Abstract
Leishmaniasis is a vector-borne disease found in many countries worldwide. The causative agent of the disease, Leishmania spp., lives as an obligate intracellular parasite within mammalian hosts. Since tissue macrophages are major target cells for parasite replication, the outcome of infection depends largely on the activation status of these cells. L-arginine is a crucial amino acid required for both nitric oxide (NO)-mediated parasite killing and polyamine-mediated parasite replication. This review highlights the significance of L-arginine as a factor determining the outcomes of Leishmania infection in vitro and its influences on host immune responses in vivo. Various therapeutic approaches targeting L-arginine metabolic pathways during infections with Leishmania are also discussed.
Keywords: L-arginine transporter, Arginase, Leishmania, Host immune response, Macrophages, Nitric oxide
Introduction
Leishmaniasis is a vector-borne disease found in 88 countries of tropical and subtropical regions worldwide. Leishmania infection can give rise to a wide spectrum of clinical manifestations, ranging from self-healing skin ulcers to disfiguring mucosal lesions and fatal visceral infections. Cutaneous leishmaniasis is the most common form of infection that leads to a development of skin papules or nodules [1]. While mucocutaneous leishmaniasis is associated with damages of soft tissues of the nasal, oral mucosa and other mucocutaneous junctures of the skin, visceral leishmaniasis often causes fever, dark spots on the skin, and splenohepatomegaly. The latter form of the disease can be fatal, if not properly treated. Leishmania parasites exhibit a dimorphic life cycle. Flagellated motile promastigotes are transmitted into a mammalian host by an infected sand fly during its bloodmeal. Once inside host cells, promastigotes transform into aflagellated amastigotes, which can multiply inside parasitophorous vacuoles (PVs) of the infected cell and eventually burst free to spread infection in the host. While many cell types, including dendritic cells (DCs) [2], fibroblasts [3], and neutrophils [4], can be infected with Leishmania parasites, macrophages (MΦs) are the major target cells for parasite replication. Since MΦs are professional phagocytes capable of killing intracellular organisms, Leishmania's ability to subvert MΦ antimicrobial mechanisms and persist for long-term infection within these cells represents an intriguing advantage for the parasite but a major challenge for the host to control this infection.
The fate of intracellular Leishmania parasites is determined by the activation status of MΦs. Fully activated MΦs can produce leishmaniacidal molecules, such as NO and oxidative mediators, and kill parasites effectively, whereas “suboptimally” and “alternatively” activated MΦs preferentially activate the arginase pathway to produce polyamines and enhance parasite replication/persistence [5, 6]. Although two distinct types of MΦ activation can lead to divergent outcomes of infection, these two pathways use L-arginine as a common substrate for their enzymatic activities. Therefore, L-arginine is situated at a crossroads between the life and death of the intracellular parasites, and its metabolism is a key determinant for infection outcome. While the roles of L-arginine have been studied extensively in cancer, trauma models (see review in [7]) and Trypanosoma cruizi infection [8], this review will focus on the roles of L-arginine in host immune responses against Leishmania infection and its biological relevance to the disease outcome.
L-arginine metabolism and transport in mammalian cells, especially in MΦs
L-arginine is involved in many metabolic pathways, including those involved in the synthesis of NO, agmatine, creatine, and urea [9]. Two major L-arginine metabolic pathways are particularly relevant to Leishmania infection due to their roles in regulating MΦ effector functions (Fig. 1). L-arginine in MΦs can either be catabolized by inducible nitric oxide synthase (iNOS) to produce NO, or by arginase for polyamine synthesis, depending on the type of extracellular stimuli. When MΦs are exposed to Th1 cytokines, including interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), together with toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS), the expression of iNOS enzyme is upregulated, driving L-arginine metabolism toward NO production [10, 11]. In addition, several Th1–favored chemokines, including MΦ inflammatory protein-1α (MIP-1α/CCL3), MΦ chemoattractant protein-1 (MCP-1/CCL2) [12, 13], and gamma interferon-inducible protein-10 (IP-10/CXCL10) [14] can also trigger iNOS activity and promote parasite killing in MΦs. In contrast, Th2 cytokines, such as interleukin (IL)-4, IL-10, IL-13, and transforming growth factor-β(TGF-β), preferentially induce L-arginine utilization toward the production of polyamines [15, 16]. These Th2 cytokines induce expression and activity of arginase, which converts L-arginine to L-ornithine, a substrate for ornithine decarboxylase (ODC) that converts it to putrescine [17]. Since polyamines (e.g., putrescine, spermidine, and spermine) are important nutrients being directly utilized by Leishmania spp., the exposure to Th2 cytokines leads to the promotion of parasite growth [15, 18].
Fig. 1.
