Abstract
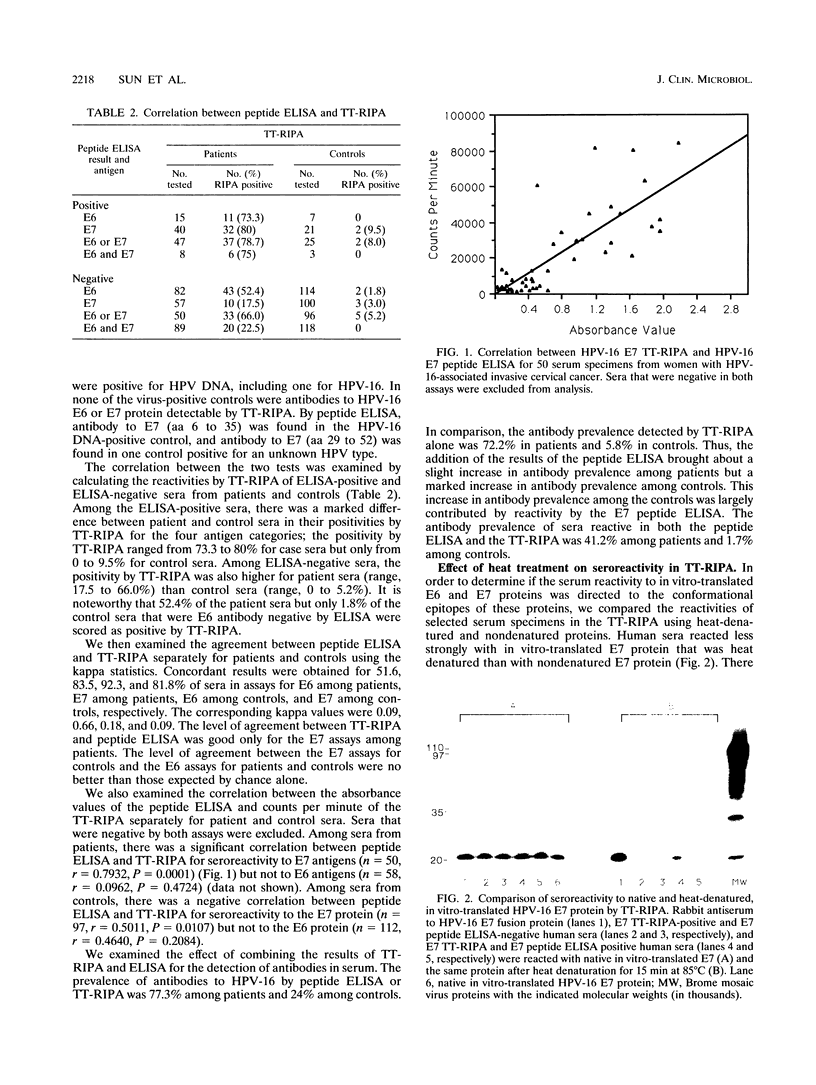
Antibodies to human papilloma virus (HPV) type 16 (HPV-16) E6 and E7 proteins in serum are markers for HPV-associated invasive cervical carcinoma. We compared two assays, a radioimmunoprecipitation assay with in vitro-translated HPV-16 E6 and E7 proteins and an enzyme-linked immunosorbent assay (ELISA) with E6 and E7 synthetic peptides, for their abilities to discriminate serologically between patients with invasive cervical cancer and controls. Among the patients, antibody prevalences were higher by the E6 radioimmunoprecipitation assay (55.7%) than by the E6 peptide ELISA (15.5%), but among the controls, they were lower by the radioimmunoprecipitation assay (1.7%) than by the E6 peptide ELISA (5%). For E7, antibody prevalences among the patients were comparable by the radioimmunoprecipitation assay (43%) and the peptide ELISA (41%), but among the controls they were higher by the E7 peptide ELISA (17.4%) than by the radioimmunoprecipitation assay (4.1%). There was good agreement between the E7 radioimmunoprecipitation assay and the E7 peptide ELISA among patients but not among controls. In tests with representative sera, heat denaturation of the translated proteins resulted in a complete loss of reactivity to the E6 protein and a marked decrease in reactivity to the E7 protein. Our study showed that the radioimmunoprecipitation assay discriminates better than the peptide ELISA between patients with invasive cervical cancer and controls and that this is related to the ability of the radioimmunoprecipitation assay to detect conformational epitopes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen N. D., Kreider J. W., Cladel N. M., Galloway D. A. Immunological cross-reactivity to laboratory-produced HPV-11 virions of polysera raised against bacterially derived fusion proteins and synthetic peptides of HPV-6b and HPV-16 capsid proteins. Virology. 1990 Mar;175(1):1–9. doi: 10.1016/0042-6822(90)90180-y. [DOI] [PubMed] [Google Scholar]
- Cowsert L. M., Lake P., Jenson A. B. Topographical and conformational epitopes of bovine papillomavirus type 1 defined by monoclonal antibodies. J Natl Cancer Inst. 1987 Nov;79(5):1053–1057. [PubMed] [Google Scholar]
- Dillner J. Mapping of linear epitopes of human papillomavirus type 16: the E1, E2, E4, E5, E6 and E7 open reading frames. Int J Cancer. 1990 Oct 15;46(4):703–711. doi: 10.1002/ijc.2910460426. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Galloway D. A. Serological assays for the detection of HPV antibodies. IARC Sci Publ. 1992;(119):147–161. [PubMed] [Google Scholar]
- Ghosh A. K., Smith N. K., Stacey S. N., Glew S. S., Connor M. E., Arrand J. R., Stern P. L. Serological response to HPV 16 in cervical dysplasia and neoplasia: correlation of antibodies to E6 with cervical cancer. Int J Cancer. 1993 Feb 20;53(4):591–596. doi: 10.1002/ijc.2910530411. [DOI] [PubMed] [Google Scholar]
- Guerrero E., Daniel R. W., Bosch F. X., Castellsagué X., Muñoz N., Gili M., Viladiu P., Navarro C., Zubiri M. L., Ascunce N. Comparison of ViraPap, Southern hybridization, and polymerase chain reaction methods for human papillomavirus identification in an epidemiological investigation of cervical cancer. J Clin Microbiol. 1992 Nov;30(11):2951–2959. doi: 10.1128/jcm.30.11.2951-2959.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochmus-Kudielka I., Schneider A., Braun R., Kimmig R., Koldovsky U., Schneweis K. E., Seedorf K., Gissmann L. Antibodies against the human papillomavirus type 16 early proteins in human sera: correlation of anti-E7 reactivity with cervical cancer. J Natl Cancer Inst. 1989 Nov 15;81(22):1698–1704. doi: 10.1093/jnci/81.22.1698. [DOI] [PubMed] [Google Scholar]
