Abstract
The interactions between Leishmania parasites and dendritic cells (DCs) are complex and involve paradoxical functions that can stimulate or halt T cell responses, leading to the control of infection or progression of disease. The magnitude and profile of DC activation vary greatly, depending upon the Leishmania species/strains, developmental stages, DC subsets, serum opsonization, and exogenous DC stimuli involved in the study. In general, the uptake of Leishmania parasites alone can trigger relatively weak and transient DC activation; however, the intracellular parasites (amastigotes) are capable of down-modulating LPS/IFN-γ-stimulated DC activation via multiple mechanisms. This review will highlight current data regarding the initial interaction of DC subsets with invading parasites, the alterations of DC signaling pathways and function by amastigotes, and the impact of DC functions on protective immunity and disease pathogenesis. Available information provides insight into the mechanisms by which DCs discriminate between the types of pathogens and regulate appropriate immune responses.
Leishmaniasis is a vector-transmitted disease distributed throughout the world's tropical and subtropical regions. At least 20 Leishmania species can give rise to a wide spectrum of clinical manifestations, ranging from self-healing skin ulcers to disfiguring mucosal lesions and fatal visceral infections. Although the determinants of parasite tissue tropism remain unclear, the diverse clinical forms are believed to be attributable to the host immune status and the species of parasite involved. Leishmania parasites have a dimorphic life cycle. Initially, the infectious promastigote form of the parasite is transmitted to the mammalian host by the bite of sand flies. Once phagocytosed by cells in the macrophage (MΦ)3 lineage, the promastigotes differentiate into a nonmotile amastigote form that replicates within the acidified parasitophorous vacuoles (1). The promastigotes interact with mammalian hosts very briefly without causing clinical manifestations, whereas amastigotes persist in their hosts for years or for a lifetime and are hence responsible for clinical diseases. Those working in this field have focused on gaining a better understanding of the processes by which the promastigotes establish the infection and the amastigotes modulate or take advantage of their host's immune system. However, investigation into the molecular details of the means by which the host cell interacts with amastigotes has been partially hampered by the limited availability of amastigotes for some Leishmania species. Although axenically cultured amastigotes are readily available for some species (e.g., Leishmania amazonensis and Leishmania mexicana), other amastigote species need to be freshly prepared from mouse/hamster lesions or generated from cultured promastigotes (via reduction of culture pH and increase of temperature).
Another challenge in defining the molecular details of the host-Leishmania interaction is the complex regulation of species- and stage-specific genes. The Leishmania genome is spread over 34∼36 chromosome pairs containing ∼8,160 genes. Global analyses of gene expression indicate that the vast majority (>95%) of genes are constitutively expressed in all life stages and that for the few dozen of the amastigote-specific genes identified, they are relatively specific for a given Leishmania species (2). Therefore, information learned from one Leishmania species may not be applicable to other species. For example, there is a great need for comparative studies of Leishmania major and Leishmania donovani (etiological agents of the Old World cutaneous and visceral leishmaniasis, respectively), as well as of L. amazonensis and Leishmania braziliensis (etiological agents of diffuse cutaneous and mucosal leishmaniasis in South America, respectively). In this regard, animal studies have clearly indicated that L. major can cause a Th2-mediated, lethal infection in BALB/c mice but a Th1-mediated, self-healing infection in C57BL/6 and C3H mice, whereas L. amazonensis often causes progressive, nonhealing lesions in all of these mouse strains (3) due to an IL-4/IL-10-independent impairment in innate and acquired immunity (4, 5). This review will highlight available evidence at the DC level that may explain the diverse outcomes of Leishmania infection.
MΦs are the main host cells for Leishmania replication and the effector cell for parasite killing. Leishmania infection does not trigger inflammatory MΦs to produce IL-12 (6), and parasite-carrying MΦs are incompetent in priming naive CD4+ T cells or stimulating Ag-specific CD4+ T cells (7). The molecular details have been reviewed elsewhere with respect to how promastigotes and amastigotes bind to MΦ surface receptors and interfere with their effector functions (8, 9). The recent advancements in the biology of DC subsets, as well as in the re-agents used, have greatly enhanced our understanding of DC-Leishmania interactions, and several reviews have summarized the critical role of myeloid DCs (mDCs) in generating protective immunity against L. major infection (10-12). New data also call for re-evaluation of the accepted paradigm in L. major infection (13, 14). This review will focus on recent data related to the initial interactions of DC subsets with promastigote infection in vivo, alterations of DC signaling pathways by amastigotes, the impact of these alterations on disease outcomes, and the potential of DC-based vaccines in animal models of cutaneous leishmaniasis. Emphasis will be placed on DC responses to the species of amastigotes in the L. mexicana complex, especially to L. amazonensis because of its association with profound T cell suppression in patients with diffuse cutaneous leishmaniasis (15, 16) and its ability to cause nonhealing skin lesions in several inbred mouse strains that are genetically resistant to L. major parasites.
