Abstract
Coliphage N4 virion-encapsidated RNA polymerase (vRNAP) is a member of the phage T7-like single-subunit RNA polymerase (RNAP) family. Its central domain (mini-vRNAP) contains all RNAP functions of the full-length vRNAP, which recognizes a five- to seven-base pair stem and three-nucleotide loop hairpin DNA promoter. Here we report the X-ray crystal structures of mini-vRNAP bound to promoters. Mini-vRNAP uses four structural motifs to recognize DNA sequences at the hairpin loop and stem, and to unwind DNA. Despite their low sequence similarity, three out of four motifs are shared with T7 RNAP that recognizes a double-stranded DNA promoter. The binary complex structure and results of engineered disulfide-linkage experiments reveal that the plug and motif B loop, which block the access of template DNA to the active site in the apo-form mini-vRNAP, undergo a large-scale conformational change upon promoter binding, explaining the restricted promoter specificity that is critical for N4 phage early transcription.
Introduction
DNA-dependent RNA polymerases (RNAPs) accurately recognize specific promoter DNA sequences and correctly position the transcription start site at the enzyme active site, while subtle differences in promoter sequences modulate the level of transcription. Therefore, knowledge of how RNAPs recognize specific DNA sequences and position the transcription start site at the active site is essential for understanding gene regulation.
The coliphage N4 virion-encapsidated DNA-dependent RNAP (vRNAP), which is present in ~4 copies per virion (Choi et al., 2008), is a member of the single-subunit T7-like RNAP family (Kazmierczak et al., 2002). However, unlike other members, N4 vRNAP binds only to single-stranded DNA containing a 5-7-base pair (bp) stem and 3-nucleotide (nt) loop hairpin (Glucksmann et al., 1992; Haynes and Rothman-Denes, 1985). Upon injection of vRNAP and the N4 double-stranded DNA genome into the E. coli host cell, the injected DNA is negatively supercoiled by E. coli DNA gyrase, which leads to extrusion of a cruciform structure that contains the vRNAP recognition sequence(Dai et al., 1997; Dai and Rothman-Denes, 1998; Falco et al., 1978; Glucksmann et al., 1992). E. coli single-stranded DNA binding protein (EcoSSB) then presents the hairpin-form promoter to vRNAP for binding and transcription of the early genes of the N4 genome (Dai and Rothman-Denes, 1998; Falco et al., 1977; Markiewicz et al., 1992). In addition, EcoSSB participates in transcription elongation; it separates the DNA/RNA hybrid from the vRNAP elongation complex to enable multiple rounds of transcription (Davydova and Rothman-Denes, 2003).
N4 vRNAP comprises three independently-folded domains; the middle domain (mini-vRNAP: 1,100 amino acids) possesses all RNAP functions (Kazmierczak et al., 2002). The apo-form mini-vRNAP structure was determined by X-ray crystallography at 2.0Å resolution (Murakami et al., 2008): the structure resembles a “fisted” right hand polymerase domain with Thumb, Fingers, and Palm sub-domains as well as an N-terminal domain, which is specific to the T7-like single-subunit RNAPs (Jeruzalmi and Steitz, 1998; Murakami et al., 2008). Furthermore, we proposed that two structural motifs – the plug module and motif B loop – in their observed position near the active site in the apo structure inactivate the enzyme. During promoter DNA binding, the plug module must change its position to open the template DNA binding cleft, and the motif B loop has to refold as a part of the O-helix to form the nucleotide binding motif (Murakami et al., 2008).
N4 vRNAP recognizes three different promoters (wt-P1, wt-P2 and wt-P3, Figure 1A) on the N4 genome; these promoters are used to direct the transcription of the phage early genes (Haynes and Rothman-Denes, 1985), and, as a result, a part of the N4 genome is pulled into the host cell (A. Demidenko and Rothman-Denes, unpublished data). The three vRNAP promoters contain a consensus sequence that includes a 5 or 7-bp stem-3-nt loop DNA hairpin (Dai and Rothman-Denes, 1998; Glucksmann et al., 1992), with subtle differences of sequence and hairpin-stem lengths (Figure 1A) that affect vRNAP binding. The P1 and P2 promoters differ in their affinity for vRNAP, with dissociation constants of 40 nM and 2 nM, respectively (Davydova et al., 2007). These binary complexes also have different salt sensitivity: the P2-vRNAP complex is much more salt-resistant than the P1-vRNAP complex (Davydova et al., 2007). The two promoters differ at the central position (−11) of the hairpin-loop, with guanine at P2 and adenine at P1; the second and third base pairs beyond the loop-closing base pair are not conserved and do not confer binding specificity (Dai and Rothman-Denes, 1998).
