Abstract
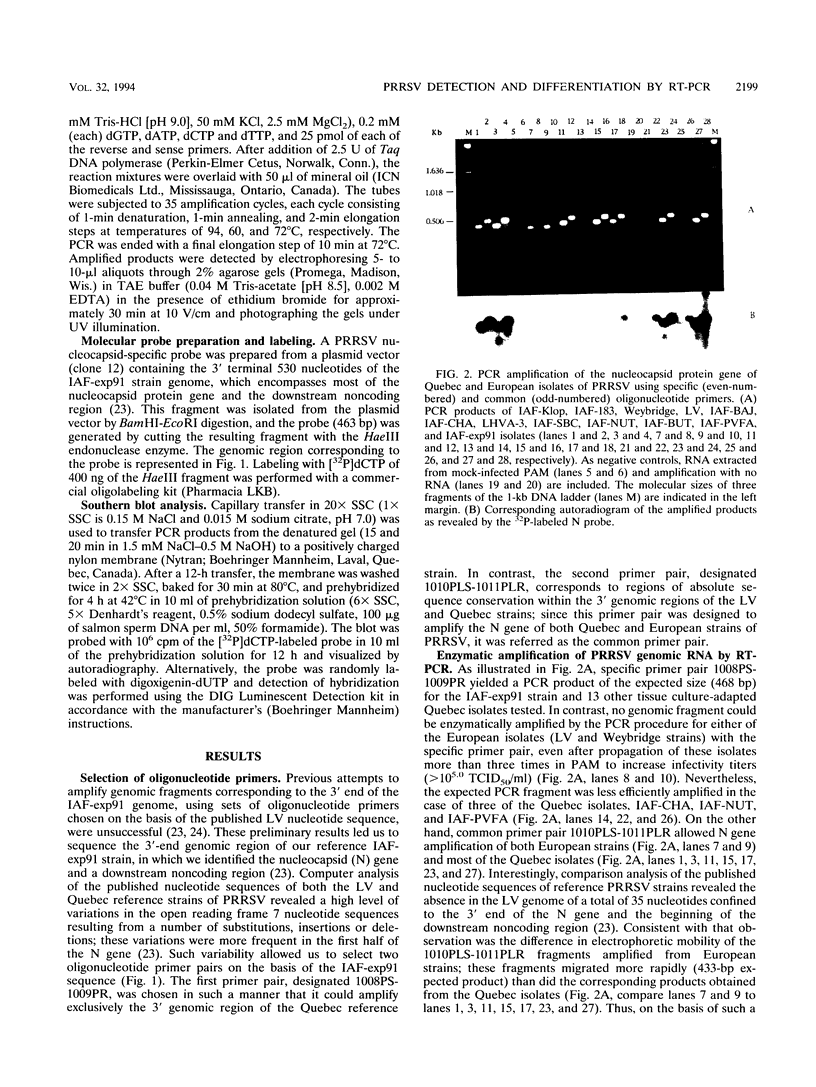
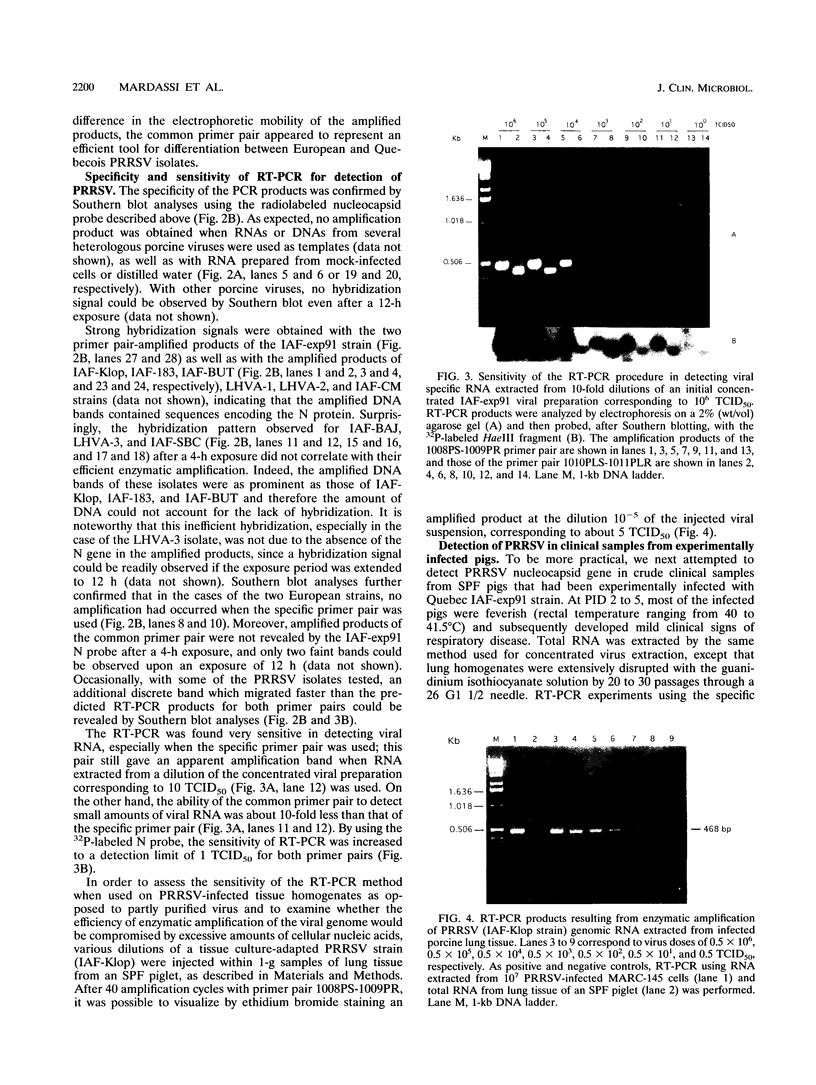
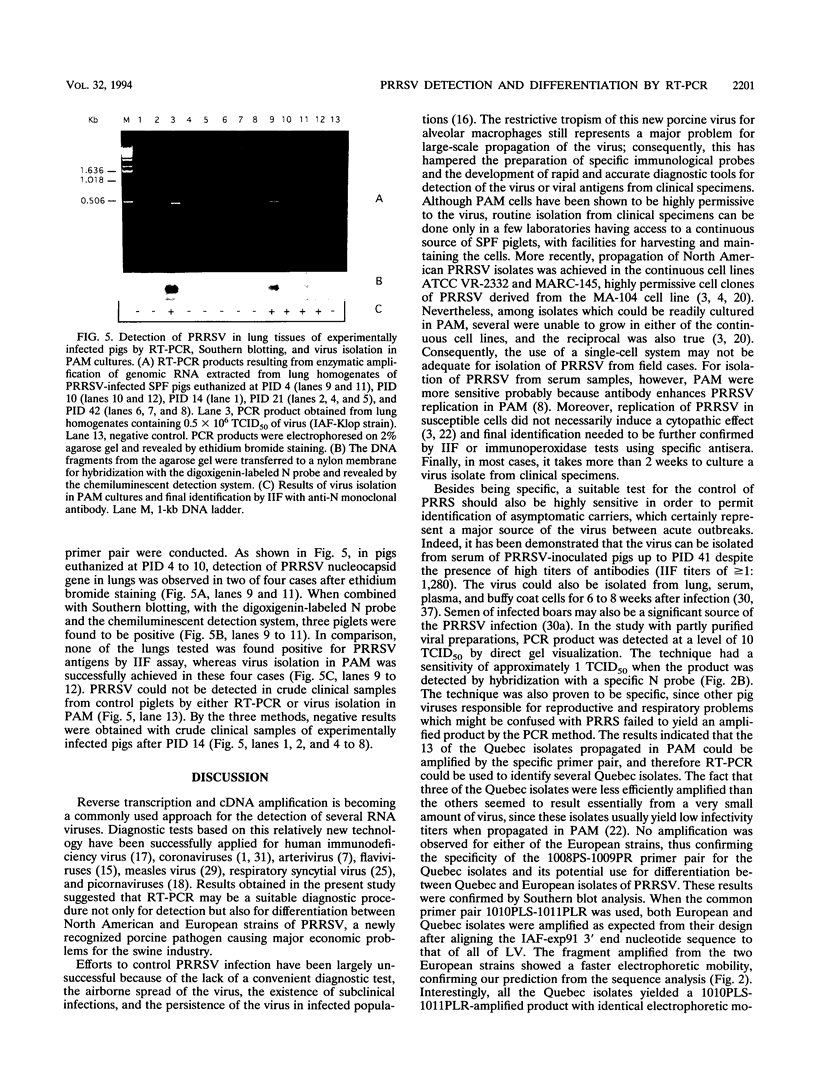
Two sets of oligonucleotide primers (1008PS-1009PR and 1010PLS-1011PLR) were designed according to the sequence of the nucleocapsid protein (N) gene of Quebec reference strain IAF-exp91 of porcine reproductive and respiratory syndrome virus (PRRSV). The primers were used in reverse transcription and PCR (RT-PCR) experiments for detection of viral genomic RNA either from infected porcine alveolar macrophages (PAM) or tissues from experimentally infected specific-pathogen-free pigs. Considering the high degree of variation detected between the nucleotide sequences of the N genes of IAF-exp91 and Lelystad virus (LV) strains of PRRSV, the primers 1008PS-1009PR were referred to as the specific primers, since they were chosen in such a manner that they could amplify only sequences from IAF-exp91 RNA and not from LV. On the other hand, the primer pair 1010PLS-1011PLR was common to both strains of PRRSV. When analyzed by agarose gel electrophoresis, the products of RT-PCR from each set of primers were resolved as single band of the predicted size, the specificity of amplified products being confirmed by Southern blotting with a specific IAF-exp91 N gene probe. No amplification was observed when RNA was extracted from uninfected PAM or from other porcine viruses. As expected, only the common primer pair was able to amplify RNA from the Quebec reference strain and two European strains (LV and Weybridge). The resulting bands displayed differences in electrophoretic mobilities due to the absence of 37 nucleotides in both European strains, thus allowing their differentiation from the IAF-exp91 strain. Most of the tissue culture-adapted Quebec isolates were detected with both primer pairs. The sensitivity of the enzymatic amplification method for detection of PRRSV from lung tissues was a 50% tissue culture infective dose of 5. RT-PCR was found to be more sensitive than indirect immunofluorescence assay for detection of PRRSV in tissues from experimentally infected pigs and as sensitive as virus isolation in PAM, especially when combined with Southern blotting with the digoxigenin-labeled N probe and chemiluminescence detection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Blue ear' disease of pigs. Vet Rec. 1991 Jun 15;128(24):574–574. doi: 10.1136/vr.128.24.574. [DOI] [PubMed] [Google Scholar]