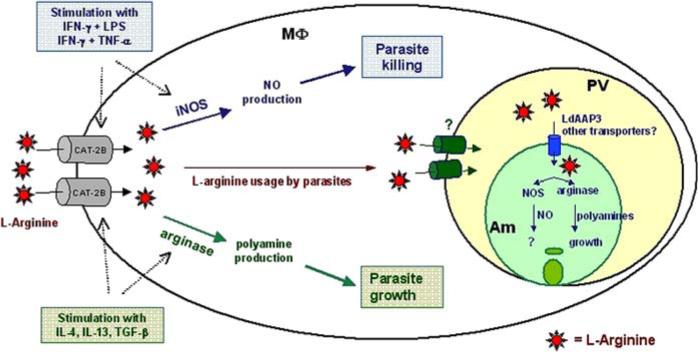
L-arginine metabolic pathways and their influence on Leishmania infection. Classical activation of MΦs via stimulation of Th1 cytokines (IFN-γ plus LPS or IFN-γ plus TNF-α) results in an enhanced expression of CAT-2B, increased L-arginine availability and iNOS activity, ultimately leading to parasite killing. Conversely, Th2-mediated activation of MΦs (via IL-4, IL-13, and TGF-β) enhances CAT-2B expression and arginase activity, resulting in parasite growth. In suboptimally activated MΦs (primed by low concentrations of IFN-γ), CAT-2B is moderately induced, but iNOS and arginase remain relatively inactivated, allowing Leishmania amastigotes (Am) to take up and use available L-arginine for their proliferation. While it is unclear which molecules are responsible for the transport of L-arginine across the parasitophorous (PV) membrane, at least one permease, LdAAP3, has been identified as a transporter of L-arginine across the membrane of L. donovani promastigotes. Once inside the amastigotes, L-arginine can be utilized by parasite-derived NOS or arginase. While Leishmania-derived arginase is involved in polyamine synthesis necessary for the parasite growth, the exact role of Leishmania-derived NOS during infection is unclear
Due to the critical roles of L-arginine pathways in determining parasite killing and proliferation, it is anticipated that these pathways are tightly regulated to permit them to compete for available intracellular L-arginine. Several counter-regulatory mechanisms between the iNOS and arginase pathways have been documented. For example, Nω-hydroxy-L-arginine (LOHA), an intermediate product of the iNOS pathway, is known to strongly inhibit arginase function [19]. Conversely, arginase can reduce iNOS activity by competing for L-arginine [20]. Thus, activities of iNOS and arginase can be greatly influenced by L-arginine availability. Although, L-arginine has been described as a non-essential dietary amino acid, because it can be synthesized in virtually all cell types from L-ornithine or L-citrulline, urea cycle intermediates [21], the endogenous synthesis of L-arginine is insufficient for certain cellular activities, including the production of NO via iNOS and polyamine synthesis [22]. In MΦs lacking an L-arginine transporter, NO and polyamine synthesis was reduced when these cells were stimulated with Th1 and Th2 cytokines, respectively [23, 24], suggesting that L-arginine availability is the bottle-neck control step for both iNOS and arginase. Therefore, L-arginine transport from the extracellular milieu is critically important for parasite growth and killing within an infected cell.
L-arginine can enter a cell via several transporters, although a Na+-independent transport system, named system y+ (y represents lysine, the first substrate described in this system; and + represents the positive charge amino acids) is postulated to be the main route for L-arginine entry in most cells [25]. Transporters in the y+ system include those of the cationic amino acid transporter (CAT) family. To date, four members in the CAT family have been identified, CAT-1 to CAT-4, encoded by Slc7A1−4 genes, respectively [26]. While CAT-1, -2, and -3 are known to transport cationic amino acids—such as L-arginine, L-ornithine, and L-lysine—the function of CAT-4 is unclear [26]. The most relevant CAT member involved in MΦ functions is CAT-2, which can be expressed in two splice variants. CAT-2A is constitutively expressed in the liver and muscle cells [27], whereas the expression level CAT-2B is inducible in MΦs and strongly unregulated by IFN-γ, IFN-γ coupling with LPS, IL-4, and GM-CSF [28-30]. In the context of Leishmania infection, the expression and function of CAT-2B in MΦs are critical, because CAT-2B-mediated L-arginine transport potentially affects both iNOS and the arginase pathways. When stimulated with IFN-γ, LPS, or IFN-γ coupled with LPS, MΦs from CAT-2 knockout mice displayed an impaired NO production [22]. These cells also produced significantly decreased levels of L-ornithine and spermine when stimulated with IL-4 and IL-10 [22]. Although these studies demonstrated the importance of CAT-2B in MΦ activities, there is limited information on a direct role of CAT-2B in Leishmania infection. Our recent study on L. amazonensis infection has shown that both Th1 (IFN-γ/LPS)-mediated killing and Th2 (IL-4)-mediated growth enhancement of amastigotes were suppressed in peritoneal MΦs derived from CAT-2B knockout mice [28]. Given that IFN-γ alone can enhance the growth of L. amazonensis amastigotes in MΦs [31], it is interesting to find that IFN-γ-mediated growth enhancement was also diminished in CAT-2B-deficient MΦs [28], suggesting that L-arginine transport via CAT-2B can significantly influence the outcome of Leishmania infection (Fig. 1). At present, it is still unclear whether the deletion of CAT-2B can alter the outcome of Leishmania infection in vivo. Since there appeared to be a variation in susceptibility to NO-mediated killing in various Leishmania species and developmental stages (promastigotes versus amastigotes) [32], it will be necessary to examine whether CAT-2B can influence the outcome of infection caused by different parasite species and developmental stages.