The interaction of DC subsets with Leishmania promastigotes in vivo
Because leishmaniasis is initiated by promastigote infection in the skin, extensive studies have been conducted in mouse models of L. major infection to define the early source of IL-12 and the role of different DC subsets in infection. Most of the reported studies, however, have used supraphysiologic doses of promastigotes in the absence of sand fly influence, which is known to modulate DC maturation/activation (17) and local immune responses (18). Following skin injection of L. major promastigotes, polymorphonuclear neutrophils (PMN) are recruited to the infection site within hours and constitute the majority of cellular infiltrates within the first day of infection (19). Although L. major-infected PMNs could constitute one of the earliest sources of IL-12 in resistant C57BL/6 mice, possibly via the activation of TLR2, 7, and 9 (20), some parasite-carrying PMNs can act as “Trojan horses” to transfer parasites to MΦs, promoting TGF-β production by MΦs and establishing the infection in MΦs (21, 22). The means by which these early events influence DC recruitment and activation remain unclear. Several DC subsets are involved in Leishmania infection. Plasmacytoid DCs (pDCs) can be detected in the infected skin; however, they may not endocytose Leishmania promastigotes but can be activated by released Leishmania genomic DNA to produce appreciable amounts of IFN-αβ and some IL-12 in a TLR9-dependent manner (23). Although adoptively transferred pDCs contribute to the control of L. major infection in mice (24), in vivo studies suggest that mDCs and IL-12, rather than pDCs and IFN-αβ, are required for NK cell cytotoxicity and IFN-γ production in cutaneous and visceral leishmaniasis (23, 25).
For some time the nature and specific functions of migratory DCs in cutaneous leishmaniasis have been matters of controversy. Although both dermal DC immigrants (MHC IIhigh, CD11c+, CD11b+, CD8α−, and CD205low) and epidermal Langerhans cells (LCs) (MHC IIhigh, CD11c+, CD11blow, CD8ainter, CD205high, and Langerin+) can home to the draining lymph nodes (LNs), they may have different capacities in engulfing promastigotes and distinct functions in experimental leishmaniasis (13, 26). In L. major infection, parasite Ags are believed to be transported from the site of infection to draining LNs by CD11c+CD8α−Langerin− dermal DCs rather than by LCs (27) because LCs are less efficient in the uptake of promastigotes. These recent data challenge our previous view on LCs (28) and support the hypothesis that dermal DCs carry parasite Ag to activate Th1 effector cells, whereas epidermal LCs take up free Ag released from parasites or damaged host cells to activate other T cell subsets (13). More recent data from L. major-infected tissues and adoptive transfer of purified monocytes have provided new evidence, indicating the importance of the kinetics of cellular recruitment during infection and the de novo differentiation of monocytes to DCs, as well as the role of this DC subset in inducing parasite-specific Th1 responses in the draining LNs (14). Collectively, these in vivo studies suggest the dynamics of the DC network during infection-induced inflammatory reactions and the importance of fully activated mDCs (CD86high, CD40high, CCR7+, and IL-12+) in TLR-9-mediated activation of NK cells and in the generation of protective Th1 responses against Leishmania parasites (Fig. 1). At present, there is still limited information on initial DC responses to other species of Leishmania at the site of infection and on the priming of pathogenic T cells.
FIGURE 1.
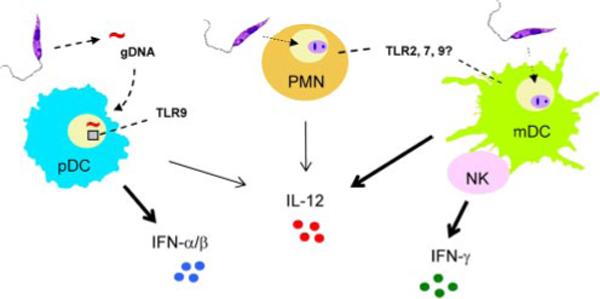
A schematic view of the hierarchy within different cell types, receptors, and cytokines in activating innate and adaptive immunity at the initial stages of infection with Leishmania promastigotes. Following the initial infection with Leishmania promastigotes, PMNs are recruited within hours to the site of infection and constitute the majority of the cellular infiltrates in the first day of infection. Parasite infection in PMNs may trigger the expression of TLR2, 7, and 9, as well as the production of some IL-12. Although pDCs do not endocytose promastigotes, they can be activated via released Leishmania genomic DNA (gDNA) to produce appreciable amounts of IFN-αβ and some IL-12 in a TLR9-dependent manner. mDCs and monocyte-derived DCs can efficiently engulf promastigotes, serving as a critical source of early IL-12 production. Activated DCs can migrate to draining LNs, activate resting NK cells via cell contact-dependent manner, and trigger NK cells to produce IFN-γ. The cognate interactions among multiple innate cell types and cytokines at the site of infection and in draining LNs are required for appropriate activation of protective Th1 responses against the invading pathogens. However, the magnitude of DC and Th1 cell activation in vivo varies greatly, depending upon the species of parasites involved.