- Köchel H. G., Sievert K., Monazahian M., Mittelstädt-Deterding A., Teichmann A., Thomssen R. Antibodies to human papillomavirus type-16 in human sera as revealed by the use of prokaryotically expressed viral gene products. Virology. 1991 Jun;182(2):644–654. doi: 10.1016/0042-6822(91)90605-b. [DOI] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Lim P. S., Jenson A. B., Cowsert L., Nakai Y., Lim L. Y., Jin X. W., Sundberg J. P. Distribution and specific identification of papillomavirus major capsid protein epitopes by immunocytochemistry and epitope scanning of synthetic peptides. J Infect Dis. 1990 Dec;162(6):1263–1269. doi: 10.1093/infdis/162.6.1263. [DOI] [PubMed] [Google Scholar]
- Mandelson M. T., Jenison S. A., Sherman K. J., Valentine J. M., McKnight B., Daling J. R., Galloway D. A. The association of human papillomavirus antibodies with cervical cancer risk. Cancer Epidemiol Biomarkers Prev. 1992 May-Jun;1(4):281–286. [PubMed] [Google Scholar]
- Müller M., Gausepohl H., de Martynoff G., Frank R., Brasseur R., Gissmann L. Identification of seroreactive regions of the human papillomavirus type 16 protein E4, E6, E7 and L1. J Gen Virol. 1990 Nov;71(Pt 11):2709–2717. doi: 10.1099/0022-1317-71-11-2709. [DOI] [PubMed] [Google Scholar]
- Müller M., Viscidi R. P., Sun Y., Guerrero E., Hill P. M., Shah F., Bosch F. X., Muñoz N., Gissmann L., Shah K. V. Antibodies to HPV-16 E6 and E7 proteins as markers for HPV-16-associated invasive cervical cancer. Virology. 1992 Apr;187(2):508–514. doi: 10.1016/0042-6822(92)90453-v. [DOI] [PubMed] [Google Scholar]
- Sasagawa T., Inoue M., Tanizawa O., Yutsudo M., Hakura A. Identification of antibodies against human papillomavirus type 16 E6 and E7 proteins in sera of patients with cervical neoplasias. Jpn J Cancer Res. 1992 Jul;83(7):705–713. doi: 10.1111/j.1349-7006.1992.tb01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey S. N., Bartholomew J. S., Ghosh A., Stern P. L., Mackett M., Arrand J. R. Expression of human papillomavirus type 16 E6 protein by recombinant baculovirus and use for detection of anti-E6 antibodies in human sera. J Gen Virol. 1992 Sep;73(Pt 9):2337–2345. doi: 10.1099/0022-1317-73-9-2337. [DOI] [PubMed] [Google Scholar]
- Strike D. G., Bonnez W., Rose R. C., Reichman R. C. Expression in Escherichia coli of seven DNA fragments comprising the complete L1 and L2 open reading frames of human papillomavirus type 6b and localization of the 'common antigen' region. J Gen Virol. 1989 Mar;70(Pt 3):543–555. doi: 10.1099/0022-1317-70-3-543. [DOI] [PubMed] [Google Scholar]
- Sun Y., Eluf-Neto J., Bosch F. X., Muñoz N., Booth M., Walboomers J. M., Shah K. V., Viscidi R. P. Human papillomavirus-related serological markers of invasive cervical carcinoma in Brazil. Cancer Epidemiol Biomarkers Prev. 1994 Jun;3(4):341–347. [PubMed] [Google Scholar]
- Viscidi R. P., Sun Y., Tsuzaki B., Bosch F. X., Muñoz N., Shah K. V. Serologic response in human papillomavirus-associated invasive cervical cancer. Int J Cancer. 1993 Nov 11;55(5):780–784. doi: 10.1002/ijc.2910550515. [DOI] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]