L-arginine metabolism in Leishmania parasites
As found in higher eukaryotes, some lower eukaryotes like Leishmania and Trypanosoma also have their own L-arginine metabolism pathways [33]. L-arginine has long been identified as an essential amino acid for Leishmania growth. As early as the 1970s, Krassner et al. demonstrated that Leishmania promastigotes could not be maintained in L-arginine-free media [34, 35], suggesting mechanisms of L-arginine uptake and utilization in Leishmania parasites. It is now confirmed that L. donovani can transport L-arginine through an amino acid permease called LdAAP3 [36]. LdAAP3 displays several distinct properties when examined in comparison to the CAT-2B found in MΦs. Unlike CAT-2B, that binds with high affinity to several basic amino acids (e.g., L-arginine, L-lysine, and L-ornithine), LdAAP3 specifically binds with high affinity to L-arginine [36]. Moreover, unlike CAT-2B that shows only 50% activity at low pH [37], LdAAP3 is highly active at pH 5.5, a physiological pH of Leishmania-containing PVs [36]. This unique ability to function at low pH makes LdAAP3 a possible key factor that can interrupt arginine metabolism in MΦs and thus outwit the host antimicrobial function (Fig. 1).
In addition to the L-arginine transporter, Leishmania also express other enzymes in the L-arginine metabolic pathway, including arginase and ODC. The expression of arginase in Leishmania was first suggested via the presence of its enzymatic activities in parasite lysates [33]. Gene-encoding arginase has been identified in L. major and L. amazonensis [38] and has been cloned from L. mexicana promastigotes. The characterization of Leishmania-derived arginase revealed that it is a single-copy gene expressed in glycosomes [39] and is essential for the parasite survival, as indicated by the failure to maintain arginase-deficient L. mexicana promastigotes in vitro in the absence of supplemented L-ornithine or polyamines [39]. Interestingly, the expression of arginase in Leishmania can be influenced directly from a host protein. As suggested by Vendrame et al., the treatment of parasites with insulin-like growth factor-I (IGF-I) can significantly induce Leishmania-derived arginase [40]. The direct correlation between arginase expression in IGF-I-treated Leishmania and the increased parasite burden in infected MΦ [40] suggests that parasite-derived arginase plays a critical role in enhancing parasite growth in vivo. Leishmania parasites also express ODC, an enzyme that converts the arginase-enzymatic product, L-ornithine, to polyamine putrescine [17]. Studies in L. donovani revealed that, as found with arginase, ODC is also a single-copy gene vital for parasite growth [41]. Targeted gene deletion of ODC is lethal, unless polyamines (e.g., putrescine or spermidine) were supplemented [41]. Interestingly, the ODC of Leishmania is much more stable than that of the mammalian homologs [42]. The slow turnover rate of Leishmania-derived ODC makes it a prime target for anti-Leishmania drugs (discussed below).
MΦ-derived iNOS and NO production are known for their pivotal roles in parasite killing. It is somewhat surprising that T. cruzi [43] and Leishmania parasites [44] also have ability to produce various amounts of NO. For example, a significant amount of NO was detected in supernatants of L. amazonensis, L. braziliensis, and L. chagasi cultures [45]. The isolation and characterization of NOS from L. donovani promastigotes [44] and L. amazonensis axenic amastigotes and promastigotes [46] revealed that this parasite-derived enzyme closely resembles its mammalian constitutive NOS (cNOS), which requires Ca2+, calmoduline, and NADPH for its activity [46]. Importantly, culture media derived from amastigotes or highly infectious metacyclics appeared to contain relatively high NOS activities [46], implying a potential correlation between Leishmania NOS and parasite infectivity [47]. However, the biological relevance of these findings in parasitic infections remains unclear.
The findings showing that Leishmania, like mammalian systems, display transporters (LdAAP3) and enzymes needed for L-arginine metabolism (arginase and NOS) highlight the common need for the parasite and host cell to compete for the same nutrients and allow us to emphasize that in a certain microenvironment (e.g., within the acidic PV), the parasite's capacity to compete for L-arginine appears to be favored. This is particularly important, given the fact that Leishmania parasites have additional strategies to suppress host cell activation and signaling pathways and to halt host cell growth (reviewed in [48, 49]). Therefore, L-arginine metabolic pathways in Leishmania may represent a novel strategy by which the parasites can compete for a limited nutrient/biological precursor with the host and evade host immune responses. Several key questions in this area remain to be addressed. How does parasite-derived arginase or iNOS compete for L-arginine? Can the parasite's L-arginine metabolic machinery interfere with the host's L-arginine metabolic pathways and affect the outcome of infection?