DC responsiveness to Leishmania promastigote infection in vitro
To define the means by which DCs respond to Leishmania parasites and Ag-specific CD4+ T cells are activated, extensive studies have been performed with bone marrow-derived mouse mDCs, skin-derived mouse LCs, or monocyte-derived human DCs. In vitro infection studies have collectively indicated that mDCs (but not LCs) can efficiently engulf promastigotes, and that infection with parasites in the absence of other stimuli can activate DCs to produce little IL-12p70 but a range of IL-12p40 and IL-10 (29-31). Nevertheless, IL-12p40 and IL-12p70 production by infected DCs can be markedly enhanced by the addition of exogenous stimuli (e.g., IL-1, IFN-γ, IFN-γ/LPS, CD40L, and anti-CD40) at the time of infection (29, 31, 32). Promastigote-infected, IL-12p40-producing mDCs have a CD11chighCD45BR−CD83+CD40+ phenotype (31). Although infection with L. donovani and L. major promastigotes can trigger the release of preformed IL-12p70 and the production of IL-12p40 (6, 33), the up-regulation of the IL-12p40 gene in L. amazonensis-infected mDCs appears to be relatively weak and transient (31) (L. Xin, K. Li, and L. Soong, submitted for publication). Although there was an ∼16- and 1300-fold induction in IL-12p40 gene expression at 8 h of infection in the absence or presence of LPS/IFN-γ, respectively, the expression levels dropped dramatically by 24 h postinfection (Fig. 2). Depending on the species of the parasites involved, promastigote infection was able to marginally or partially reduce DC responsiveness to exogenous stimuli (29, 31) and to impair DC differentiation in vitro (34).
FIGURE 2.
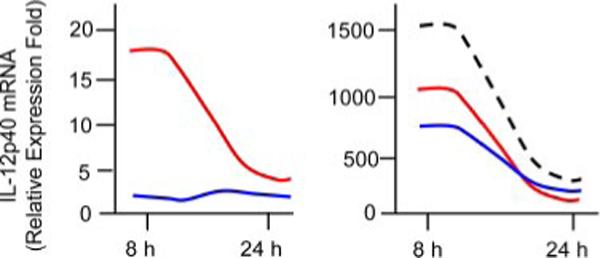
Transient expression of the IL-12p40 gene in DCs following infection with L. amazonensis promastigotes. Bone marrow-derived C57BL/6 DCs were infected with promastigotes (red lines) and amastigotes (blue lines) for 8 and 24 h, respectively. To examine the effect of parasitic infection on DC responsiveness to other stimuli, cells were infected with parasites for 6 h and then treated with IFN-γ (100 ng/ml) plus LPS (100 ng/ml) for an additional 2 or 18 h, respectively. LPS/IFN-γ-treated DCs are denoted by a dashed line. The levels of IL-12p40 mRNA were quantified by real-time RT-PCR (normalized to the β-actin gene) and are presented as relative expression fold in comparison to those of untreated DCs.
The responsiveness of mDCs to different Leishmania promastigotes can be roughly ranked in the order of L. major and L. braziliensis > L. donovani and Leishmania infantum > L. mexicana and L. amazonensis as judged by the expression levels of surface markers and cytokines and APC potential in priming naive CD4+ T cells (31, 32, 35) (D. A. Vargas-Inchaustegui, L. Xin, and L. Soong, submitted for publication). It is well known that promastigote surface molecules, such as lipophosphoglycan (LPG), can suppress MΦ functions (36); however, LPG, but not the lipid-free LPG, can also activate murine mDCs and NK cells through TLR2 (37). Given the marked multiplicity in LPG glycan side chains (composition and position on the LPG core structure) (38), it will be important to examine whether diverse DC responses to promastigotes are solely due to LPG (32) or to other undefined pattern-recognition molecules. In comparison to other pathogens that have chemically defined ligands for multiple TLRs (39), TLR ligand involvement is less clear for Leishmania parasites. Although circumstantial evidence suggests the involvement of LPG as well as TLR2, 3, 4, 7, and 9, in Leishmania infection (20, 40-42), the lack of strong TLR ligands in Leishmania infection may partially explain the relatively weak DC activation induced by promastigote infection alone.