- Andreasen J. R., Jr, Jackwood M. W., Hilt D. A. Polymerase chain reaction amplification of the genome of infectious bronchitis virus. Avian Dis. 1991 Jan-Mar;35(1):216–220. [PubMed] [Google Scholar]
- Baron T., Albina E., Leforban Y., Madec F., Guilmoto H., Plana Duran J., Vannier P. Report on the first outbreaks of the porcine reproductive and respiratory syndrome (PRRS) in France. Diagnosis and viral isolation. Ann Rech Vet. 1992;23(2):161–166. [PubMed] [Google Scholar]
- Bautista E. M., Goyal S. M., Yoon I. J., Joo H. S., Collins J. E. Comparison of porcine alveolar macrophages and CL 2621 for the detection of porcine reproductive and respiratory syndrome (PRRS) virus and anti-PRRS antibody. J Vet Diagn Invest. 1993 Apr;5(2):163–165. doi: 10.1177/104063879300500204. [DOI] [PubMed] [Google Scholar]
- Benfield D. A., Nelson E., Collins J. E., Harris L., Goyal S. M., Robison D., Christianson W. T., Morrison R. B., Gorcyca D., Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J Vet Diagn Invest. 1992 Apr;4(2):127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- Bilodeau R., Dea S., Sauvageau R. A., Martineau G. P. 'Porcine reproductive and respiratory syndrome' in Quebec. Vet Rec. 1991 Aug 3;129(5):102–103. doi: 10.1136/vr.129.5.102. [DOI] [PubMed] [Google Scholar]
- Carman W. F., Williamson C., Cunliffe B. A., Kidd A. H. Reverse transcription and subsequent DNA amplification of rubella virus RNA. J Virol Methods. 1989 Jul;25(1):21–29. doi: 10.1016/0166-0934(89)90097-9. [DOI] [PubMed] [Google Scholar]
- Chirnside E. D., Spaan W. J. Reverse transcription and cDNA amplification by the polymerase chain reaction of equine arteritis virus (EAV). J Virol Methods. 1990 Nov;30(2):133–140. doi: 10.1016/0166-0934(90)90014-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conzelmann K. K., Visser N., Van Woensel P., Thiel H. J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993 Mar;193(1):329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S., Bilodeau R., Athanaseous R., Sauvageau R. A., Martineau G. P. PRRS syndrome in Quebec: isolation of a virus serologically related to Lelystad virus. Vet Rec. 1992 Feb 22;130(8):167–167. doi: 10.1136/vr.130.8.167-b. [DOI] [PubMed] [Google Scholar]