The influences of L-arginine and its metabolism on other host immune components
L-arginine not only serves as a crucial amino acid for MΦ activation, but also influences other arms of host immune responses, including T and B cells [50]. Although the role of L-arginine in Leishmania-specific T cell responses is unclear, the crucial roles of L-arginine in T-cell proliferation and activation have been demonstrated in other disease models, such as certain tumors [51] and in cases of Helicobactor pylori infection [52]. Using mouse tumor-associated myeloid cells (CD11b+, CD16+/CD32+, I-A/I-E+) that express high levels of arginase, Rodriquez et al. demonstrated that L-arginine depletion, due to the activation of arginase in these MΦ-like myeloid cells, led to the suppression of T-cell responses [51]. Specifically, the CD3ζ chain, the main signaling chain of the T-cell receptor, was significantly downregulated in reduced concentrations of extracellular L-arginine [51, 53]. A similar suppression was documented when extracellular L-arginine concentration was reduced in the presence of H. pylori-derived arginase [52]. Moreover, L-arginine starvation can arrest stimulated T cells in the G0–G1 phase of the cell cycle [54]. These studies suggest that the co-localization of T cells with cells displaying high arginase activities may render T cells hyporesponsive. Defective T-cell proliferation and activation are hallmarks in L. amazonensis-infected mice [55] and humans [56]. Since L. amazonensis infection induces the production of MΦ-derived IL-10 [18, 57] that can activate arginase function [15], local L-arginine depletion, together with suppressed antigen-presentation function of DCs [58], may collectively contribute to T-cell hyporesponsiveness in infected hosts.
L-arginine availability also plays a role in regulating functions of B cells. Using F/A-2++ mice that over expressed arginase, but reduced tissue L-arginine concentration, de Jonge et al. demonstrated that L-arginine deficiency was correlated with a dramatic reduction of B-cell maturation and a significant decrease in serum IgM, due to an impaired transition from pro- to pre-B cells [59]. The findings that oral supplementation of L-arginine enhanced antigen-specific IgA in mice immunized with tetanus toxoid further support a role for L-arginine in B-cell regulation [60]. Studies using CAT-2-deficient mice revealed that the lack of an L-arginine transporter led to an increase in DC activation in the lungs [59], suggesting that L-arginine concentrations in vivo can influence DC function. In addition, CAT-2-deficient mice displayed marked eosinophilia and neutrophilia, before and after weaning, respectively [59], suggesting that L-arginine plays a role in cellular migration. This notion was supported by a study showing that a correlation among L-arginine concentration, expression of adhesion molecules on endothelial cells, and neutrophil trans-endothelial migration [61]. These studies collectively indicate multiple roles of L-arginine in regulating host immune functions [50].
Targeting L-arginine metabolic pathways as a therapeutic approach for the control of Leishmania infection
Since L-arginine metabolic pathways are crucial for parasite growth and host defense, these pathways may be explored as therapeutic approaches for the control of Leishmania infection. One approach is to interfere with arginase activity using a physiological inhibitor, LOHA. Treatment of bone marrow-derived MΦs containing either L. major or L. infantum promastigotes with LOHA results in a dramatic decrease in both the number of intracellular amastigotes per cell and the percentage of infected cells [19]. Interestingly, LOHA appeared to suppress both MΦ- and Leishmania-derived arginases [19]. Inhibition of arginase activity can also reduce the severity of leishmaniasis in vivo. For example, Kropf et al. demonstrated that administration of the synthetic arginase inhibitor Nω-hydroxy-nor-L-arginine (nor-NOHA) to L. major-infected BALB/c mice during infection significantly reduced arginase activity, lesion sizes, and tissue parasite burdens [62].
While inhibition of arginase is a possible means of controlling Leishmania infection, the stimulation of iNOS-mediated parasite killing is a more attractive approach because iNOS induction is known to be correlated with the effectiveness of anti-Leishmania treatment. For example, high levels of iNOS induction were observed following successful treatment of L. donovani-infected BALB/c mice with IFN-γ coupled with synthetic peptide containing an amino acid sequence of a cysteine protease inhibitor, cystatin [63]. Similarly, a reduced parasite burden in L. donovani-infected BALB/c mice treated with the phosphotyrosine phosphatase inhibitor bpV(phen) was associated with the ability of bpV(phen) to induce high levels of iNOS activity [64]. Of note, iNOS function in vivo can be enhanced when L-arginine availability is increased. Treatment of L. donovani-infected hamsters with an iNOS inducer, polyinosini-polycytidylic acid stabilized with polylysine and carboxymethylcellulose (poly ICLC), was improved when L-arginine was supplemented [65], highlighting the importance of L-arginine in iNOS-mediated control of leishmaniasis.
Since Leishmania parasites exhibit L-arginine transporters and enzymes of L-arginine metabolism, some drugs are designed to specifically target Leishmania-derived ODC. Since this parasite enzyme has a lower turnover rate, when compared to mammalian homologs [42, 66], treatment of experimental leishmaniasis with ODC inhibitors, such as D, L-α-difluoromethylornithine (DFMO) [67] and 3-aminooxy-1-aminopropane (APA) [68-70], is an effective measure for controlling parasitic infection [42, 71]. In addition to the enzymes in L-arginine metabolic pathways, the L-arginine transporter may serve as a potential target for anti-leishmania drugs. Although there is no known difference in the turnover rates of Leishmania and host L-arginine transporters, current anti-Leishmania drugs, i.e., the diamidines, partially exert their antileishmanial activities by targeting Leishmania's L-arginine transporter. Kandpal et al. demonstrated a direct correlation between the inhibition of L-arginine uptake by L. donovani promastigotes and the antileishmanial activities caused by pentamidine and dibromopropamidine, a highly potent antileishmanial diamidine [72]. Treatment of L. donovani and L. amazonensis promastigotes with pentamidine also resulted in a significant decrease in the intracellular pool of L-arginine [73]. Since L-arginine is essential for the survival of intracellular parasites, a reduced concentration of intracellular L-arginine may exert a detrimental effect on these parasites.