Down-modulation of DC functions by Leishmania amastigotes
The molecular details of how DCs deal with the disease-forming amastigotes are still convoluted. In contrast to promastigotes, Leishmania amastigotes are largely deficient in LPG and have several unique biological features (9). First, the avidity of Leishmania for the surface C-type lectin called DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN or CD209) and DC-SIGN-related molecules varies greatly with the parasite species and maturation stage in question. For example, amastigotes of L. amazonensis, L. mexicana, L. donovani, but not L. major, can bind to DC-SIGN (43, 44). Because this binding is independent of LPG and does not induce DC maturation (44), the benefits of engaging to DC-SIGN in these DCs remain enigmatic (45). Second, lesion-derived amastigotes are commonly coated with host Abs. Whereas Ab opsonization promotes the uptake of L. major amastigotes by murine mDCs and skin-derived LCs (6, 46), amastigotes of L. amazonensis (47), L. mexicana (48), and L. braziliensis (D. A. Vargas-Inchaustegui, L. Xin, and L.Soong, submitted for publication) are highly efficient in infecting mDCs, even in the absence of any Abs. Nevertheless, Ab opsonization of amastigotes (and promastigotes) significantly enhances DC activation and the production of IL-10 (and IL-12p40, to some extent) (6, 47) via the FcγR-mediated pathway (49). Consequently, such DCs preferentially prime IL-10-producing CD4+ T cells and promote lesion progression in mice (47, 48). In contrast, other studies have shown that the uptake of Ab-opsonized L. major amastigotes by murine mDCs or lesion-derived L. donovani amastigotes by human mDCs activates DCs to produce IL-12 and to induce protective immunity (46, 50). In further contrast, uptake of opsonized L. major amastigotes by BALB/c mDCs preferentially stimulates the production of IL-12p40 and the formation of inhibitory IL-12p40 homodimers (51). The differences in DC cytokine profiles may partially explain the complex roles of Ab in leishmaniasis (12, 47, 52).
Although L. amazonensis amastigotes can infect >80% of CD11c+ mDCs in <30 min, they are especially poor at activating these cells (35, 48). Live L. amazonensis amastigotes also markedly alter DC responsiveness to exogenous stimuli (IFN-γ, LPS, and LPS/IFN-γ), and amastigote-infected DCs are poor APCs in priming naive CD4+ T cells in vitro and in vivo (35) (L. Xin, J. Li, and L. Soong, submitted for publication). Although IL-10 can be readily detected in amastigote-infected mDCs (30), current evidence suggests that the down-modulation of DC activation following amastigote infection is not due to endogenous IL-10 but rather to reduced phosphorylation and/or accelerated degradation of key molecules in the JAK/STAT, NF-κB, and IFN regulatory factor (IRF) pathways via parasite-derived proteinases and oligopeptidases (Fig. 3). This hypothesis is supported by several lines of evidence. First, heat inactivation (56°C for 30 min) or pretreatment of amastigotes with protease inhibitors averts host protein degradation, permitting DC activation to some extent (30)(L. Xin, K. Li, and L. Soong, submitted for publication). Second, Leishmania parasites are known to contain a vast repertoire of proteolytic enzymes encoding at least 154 peptidases (aspartic, cysteine, metallo, serine, and threonine peptidases) (53), and amastigotes of L. amazonensis and L. mexicana are notorious for their high cysteine protease/oligopeptidase B activities in specialized structures called megasomes (54, 55). The cathepsin B-like cysteine proteinase is central to the parasites’ ability to modulate NF-κB signaling and to consequently inhibit IL-12 production by murine MΦs (56), and targeted depletion of its encoding gene greatly reduces parasite virulence (57). At present, it is unclear how parasite components travel through the parasitophorous vacuole membrane to interfere with host signaling pathways and whether such down-modulation is specific to activation-related pathways. Nevertheless, alterations in DC activation/migration may be a general phenomenon in Leishmania infection, especially during the chronic stages (58, 59).
FIGURE 3.
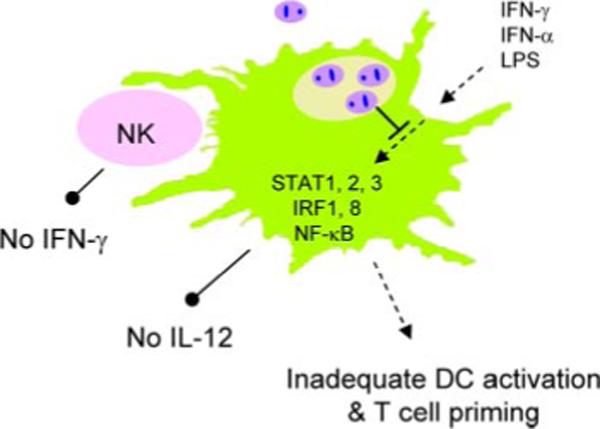
Down-modulation of cytokine- and TLR4-mediated DC activation and IL-12 production in DCs by Leishmania amastigotes. L. amazonensis amastigotes can efficiently enter mDCs, preventing and actively suppressing DC maturation/activation and IL-12 production. Alterations in amastigote-infected, LPS/IFN-stimulated DCs are mostly attributed to suppressed activation and/or accelerated degradation of components in the JAK/STAT, NF-κB, and IFN regulator factor (IRF) pathways via parasite-derived proteinases and oligopeptidases rather than to the endogenous production of IL-10. Alterations in DC activation and function can result in an impaired activation of NK cells and parasite-specific Th1 cells, leading to uncontrolled parasite replication and persistent infection.