- Dea S., Bilodeau R., Athanassious R., Sauvageau R., Martineau G. P. Swine reproductive and respiratory syndrome in Québec: Isolation of an enveloped virus serologically-related to Lelystad virus. Can Vet J. 1992 Dec;33(12):801–808. [PMC free article] [PubMed] [Google Scholar]
- Dea S., Bilodeau R., Sauvageau R., Martineau G. P. Outbreaks in Quebec pig farms of respiratory and reproductive problems associated with encephalomyocarditis virus. J Vet Diagn Invest. 1991 Oct;3(4):275–282. doi: 10.1177/104063879100300401. [DOI] [PubMed] [Google Scholar]
- Dea S., Garzon S. Identification of coronaviruses by the use of indirect protein A-gold immunoelectron microscopy. J Vet Diagn Invest. 1991 Oct;3(4):297–305. doi: 10.1177/104063879100300405. [DOI] [PubMed] [Google Scholar]
- Eldadah Z. A., Asher D. M., Godec M. S., Pomeroy K. L., Goldfarb L. G., Feinstone S. M., Levitan H., Gibbs C. J., Jr, Gajdusek D. C. Detection of flaviviruses by reverse-transcriptase polymerase chain reaction. J Med Virol. 1991 Apr;33(4):260–267. doi: 10.1002/jmv.1890330410. [DOI] [PubMed] [Google Scholar]
- Goyal S. M. Porcine reproductive and respiratory syndrome. J Vet Diagn Invest. 1993 Oct;5(4):656–664. doi: 10.1177/104063879300500435. [DOI] [PubMed] [Google Scholar]
- Holodniy M., Katzenstein D. A., Sengupta S., Wang A. M., Casipit C., Schwartz D. H., Konrad M., Groves E., Merigan T. C. Detection and quantification of human immunodeficiency virus RNA in patient serum by use of the polymerase chain reaction. J Infect Dis. 1991 Apr;163(4):862–866. doi: 10.1093/infdis/163.4.862. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Auvinen P., Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989 Dec;70(Pt 12):3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Kwang J., Yoon I. J., Joo H. S., Frey M. L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol. 1993;133(3-4):477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- Lunkenheimer K., Hufert F. T., Schmitz H. Detection of Lassa virus RNA in specimens from patients with Lassa fever by using the polymerase chain reaction. J Clin Microbiol. 