Concluding remarks
L-arginine is an important amino acid involved in many crucial pathways in host MΦs and Leishmania parasites. L-arginine metabolic pathways not only participate in the regulation of iNOS-mediated parasite killing and arginase-mediated parasite growth, but also are involved in the regulation of other immune components, including T cells, B cells, DCs, and neutrophils. Although several anti-Leishmania reagents which target L-arginine metabolic pathways have been studied, more research in this area is still needed. Given that several Leishmania-specific proteins in L-arginine pathways have been characterized (i.e., LdAAP3, Leishmania-derived arginase, and NOS), and that more of such proteins are likely to be identified in the near future due to the completion of the Leishmania genome project, research focusing on disrupting functions of L-arginine metabolic pathways in Leishmania may provide a novel and potentially effective therapeutic approach for the control of leishmaniasis.
Abbreviations
- DCs
Dendritic cells
- MΦs
Macrophages
- NO
Nitric oxide
- iNOS
Inducible nitric oxide synthase
- IFN-γ
Interferon-gamma
- TNF-α
Tumor necrosis factor
- IL
Interleukin
- LPS
Lipopolysaccharide
- ODC
Ornithine decarboxylase
- CAT
Cationic amino acid transporter
- LOHA
Nω-Hydroxy-L-arginine
- SNAP
S-nitroso-acetyl-penicillamine
Contributor Information
Nanchaya Wanasen, Departments of Microbiology & Immunology, Institute for Human Infections and Immunity, Center for Biodefense and Emerging Infections, Sealy Center for Vaccine Development, University of Texas Medical Branch, Medical Research Building, Room 3.132, 301 University Blvd, Galveston, TX 77555−1070, USA.
Lynn Soong, Departments of Microbiology & Immunology, Institute for Human Infections and Immunity, Center for Biodefense and Emerging Infections, Sealy Center for Vaccine Development, University of Texas Medical Branch, Medical Research Building, Room 3.132, 301 University Blvd, Galveston, TX 77555−1070, USA e-mail: lysoong@utmb.edu, Department of Pathology, Institute for Human Infections and Immunity, Center for Biodefense and Emerging Infections, Sealy Center for Vaccine Development, University of Texas Medical Branch, Galveston, TX 77555−1070, USA.
References
- 1.Bogitsh BJ, Carter CE, Oeltmann TN. Human Parastiology. 3rd ed. Elsevier Academic Press; Burlington: 2005. Blood and tissue protozoa I: hemaflagellates. pp. 101–14. Chapter 6. [Google Scholar]
- 2.Moll H, Flohe S, Rollinghoff M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol. 1995;25:693–9. doi: 10.1002/eji.1830250310. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan C, Donhauser N, Doring R, Rollinghoff M, Diefenbach A, Rittig MG. Fibroblasts as host cells in latent leishmaniosis. J Exp Med. 2000;191:2121–30. doi: 10.1084/jem.191.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–5. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 5.Noel W, Raes G, Hassanzadeh GG, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–33. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Kreider T, Anthony RM, Urban JF, Jr., Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–53. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popovic PJ, Zeh HJ, 3rd, Ochoa JB. Arginine and immunity. J Nutr. 2007;137:1681S–6S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 8.Peluffo G, Piacenza L, Irigoin F, Alvarez MN, Radi R. L-arginine metabolism during interaction of Trypanosoma cruzi with host cells. Trends Parasitol. 2004;20:363–9. doi: 10.1016/j.pt.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Morris SM., Jr Enzymes of arginine metabolism. J Nutr. 2004;134:2743S–7S. doi: 10.1093/jn/134.10.2743S. discussion 2765S–7S. [DOI] [PubMed] [Google Scholar]
- 10.Green SJ, Crawford RM, Hockmeyer JT, Meltzer MS, Nacy CA. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990;145:4290–7. [PubMed] [Google Scholar]
- 11.Liew FY, Li Y, Moss D, Parkinson C, Rogers MV, Moncada S. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991;21:3009–14. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya S, Ghosh S, Dasgupta B, Mazumder D, Roy S, Majumdar S. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J Infect Dis. 2002;185:1704–8. doi: 10.1086/340820. [DOI] [PubMed] [Google Scholar]
- 13.Brandonisio O, Panaro MA, Fumarola I, Sisto M, Leogrande D, Acquafredda A, Spinelli R, Mitolo V. Macrophage chemotactic protein–1 and macrophage inflammatory protein-1 alpha induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clin Exp Med. 2002;2:125–9. doi: 10.1007/s102380200017. [DOI] [PubMed] [Google Scholar]