DC-based immunotherapy in murine models of leishmaniasis
Examinations of the utility of DC vaccination as cellular immunotherapy for cancers have yielded some encouraging results and prompted similar studies in animal models of leishmaniasis. Although DC-based vaccination may lack a conspicuous application for parasitic diseases endemic in developing countries, it serves as a valuable tool for defining DC-targeted control methods. Using L. major infection as a model, Moll and colleagues have shown that a single i.v. injection of ex vivo Ag-pulsed LCs (60), mDCs (61), or pDCs (24) (5 × 104 ∼ 5 × 105 cells per mouse) is sufficient to induce protective immunity against single or repeated parasite challenges in susceptible BALB/c mice. Interestingly, Ag-pulsed LCs and pDCs can mediate protection independent of additional DC stimuli (CpG, TNF-α, LPS, or CD40 ligation); however, overnight exposure to CpG motifs is required for Ag-loaded mDCs to induce specific Th1 responses (61), a phenomenon also observed for mDCs pulsed with L. infantum Ag (62). In addition, while a polarized Th1 cytokine profile is linked to protection in LC-and CpG/mDC-immunized mice, no strong correlation (or specific roles for IL-12 and IFN-α) is observed in the case of pDC-immunized mice (24). These findings indicate the functional diversity of DC subsets in the control of L. major infection and the importance of mounting adequate local immune responses to control the infection (63). However, direct lesional injection of L. amazonensis Ag-pulsed mDCs (106 cells per mouse) together with rIL-12 failed to promote healing in mice chronically infected with L. amazonensis (35). Therefore, simply providing in vitro-activated DCs or primed Th1 cells is not sufficient to ensure healing in L. amazonensis-infected hosts (35, 64). Additional treatments aimed at reducing intracellular parasite loads and/or blocking a parasite's enzymatic activities are needed to outwit the parasites and thereby stem their persistence.
The development of DC-based immunotherapy for the control of cutaneous leishmaniasis and other infectious diseases requires a better understanding of the following: 1) the differences in the homing of transferred DCs to draining LNs and into a particular T cell activation compartment (13); 2) the requirement for additional DC stimuli in vaccination protocols (63); and 3) the contribution of microenvironmental factors in DC functions during the early and late phases of infection (65). Given the unique features of DC-amastigote interaction, DC-based vaccination studies should be extended to those hosts having an established infection. Targeted delivery of leishmanial Ag to endogenous DCs (66) and the use of genetically engineered parasites that express the DC activator (67) are other attractive approaches.
Conclusions
Leishmania parasites, especially their intracellular forms, have evolved complex strategies to evade DC functions. While infection with promastigotes alone can induce dermal DCs and mDCs to mature, these activated DCs only transiently produce relatively low amounts of proinflammatory cytokines. In vitro studies have suggested that promastigote-infected DCs retain their responsiveness to exogenous DC stimuli, producing appreciable levels of IL-1, TNF-α, IL-12p70, and IL-10, depending upon the nature and combination of stimuli. Infection with Leishmania amastigotes, however, often fails to induce DC activation, presumably due to the general lack of LPG and the direct effect of amastigote proteinases/oligopeptidases in host cell signaling pathways. There is an increasing awareness of species- and strain-dependent differences in DC responsiveness to Leishmania parasites. Likewise, marked differences are evident in the biological roles of different DC subsets. The molecular basis of parasite-derived and DC-derived differences remains enigmatic, although DC-based studies have provided valuable information. The details are largely unexplored with regard to how different DC subsets interact with promastigotes during natural infection in vivo and with amastigotes, especially at the chronic stages of infection. The availability of molecularly engineered, traceable, and suicidal parasites (68, 69) would provide additional tools to advance our understanding of host-parasite interactions.
Acknowledgments
I thank David Sacks and Mary Ann McDowell for insightful discussion and critiques and additional thank Mardelle Susman for proofreading the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants AI043003 and AI039540).
Abbreviations used in this paper: MΦ, macrophage; DC, dendritic cell; DC-SIGN, dendritic cell-specific ICAM-3-grabbing nonintegrin; LC, Langerhans cell; LN, lymph node; LPG, lipophosphoglycan; mDC, myeloid DC; pDC, plasmacytoid DC; PMN, polymorphonuclear neutrophil.
Disclosures
The authors have no financial conflict of interest.
