Rimanoczy A, Bar N, Schindler CJ, Vathy I. Density of mu-opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus. 2003;13:461–471. doi: 10.1002/hipo.10076. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27:404–414. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J. Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Shiotsu G, Stein L. Hippocampal µ-receptors mediate opioid reinforcement in the CA3 region. Brain Res. 1991;545:8–16. doi: 10.1016/0006-8993(91)91263-z. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, McEwen BS, Milner TA. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2004;130:151–163. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Timiras PS. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83:207–216. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- Thureson-Klein AK, Klein RL. Exocytosis from neuronal large dense-core vesicles. Int. Rev. Cytol. 1990;121:67–126. doi: 10.1016/s0074-7696(08)60659-2. [DOI] [PubMed] [Google Scholar]
- Tibbetts TA, DeMayo F, Rich S, Conneely OM, O'Malley BW. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12021–12026. doi: 10.1073/pnas.96.21.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Hurd YL, Dow-Edwards DL. Gender differences in prodynorphin but not proenkephalin mRNA expression in the striatum of adolescent rats exposed to prenatal cocaine. Neurosci. Lett. 2007a;421:213–217. doi: 10.1016/j.neulet.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Drake CT, Milner TA. Neuroscience Meeting Planner. Atlanta GA: Society for Neuroscience; 2006. Enkephalin and dynorphin immunoreactivities increase in the dorsal hippocampus in response to estrogens and colocalize with estrogen receptor beta in mossy fiber terminals. 724.6. [Google Scholar]
- Torres-Reveron A, Williams TJ, Prasad P, Drake CT, Milner TA. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007; 2007b. Ovarian steroids affect the subcellular distribution of mu opioid receptors in parvalbumin interneurons of the dentate gyrus. Online 729.6. [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S. Cholinergic terminals in rat hippocampal formation contain estrogen receptor α. Society for Neuroscience Abstracts 28. 2002 [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Cellular relationships between cholinergic terminals and estrogen receptor alpha in the dorsal hippocampus. The Journal of Comparitive Neurology. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Traish AM, Wotiz HH. Monoclonal and polyclonal antibodies to human progesterone receptor peptide-(533–547) recognize a specific site in unactivated (8S) and activated (4S) progesterone receptor and distinguish between intact and proteolyzed receptors. Endocrinology. 1990;127:1167–1175. doi: 10.1210/endo-127-3-1167. [DOI] [PubMed] [Google Scholar]
- Turner CD, Bagnara JT. General Endocrinology. Philadelphia: W.B. Saunders; 1971. [Google Scholar]
- Vasudevan N, Koibuchi N, Chin WW, Pfaff DW. Differential crosstalk between estrogen receptor (ER)alpha and ERbeta and the thyroid hormone receptor isoforms results in flexible regulation of the consensus ERE. Brain Res. Mol. Brain Res. 2001a;95:9–17. doi: 10.1016/s0169-328x(01)00165-6. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr. Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Zhu YS, Daniel S, Koibuchi N, Chin WW, Pfaff D. Crosstalk between oestrogen receptors and thyroid hormone receptor isoforms results in differential regulation of the preproenkephalin gene. J. Neuroendocrinol. 2001b;13:779–790. doi: 10.1046/j.1365-2826.2001.00693.x. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M, Sanchez-Barrera M, Rodriguez-Fernandez A, Gomez-Rio M. Neuroimaging and drug addiction: neuroanatomical correlates of cocaine, opiates, cannabis and ecstasy abuse. Rev. Neurol. 2007;44:432–439. [PubMed] [Google Scholar]
- Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, Herrick SP, Hayashi S, Warrier S, Iadecola C, Milner TA. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094:163–178. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Waters EM, Torres-Reveron A, McEwen B, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J. Comp. Neurol. 2008 doi: 10.1002/cne.21826. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: From form to function. Brain Res. Rev. 2007 doi: 10.1016/j.brainresrev.2007.05.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estradiol facilitates kainic acid-induced, but not flurothyl-induced, behavioral seizure activity in adult female rats. Epilepsia. 2000;41:510–515. doi: 10.1111/j.1528-1157.2000.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Schwartzkroin PA. Hormonal effects on the brain. Epilepsia. 1998;39 Suppl 8:S2–S8. doi: 10.1111/j.1528-1157.1998.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekstrom TJ, Hauser KF, Pickel VM, Bakalkin G. Prodynorphin storage and processing in axon terminals and dendrites. FASEB J. 2006;20:2124–2126. doi: 10.1096/fj.06-6174fje. [DOI] [PubMed] [Google Scholar]
- Zhang H, Torregrossa MM, Jutkiewicz EM, Shi YG, Rice KC, Woods JH, Watson SJ, Ko MC. Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta-and micro-opioid receptors independent of antidepressant-like effects. Eur. J. Neurosci. 2006;23:984–994. doi: 10.1111/j.1460-9568.2006.04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YS, Cai LQ, You X, Duan Y, Imperato-McGinley J, Chin WW, Pfaff DW. Molecular analysis of estrogen induction of preproenkephalin gene expression and its modulation by thyroid hormones. Brain Res. Mol. Brain Res. 2001;91:23–33. doi: 10.1016/s0169-328x(01)00109-7. [DOI] [PubMed] [Google Scholar]
- Zhu YS, Pfaff DW. DNA binding of hypothalamic nuclear proteins on estrogen response element and preproenkephalin promoter: modification by estrogen. Neuroendocrin. 1995;62:454–466. doi: 10.1159/000127035. [DOI] [PubMed] [Google Scholar]