- 14.Vasquez RE, Soong L. CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice. Infect Immun. 2006;74:6769–77. doi: 10.1128/IAI.01073-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iniesta V, Gomez-Nieto LC, Molano I, Mohedano A, Carcelen J, Miron C, Alonso C, Corraliza I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002;24:113–8. doi: 10.1046/j.1365-3024.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 16.Barksdale AR, Bernard AC, Maley ME, Gellin GL, Kearney PA, Boulanger BR, Tsuei BJ, Ochoa JB. Regulation of arginase expression by T-helper II cytokines and isoproterenol. Surgery. 2004;135:527–35. doi: 10.1016/j.surg.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Raina A, Janne J. Biosynthesis of putrescine: characterization of ornithine decarboxylase from regenerating rat liver. Acta Chem Scand. 1968;22:2375–8. doi: 10.3891/acta.chem.scand.22-2375. [DOI] [PubMed] [Google Scholar]
- 18.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–7. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 19.Iniesta V, Gomez-Nieto LC, Corraliza I. The inhibition of arginase by N(omega)-hydroxy-l-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777–84. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotoh T, Mori M. Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. J Cell Biol. 1999;144:427–34. doi: 10.1083/jcb.144.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peck MD, Babcock GF, Alexander JW, Billiar T, Ochoa J. High doses of dietary arginine during repletion impair weight gain and increase infectious mortality in protein-malnourished mice. Br J Nutr. 1995;74:787–95. [PubMed] [Google Scholar]
- 22.Yeramian A, Martin L, Serrat N, Arpa L, Soler C, Bertran J, McLeod C, Palacin M, Modolell M, Lloberas J, Celada A. Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J Immunol. 2006;176:5918–24. doi: 10.4049/jimmunol.176.10.5918. [DOI] [PubMed] [Google Scholar]
- 23.Stevens BR, Kakuda DK, Yu K, Waters M, Vo CB, Raizada MK. Induced nitric oxide synthesis is dependent on induced alternatively spliced CAT-2 encoding L-arginine transport in brain astrocytes. J Biol Chem. 1996;271:24017–22. doi: 10.1074/jbc.271.39.24017. [DOI] [PubMed] [Google Scholar]
- 24.Yeramian A, Martin L, Arpa L, Bertran J, Soler C, McLeod C, Modolell M, Palacin M, Lloberas J, Celada A. Macrophages require distinct arginine catabolism and transport systems for proliferation and for activation. Eur J Immunol. 2006;36:1516–26. doi: 10.1002/eji.200535694. [DOI] [PubMed] [Google Scholar]
- 25.Closs EI, Simon A, Vekony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr. 2004;134:2752S–9S. doi: 10.1093/jn/134.10.2752S. discussion 2765S–7S. [DOI] [PubMed] [Google Scholar]
- 26.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–42. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 27.Closs EI, Albritton LM, Kim JW, Cunningham JM. Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J Biol Chem. 1993;268:7538–44. [PubMed] [Google Scholar]
- 28.Wanasen N, MacLeod CL, Ellies LG, Soong L. L-arginine and cationic amino acid transporter 2B regulate growth and survival of Leishmania amazonensis amastigotes in macrophages. Infect Immun. 2007;75:2802–10. doi: 10.1128/IAI.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammermann R, Dreissig MD, Mossner J, Fuhrmann M, Berrino L, Gothert M, Racke K. Nuclear factor-kappaB mediates simultaneous induction of inducible nitric-oxide synthase and up-regulation of the cationic amino acid transporter CAT-2B in rat alveolar macrophages. Mol Pharmacol. 2000;58:1294–302. [PubMed] [Google Scholar]
- 30.Martin L, Comalada M, Marti L, Closs EI, MacLeod CL, Martin del Rio R, Zorzano A, Modolell M, Celada A, Palacin M, Bertran J. Granulocyte-macrophage colony-stimulating factor increases L-arginine transport through the induction of CAT2 in bone marrow-derived macrophages. Am J Physiol Cell Physiol. 2006;290:C1364–72. doi: 10.1152/ajpcell.00520.2005. [DOI] [PubMed] [Google Scholar]
- 31.Qi H, Ji J, Wanasen N, Soong L. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect Immun. 2004;72:988–95. doi: 10.1128/IAI.72.2.988-995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemesre JL, Sereno D, Daulouede S, Veyret B, Brajon N, Vincendeau P. Leishmania spp.: nitric oxide-mediated metabolic inhibition of promastigote and axenically grown amastigote forms. Exp Parasitol. 1997;86:58–68. doi: 10.1006/expr.1997.4151. [DOI] [PubMed] [Google Scholar]
- 33.Camargo EP, Coelho JA, Moraes G, Figueiredo EN. Trypanosoma spp., Leishmania spp. and Leptomonas spp.: enzymes of ornithine-arginine metabolism. Exp Parasitol. 1978;46:141–4. doi: 10.1016/0014-4894(78)90125-x. [DOI] [PubMed] [Google Scholar]
- 34.Krassner SM, Flory B. Essential amino acids in the culture of Leishmania tarentolae. J Parasitol. 1971;57:917–20. [PubMed] [Google Scholar]