References
- 1.McConville MJ, Souza D. d., Saunders E, Likic VA, Naderer T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007;23:368–375. doi: 10.1016/j.pt.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Cohen-Freue G, Holzer TR, Forney JD, McMaster WR. Global gene expression in Leishmania. Int. J. Parasitol. 2007;37:1077–1086. doi: 10.1016/j.ijpara.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 3.McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 2004;201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 4.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 2003;71:4278–4288. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect. Immun. 2002;70:2151–2158. doi: 10.1128/IAI.70.4.2151-2158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kima PE, Soong L, Chicharro C, Ruddle NH, McMahon-Pratt D. Sequestration in Leishmania-infected macrophages sequester endogenously synthesized parasite antigens from presentation to CD4+ T cells. Eur. J. Immunol. 1996;26:3163–3169. doi: 10.1002/eji.1830261249. [DOI] [PubMed] [Google Scholar]
- 8.Vannier-Santos MA, Martiny A, de Souza W. Cell biology of Leishmania spp.: invading and evading. Curr. Pharm. Des. 2002;8:297–318. doi: 10.2174/1381612023396230. [DOI] [PubMed] [Google Scholar]
- 9.Kima PE. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 2007;37:1087–1096. doi: 10.1016/j.ijpara.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat. Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 11.Sher A, Pearce E, Kaye P. Shaping the immune response to parasites: role of dendritic cells. Curr. Opin. Immunol. 2003;15:421–429. doi: 10.1016/s0952-7915(03)00072-4. [DOI] [PubMed] [Google Scholar]
- 12.Von Stebut E. Immunology of cutaneous leishmaniasis: the role of mast cells, phagocytes and dendritic cells for protective immunity. Eur. J. Dermatol. 2007;17:115–122. doi: 10.1684/ejd.2007.0122. [DOI] [PubMed] [Google Scholar]
- 13.Ritter U, Osterloh A. A new view on cutaneous dendritic cell subsets in experimental leishmaniasis. Med. Microbiol. Immunol. 2007;196:51–59. doi: 10.1007/s00430-006-0023-0. [DOI] [PubMed] [Google Scholar]
- 14.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. (see comment) [DOI] [PubMed] [Google Scholar]
- 15.Silveira FT, Blackwell JM, Ishikawa EA, Braga R, Shaw JJ, Quinnell RJ, Soong L, Kima P, McMahon-Pratt D, Black GF, Shaw MA. T cell responses to crude and defined leishmanial antigens in patients from the lower Amazon region of Brazil infected with different species of Leishmania of the subgenera Leishmania and Viannia. Parasite Immunol. 1998;20:19–26. doi: 10.1046/j.1365-3024.1998.t01-1-00126.x. [DOI] [PubMed] [Google Scholar]
- 16.Silveira FT, Lainson R, Corbett CE. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem. Inst. Oswaldo Cruz. 2004;99:239–251. doi: 10.1590/s0074-02762004000300001. [DOI] [PubMed] [Google Scholar]
- 17.Costa DJ, Favali C, Clarencio J, Afonso L, Conceicao V, Miranda JC, Titus RG, Valenzuela J, Barral-Netto M, Barral A, Brodskyn CI. Lutzomyia longipalpis salivary gland homogenate impairs cytokine production and costimulatory molecule expression on human monocytes and dendritic cells. Infect. Immun. 2004;72:1298–1305. doi: 10.1128/IAI.72.3.1298-1305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrade BB, de Oliveira CI, Brodskyn CI, Barral A, Barral-Netto M. Role of sand fly saliva in human and experimental leishmaniasis: current insights. Scand. J. Immunol. 2007;66:122–127. doi: 10.1111/j.1365-3083.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- 19.Beil WJ, Meinardus-Hager G, Neugebauer DC, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J. Leukocyte Biol. 1992;52:135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 20.Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J. Leukocyte Biol. 2007;82:288–284. doi: 10.1189/jlb.0706440. [DOI] [PubMed] [Google Scholar]
- 21.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J. Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 22.Gueirard P, Laplante A, Rondeau C, Milon G, Desjardins M. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cell Microbiol. 2008;10:100–111. doi: 10.1111/j.1462-5822.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 23.Schleicher U, Liese J, Knippertz I, Kurzmann C, Hesse A, Heit A, Fischer JA, Weiss S, Kalinke U, Kunz S, Bogdan C. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remer KA, Apetrei C, Schwarz T, Linden C, Moll H. Vaccination with plasmacytoid dendritic cells induces protection against infection with Leishmania major in mice. Eur. J. Immunol. 2007;37:2463–2473. doi: 10.1002/eji.200636780. [DOI] [PubMed] [Google Scholar]
- 25.Bajenoff M, Breart B, Huang AYC, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misslitz AC, Bonhagen K, Harbecke D, Lippuner C, Kamradt T, Aebischer T. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur. J. Immunol. 2004;34:715–725. doi: 10.1002/eji.200324391. [DOI] [PubMed] [Google Scholar]