1990 Dec;28(12):2689–2692. doi: 10.1128/jcm.28.12.2689-2692.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardassi H., Athanassious R., Mounir S., Dea S. Porcine reproductive and respiratory syndrome virus: morphological, biochemical and serological characteristics of Quebec isolates associated with acute and chronic outbreaks of porcine reproductive and respiratory syndrome. Can J Vet Res. 1994 Jan;58(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- Mardassi H., Mounir S., Dea S. Identification of major differences in the nucleocapsid protein genes of a Québec strain and European strains of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1994 Mar;75(Pt 3):681–685. doi: 10.1099/0022-1317-75-3-681. [DOI] [PubMed] [Google Scholar]
- Meulenberg J. J., Hulst M. M., de Meijer E. J., Moonen P. L., den Besten A., de Kluyver E. P., Wensvoort G., Moormann R. J. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993 Jan;192(1):62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton A. W., Paton J. C., Lawrence A. J., Goldwater P. N., Harris R. J. Rapid detection of respiratory syncytial virus in nasopharyngeal aspirates by reverse transcription and polymerase chain reaction amplification. J Clin Microbiol. 1992 Apr;30(4):901–904. doi: 10.1128/jcm.30.4.901-904.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton D. J., Brown I. H., Scott A. C., Done S. H., Edwards S. Isolation of a Lelystad virus-like agent from British pigs and scanning electron microscopy of infected macrophages. Vet Microbiol. 1992 Nov;33(1-4):195–201. doi: 10.1016/0378-1135(92)90047-w. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Moennig V. Lactate dehydrogenase-elevating virus, equine arteritis virus, and simian hemorrhagic fever virus: a new group of positive-strand RNA viruses. Adv Virus Res. 1992;41:99–192. doi: 10.1016/S0065-3527(08)60036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana J., Vayreda M., Vilarrasa J., Bastons M., Rosell R., Martinez M., San Gabriel A., Pujols J., Badiola J. L., Ramos J. A. Porcine epidemic abortion and respiratory syndrome (mystery swine disease). Isolation in Spain of the causative agent and experimental reproduction of the disease. Vet Microbiol. 1992 Nov;33(1-4):203–211. doi: 10.1016/0378-1135(92)90048-x. [DOI] [PubMed] [Google Scholar]