- 35.Steiger RF, Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977;24:437–41. doi: 10.1111/j.1550-7408.1977.tb04771.x. [DOI] [PubMed] [Google Scholar]
- 36.Shaked-Mishan P, Suter-Grotemeyer M, Yoel-Almagor T, Holland N, Zilberstein D, Rentsch D. A novel high-affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol Microbiol. 2006;60:30–8. doi: 10.1111/j.1365-2958.2006.05060.x. [DOI] [PubMed] [Google Scholar]
- 37.Closs EI, Graf P, Habermeier A, Cunningham JM, Forstermann U. Human cationic amino acid transporters hCAT-1, hCAT-2A, hCAT-2B: three related carriers with distinct transport properties. Biochemistry. 1997;36:6462–8. doi: 10.1021/bi962829p. [DOI] [PubMed] [Google Scholar]
- 38.da Silva ER, Castilho TM, Pioker FC, Silva CHTP, Floeter-Winter LM. Genomic organisation and transcription characterisation of the gene encoding Leishmania (Leishmania) amazonensis arginase and its protein structure prediction. Int J Parasitol. 2002;32:727–37. doi: 10.1016/s0020-7519(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 39.Roberts SC, Tancer MJ, Polinsky MR, Gibson KM, Heby O, Ullman B. Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J Biol Chem. 2004;279:23668–78. doi: 10.1074/jbc.M402042200. [DOI] [PubMed] [Google Scholar]
- 40.Vendrame CM, Carvalho MD, Rios FJ, Manuli ER, Petitto-Assis F, Goto H. Effect of insulin-like growth factor-I on Leishmania amazonensis promastigote arginase activation and reciprocal inhibition of NOS2 pathway in macrophage in vitro. Scand J Immunol. 2007;66:287–96. doi: 10.1111/j.1365-3083.2007.01950.x. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Roberts SC, Jardim A, Carter NS, Shih S, Ariyanayagam M, Fairlamb AH, Ullman B. Ornithine decarboxylase gene deletion mutants of Leishmania donovani. J Biol Chem. 1999;274:3781–8. doi: 10.1074/jbc.274.6.3781. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez CP, Gonzalez NS, Algranati ID. Stable ornithine decarboxylase in promastigotes of Leishmania mexicana mexicana. Biochem Biophys Res Commun. 1989;161:754–61. doi: 10.1016/0006-291x(89)92664-8. [DOI] [PubMed] [Google Scholar]
- 43.Paveto C, Pereira C, Espinosa J, Montagna AE, Farber M, Esteva M, Flawia MM, Torres HN. The nitric oxide transduction pathway in Trypanosoma cruzi. J Biol Chem. 1995;270:16576–9. doi: 10.1074/jbc.270.28.16576. [DOI] [PubMed] [Google Scholar]
- 44.Basu NK, Kole L, Ghosh A, Das PK. Isolation of a nitric oxide synthase from the protozoan parasite, Leishmania donovani. FEMS Microbiol Lett. 1997;156:43–7. doi: 10.1111/j.1574-6968.1997.tb12703.x. [DOI] [PubMed] [Google Scholar]
- 45.Genestra M, de Souza WJ, Cysne-Finkelstein L, Leon LL. Comparative analysis of the nitric oxide production by Leishmania sp. Med Microbiol Immunol. 2003;192:217–23. doi: 10.1007/s00430-003-0176-z. [DOI] [PubMed] [Google Scholar]
- 46.Genestra M, Guedes-Silva D, Souza WJ, Cysne-Finkelstein L, Soares-Bezerra RJ, Monteiro FP, Leon LL. Nitric oxide synthase (NOS) characterization in Leishmania amazonensis axenic amastigotes. Arch Med Res. 2006;37:328–33. doi: 10.1016/j.arcmed.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Genestra M, Souza WJ, Guedes-Silva D, Machado GM, Cysne-Finkelstein L, Bezerra RJ, Monteiro F, Leon LL. Nitric oxide biosynthesis by Leishmania amazonensis promastigotes containing a high percentage of metacyclic forms. Arch Microbiol. 2006;185:348–54. doi: 10.1007/s00203-006-0105-9. [DOI] [PubMed] [Google Scholar]
- 48.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kima PE. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int J Parasitol. 2007;37:1087–96. doi: 10.1016/j.ijpara.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–52. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 52.Zabaleta J, McGee DJ, Zea AH, Hernandez CP, Rodriguez PC, Sierra RA, Correa P, Ochoa AC. Helicobacter pylori arginase inhibits T cell proliferation and reduces the expression of the TCR zeta-chain (CD3zeta). J Immunol. 2004;173:586–93. doi: 10.4049/jimmunol.173.1.586. [DOI] [PubMed] [Google Scholar]