- 27.Ritter U, Meissner A, Scheidig C, Korner H. CD8α- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 2004;34:1542–1550. doi: 10.1002/eji.200324586. [DOI] [PubMed] [Google Scholar]
- 28.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 29.von Stebut E, Belkaid Y, Nguyen BV, Cushing M, Sacks DL, Udey MC. Leishmania major-infected murine Langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur. J. Immunol. 2000;30:3498–3506. doi: 10.1002/1521-4141(2000012)30:12<3498::AID-IMMU3498>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Qi H, Popov V, Soong L. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4+ T cells in vivo. J. Immunol. 2001;167:4534–4542. doi: 10.4049/jimmunol.167.8.4534. [DOI] [PubMed] [Google Scholar]
- 31.Xin L, Li Y, Soong L. The role of IL-1β in activating CD11chighCD45RB− DC subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect. Immun. 2007;75:5018–5026. doi: 10.1128/IAI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect. Immun. 2002;70:3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinones M, Ahuja SK, Melby PC, Pate L, Reddick RL, Ahuja SS. Preformed membrane-associated stores of interleukin (IL)-12 are a previously unrecognized source of bioactive IL-12 that is mobilized within minutes of contact with an intracellular parasite. J. Exp. Med. 2000;192:507–516. doi: 10.1084/jem.192.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Favali C, Tavares N, Clarencio J, Barral A, Barral-Netto M, Brodskyn C. Leishmania amazonensis infection impairs differentiation and function of human dendritic cells. J. Leukocyte Biol. 2007;82:1401–1406. doi: 10.1189/jlb.0307187. [DOI] [PubMed] [Google Scholar]
- 35.Vanloubbeeck YF, Ramer AE, Jie F, Jones DE. CD4+ Th1 cells induced by dendritic cell-based immunotherapy in mice chronically infected with Leishmania amazonensis do not promote healing. Infect. Immun. 2004;72:4455–4463. doi: 10.1128/IAI.72.8.4455-4463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naderer T, Vince JE, McConville MJ. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr Mol. Med. 2004;4:649–665. doi: 10.2174/1566524043360069. [DOI] [PubMed] [Google Scholar]
- 37.Brandonisio O, Spinelli R, Pepe M. Dendritic cells in Leishmania infection. Microbes Infect. 2004;6:1402–1409. doi: 10.1016/j.micinf.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Turco SJ, Spath GF, Beverley SM. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 2001;17:223–226. doi: 10.1016/s1471-4922(01)01895-5. [DOI] [PubMed] [Google Scholar]
- 39.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 40.Kropf P, Freudenberg MA, Modolell M, Price HP, Herath S, Antoniazi S, Galanos C, Smith DF, Muller I. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 2004;72:1920–1928. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flandin JF, Chano F, Descoteaux A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-γ-primed macrophages. Eur. J. Immunol. 2006;36:411–420. doi: 10.1002/eji.200535079. [DOI] [PubMed] [Google Scholar]
- 42.Liese J, Schleicher U, Bogdan C. TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur. J. Immunol. 2007;37:3424–3434. doi: 10.1002/eji.200737182. [DOI] [PubMed] [Google Scholar]
- 43.Colmenares M, Corbi AL, Turco SJ, Rivas L. The dendritic cell receptor DC-SIGN discriminates among species and life cycle forms of Leishmania. J. Immunol. 2004;172:1186–1190. doi: 10.4049/jimmunol.172.2.1186. [DOI] [PubMed] [Google Scholar]
- 44.Caparros E, Serrano D, Puig-Kroger A, Riol L, Lasala F, Martinez I, Vidal-Vanaclocha F, Delgado R, Rodriguez-Fernandez JL, Rivas L, et al. Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes. Immunobiology. 2005;210:185–193. doi: 10.1016/j.imbio.2005.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garg R, Trudel N, Tremblay MJ. Consequences of the natural propensity of Leishmania and HIV-1 to target dendritic cells. Trends Parasitology. 2007;23:317–324. doi: 10.1016/j.pt.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Woelbing F, Kostka SL, Moelle K, Belkaid Y, Sunderkoetter C, Verbeek S, Waisman A, Nigg AP, Knop J, Udey MC, von Stebut E. Uptake of Leishmania major by dendritic cells is mediated by Fc-γ receptors and facilitates acquisition of protective immunity. J. Exp. Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wanasen N, Xin L, Soong L. Pathogenic role of B cells and antibodies in murine Leishmania amazonensis infection. Int. J. Parasitol. doi: 10.1016/j.ijpara.2007.08.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prina E, Abdi SZ, Lebastard M, Perret E, Winter N, Antoine JC. Dendritic cells as host cells for the promastigote and amastigote stages of Leishmania amazonensis: the role of opsonins in parasite uptake and dendritic cell maturation. J. Cell Sci. 2004;117:315–325. doi: 10.1242/jcs.00860. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J. Immunol. 2007;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh M, Mandal L, Maitra S, Rakshit S, Paul K, Bagchi J, Ganguly D, Pal C, Bandyopadhyay S. Leishmania donovani infection of human myeloid dendritic cells leads to a Th1 response in CD4+ T cells from healthy donors and patients with Kala-Azar. J. Infect. Dis. 2006;194:294–301. doi: 10.1086/505228. [DOI] [PubMed] [Google Scholar]