- Shimizu H., McCarthy C. A., Smaron M. F., Burns J. C. Polymerase chain reaction for detection of measles virus in clinical samples. J Clin Microbiol. 1993 May;31(5):1034–1039. doi: 10.1128/jcm.31.5.1034-1039.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. W., Van Alstine W. G., Kanitz C. L., Keffaber K. K. Endemic porcine reproductive and respiratory syndrome virus infection of nursery pigs in two swine herds without current reproductive failure. J Vet Diagn Invest. 1993 Jul;5(3):432–434. doi: 10.1177/104063879300500322. [DOI] [PubMed] [Google Scholar]
- Verbeek A., Tijssen P. Polymerase chain reaction for probe synthesis and for direct amplification in detection of bovine coronavirus. J Virol Methods. 1990 Sep;29(3):243–255. doi: 10.1016/0166-0934(90)90052-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensvoort G., de Kluyver E. P., Luijtze E. A., den Besten A., Harris L., Collins J. E., Christianson W. T., Chladek D. Antigenic comparison of Lelystad virus and swine infertility and respiratory syndrome (SIRS) virus. J Vet Diagn Invest. 1992 Apr;4(2):134–138. doi: 10.1177/104063879200400203. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., de Kluyver E. P., Pol J. M., Wagenaar F., Moormann R. J., Hulst M. M., Bloemraad R., den Besten A., Zetstra T., Terpstra C. Lelystad virus, the cause of porcine epidemic abortion and respiratory syndrome: a review of mystery swine disease research at Lelystad. Vet Microbiol. 1992 Nov;33(1-4):185–193. doi: 10.1016/0378-1135(92)90046-v. [DOI] [PubMed] [Google Scholar]
- Yoon I. J., Joo H. S., Christianson W. T., Kim H. S., Collins J. E., Carlson J. H., Dee S. A. Isolation of a cytopathic virus from weak pigs on farms with a history of swine infertility and respiratory syndrome. J Vet Diagn Invest. 1992 Apr;4(2):139–143. doi: 10.1177/104063879200400204. [DOI] [PubMed] [Google Scholar]
- Yoon I. J., Joo H. S., Christianson W. T., Kim H. S., Collins J. E., Morrison R. B., Dial G. D. An indirect fluorescent antibody test for the detection of antibody to swine infertility and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1992 Apr;4(2):144–147. doi: 10.1177/104063879200400205. [DOI] [PubMed] [Google Scholar]