- 53.Kropf P, Baud D, Marshall SE, Munder M, Mosley A, Fuentes JM, Bangham CR, Taylor GP, Herath S, Choi BS, et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol. 2007;37:935–45. doi: 10.1002/eji.200636542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–73. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ji J, Sun J, Soong L. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect Immun. 2003;71:4278–88. doi: 10.1128/IAI.71.8.4278-4288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 57.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2007;178:1077–85. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xin L, Li Y, Soong L. Role of interleukin-1beta in activating the CD11chigh CD45RB- dendritic cell subset and priming Leishmania amazonensis-specific CD4+ T cells in vitro and in vivo. Infect Immun. 2007;75:5018–26. doi: 10.1128/IAI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Jonge WJ, Kwikkers KL, te Velde AA, van Deventer SJ, Nolte MA, Mebius RE, Ruijter JM, Lamers MC, Lamers WH. Arginine deficiency affects early B cell maturation and lymphoid organ development in transgenic mice. J Clin Invest. 2002;110:1539–48. doi: 10.1172/JCI16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi T, Yamamoto M, Hiroi T, McGhee J, Takeshita Y, Kiyono H. Arginine enhances induction of T helper 1 and T helper 2 cytokine synthesis by Peyer's patch alpha beta T cells and antigen-specific mucosal immune response. Biosci Biotechnol Biochem. 1998;62:2334–40. doi: 10.1271/bbb.62.2334. [DOI] [PubMed] [Google Scholar]
- 61.Yeh CL, Hsu CS, Chen SC, Hou YC, Chiu WC, Yeh SL. Effect of arginine on cellular adhesion molecule expression and leukocyte transmigration in endothelial cells stimulated by biological fluid from surgical patients. Shock. 2007;28:39–44. doi: 10.1097/shk.0b013e31802f0190. [DOI] [PubMed] [Google Scholar]
- 62.Kropf P, Fuentes JM, Fahnrich E, Arpa L, Herath S, Weber V, Soler G, Celada A, Modolell M, Muller I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. Faseb J. 2005;19:1000–2. doi: 10.1096/fj.04-3416fje. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee S, Ukil A, Das PK. Immunomodulatory peptide from cystatin, a natural cysteine protease inhibitor, against leishmaniasis as a model macrophage disease. Antimicrob Agents Chemother. 2007;51:1700–7. doi: 10.1128/AAC.01555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matte C, Marquis JF, Blanchette J, Gros P, Faure R, Posner BI, Olivier M. Peroxovanadium-mediated protection against murine leishmaniasis: role of the modulation of nitric oxide. Eur J Immunol. 2000;30:2555–64. doi: 10.1002/1521-4141(200009)30:9<2555::AID-IMMU2555>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 65.Bhakuni V, Singha UK, Dutta GP, Levy HB, Maheshwari RK. Killing of Leishmania donovani amastigotes by poly ICLC in hamsters. J Interferon Cytokine Res. 1996;16:321–5. doi: 10.1089/jir.1996.16.321. [DOI] [PubMed] [Google Scholar]
- 66.Ghoda L, Phillips MA, Bass KE, Wang CC, Coffino P. Trypanosome ornithine decarboxylase is stable because it lacks sequences found in the carboxyl terminus of the mouse enzyme which target the latter for intracellular degradation. J Biol Chem. 1990;265:11823–6. [PubMed] [Google Scholar]
- 67.Mamont PS, Duchesne MC, Grove J, Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978;81:58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- 68.Dufe VT, Ingner D, Heby O, Khomutov AR, Persson L, Al-Karadaghi S. A structural insight into the inhibition of human and Leishmania donovani ornithine decarboxylases by 1-amino-oxy-3-aminopro-pane. Biochem J. 2007;405:261–8. doi: 10.1042/BJ20070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mett H, Stanek J, Lopez-Ballester JA, Janne J, Alhonen L, Sinervirta R, Frei J, Regenass U. Pharmacological properties of the ornithine decarboxylase inhibitor 3-aminooxy-1-propanamine and several structural analogues. Cancer Chemother Pharmacol. 1993;32:39–45. doi: 10.1007/BF00685874. [DOI] [PubMed] [Google Scholar]
- 70.Khomutov RM, Hyvonen T, Karvonen E, Kauppinen L, Paalanen T, Paulin L, Eloranta T, Pajula RL, Andersson LC, Poso H. 1-Aminooxy-3-aminopropane, a new and potent inhibitor of polyamine biosynthesis that inhibits ornithine decarboxylase, adenosylmethionine decarboxylase and spermidine synthase. Biochem Biophys Res Commun. 1985;130:596–602. doi: 10.1016/0006-291x(85)90458-9. [DOI] [PubMed] [Google Scholar]
- 71.Mukhopadhyay R, Madhubala R. Effect of a bis(benzyl)polyamine analogue, and DL-alpha-difluoromethylornithine on parasite suppression and cellular polyamine levels in golden hamster during Leishmania donovani infection. Pharmacol Res. 1993;28:359–65. doi: 10.1006/phrs.1993.1138. [DOI] [PubMed] [Google Scholar]
- 72.Kandpal M, Tekwani BL, Chauhan PM, Bhaduri AP. Correlation between inhibition of growth and arginine transport of Leishmania donovani promastigotes in vitro by diamidines. Life Sci. 1996;59:PL75–80. doi: 10.1016/0024-3205(96)00341-4. [DOI] [PubMed] [Google Scholar]
- 73.Basselin M, Badet-Denisot MA, Lawrence F, Robert-Gero M. Effects of pentamidine on polyamine level and biosynthesis in wild-type, pentamidine-treated, and pentamidine-resistant Leishmania. Exp Parasitol. 1997;85:274–82. doi: 10.1006/expr.1996.4131. [DOI] [PubMed] [Google Scholar]