- 51.Nigg AP, Zahn S, Ruckerl D, Holscher C, Yoshimoto T, Ehrchen JM, Wolbing F, Udey MC, von Stebut E. Dendritic cell-derived IL-12p40 homodimer contributes to susceptibility in cutaneous leishmaniasis in BALB/c mice. J. Immunol. 2007;178:7251–7258. doi: 10.4049/jimmunol.178.11.7251. [DOI] [PubMed] [Google Scholar]
- 52.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Besteiro S, Williams RA, Coombs GH, Mottram JC. Protein turnover and differentiation in Leishmania. Int. J. Parasitol. 2007;37:1063–1075. doi: 10.1016/j.ijpara.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueda-Nakamura T, Attias M, de Souza W. Comparative analysis of megasomes in members of the Leishmania mexicana complex. Res. Microbiol. 2007;158:456–462. doi: 10.1016/j.resmic.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 55.de Matos Guedes HL, Carneiro MPD, de Oliveira Gomes DC, Rossi-Bergmann B, de Simone SG. Oligopeptidase B from L. amazonensis: molecular cloning, gene expression analysis and molecular model. Parasitol. Res. 2007;101:866–875. doi: 10.1007/s00436-007-0552-5. [DOI] [PubMed] [Google Scholar]
- 56.Cameron P, McGachy A, Anderson M, Paul A, Coombs GH, Mottram JC, Alexander J, Plevin R. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-κB signaling pathway. J. Immunol. 2004;173:3297–3304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- 57.Saravia NG, Escorcia B, Osorio Y, Valderrama L, Brooks D, Arteaga L, Coombs G, Mottram J, Travi BL. Pathogenicity and protective immunogenicity of cysteine proteinase-deficient mutants of Leishmania mexicana in nonmurine models. Vaccine. 2006;24:4247–4259. doi: 10.1016/j.vaccine.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 58.Ato M, Stager S, Engwerda CR, Kaye PM. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat. Immunol. 2002;3:1185–1191. doi: 10.1038/ni861. [DOI] [PubMed] [Google Scholar]
- 59.Steigerwald M, Moll H. Leishmania major modulates chemokine and chemokine receptor expression by dendritic cells and affects their migratory capacity. Infect. Immun. 2005;73:2564–2567. doi: 10.1128/IAI.73.4.2564-2567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berberich C, Ramirez-Pineda JR, Hambrecht C, Alber G, Skeiky YA, Moll H. Dendritic Cell (DC)-based protection against an intracellular pathogen is dependent upon DC-derived IL-12 and can be induced by molecularly defined antigens. J. Immunol. 2003;170:3171–3179. doi: 10.4049/jimmunol.170.6.3171. [DOI] [PubMed] [Google Scholar]
- 61.Ramirez-Pineda JR, Frohlich A, Berberich C, Moll H. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J. Immunol. 2004;172:6281–6289. doi: 10.4049/jimmunol.172.10.6281. [DOI] [PubMed] [Google Scholar]
- 62.Carrion J, Nieto A, Soto M, Alonso C. Adoptive transfer of dendritic cells pulsed with Leishmania infantum nucleosomal histones confers protection against cutaneous leishmaniosis in BALB/c mice. Microbes Infect. 2007;9:735–743. doi: 10.1016/j.micinf.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Fajardo-Moser M, Berzel S, Moll H. Mechanisms of dendritic cell-based vaccination against infection. Int. J. Med. Microbiol. 2008;298:11–20. doi: 10.1016/j.ijmm.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Ji J, Sun J, Qi H, Soong L. Analysis of T helper cell responses during infection with Leishmania amazonensis. Am. J. Trop. Med. Hyg. 2002;66:338–345. doi: 10.4269/ajtmh.2002.66.338. [DOI] [PubMed] [Google Scholar]
- 65.Svensson M, Kaye PM. Stromal-cell regulation of dendritic-cell differentiation and function. Trends Immunol. 2006;27:580–587. doi: 10.1016/j.it.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Zubairi S, Sanos SL, Hill S, Kaye PM. Immunotherapy with OX40L-Fc or anti-CTLA-4 enhances local tissue responses and killing of Leishmania donovani. Eur. J. Immunol. 2004;34:1433–1440. doi: 10.1002/eji.200324021. [DOI] [PubMed] [Google Scholar]
- 67.Field AE, Wagage S, Conrad SM, Mosser DM. Reduced pathology following infection with transgenic Leishmania major expressing murine CD40 ligand. Infect. Immun. 2007;75:3140–3149. doi: 10.1128/IAI.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robinson KA, Goyard S, Beverley SM. In vitro shuttle mutagenesis using engineered mariner transposons. Methods Mol. Biol. 2004;270:299–318. doi: 10.1385/1-59259-793-9:299. [DOI] [PubMed] [Google Scholar]
- 69.Dutta S, Ray D, Kolli BK, Chang KP. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phthalocyanine chloride for photolysis in vitro. Antimicrob. Agents Chemother. 2005;49:4474–4484. doi: 10.1128/AAC.49.11.4474-4484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