Figure 1. Promoter DNA sequences and the overall structure of binary complex.
(A) Sequences and secondary structures of the three vRNAP promoters (wt-P1, wt-P2 and wt-P3) in the N4 genome. The transcription start site (+1) is colored in green and the direction of transcription is shown by an arrow.
(B) Promoter DNA constructs used for crystallization. The downstream region highlighted by the grey box is disordered in the binary complex structures. The transcription start site (+1) is colored in green.
(C) Overall structure of the N4 mini-vRNAP bound to the P2 promoter (top). The promoter DNA hairpin is depicted by a pink ribbon. The thick bar (bottom) represents the N4 mini-vRNAP primary sequence with amino acid numbering. Domains, sub-domains and structural motifs are labeled and color-coded as in the top panel; the color scheme is maintained throughout this paper.
Results of DNA cross-linking studies together with site-specific mutagenesis of the promoter identified positions −11G (center of the hairpin loop) and −8G (at the hairpin-stem) as the two major determinants of the vRNAP-promoter interaction; the 6-keto and N7 groups of both guanines determine the high-affinity and salt-resistance of vRNAP-promoter complex (Davydova et al., 2007; Murakami et al., 2008). Based on these results and the apo-form mini-vRNAP crystal structure, a “docked” model of the vRNAP binary complex was constructed (Murakami et al., 2008).
Here we report the X-ray crystal structures of mini-vRNAP bound to three different promoters, which explain: 1) how N4 vRNAP structurally accommodates three different types of hairpin-form DNA promoter; 2) how subtle differences of promoter sequence determine the affinity and salt-stability of the binary complex; and 3) how the N4 vRNAP changes its conformation from the transcription inactive apo-form (Murakami et al., 2008) to a transcription-competent binary complex. These findings provide a structural explanation for the exquisite selectivity of N4 RNAP for its cognate promoter, which is essential to allow the few injected copies of vRNAP to discriminate in a host cell the single copy of the N4 genome from host DNA. In addition, the results presented provide insights into the mechanism of transcriptional initiation by N4 vRNAP.
Results
Overall Structure
We have determined the structures of three different binary complexes (N4 mini-vRNAP and promoter DNA) by X-ray crystallography at as high as 2-Å resolution (STable 2). They correspond to one binary complex each with the P1 and P2 promoters and one complex with the P2-7a promoter (promoters shown in Figure 1B). The single-strand DNA oligonucleotides used for crystallization contain a promoter hairpin at its 3′-end (the 3-nt loop and 5 or 7-bp stem), 5 or 7 bases of single-stranded template DNA to position +3 and another DNA hairpin at the 5′-end to mimic downstream duplex DNA (Figure 1B). The sequence and/or hairpin length of each DNA represent the key differences between the three N4 genome vRNAP promoters (wt-P1, wt-P2 and wt-P3, Figure 1A).
All three structures crystallized in space group P212121 with two binary complexes per asymmetric unit (SFigure 1); they have almost identical structures (0.438 Å root mean square deviation) with some minor deviations in the position of the Fingers at residues 657–661 (motif B), 689–729 (C-terminus of helix Y), and 756–764 (surface exposed region). We observed as many as 967 water molecules and 2 monophosphates (one monophosphate for each binary complex) in the electron density map (SFigure 2, STable 2). We did not find extra electron density corresponding to the divalent catalytic metals (Mg2+) at the enzyme active site, even when crystals were soaked in 10 mM MgCl2, suggesting that the catalytic metals are only bound to the enzyme in the presence of the incoming nucleotide at the active site. This is consistent with what has been seen with T7 RNAP (Yin and Steitz, 2004).
The overall structure of the mini-vRNAP binary complex resembles a “right hand” as observed in the apo-form structure; however the apo structure “fist” is now relaxed to a “grasping hand” in the binary complex (Figure 1C) to accommodate promoter DNA, with important conformational changes that are described below.
The resolved regions of DNA include the hairpin promoter and transcription start site (Figures 1B and 1C). All structures show strong and clear electron density for the promoter hairpin DNA (−17 to −5), moderate but interpretable electron density for the single-stranded template DNA (−4 to +2 or −4 to +3), and no definable electron density for DNA downstream of +3 or +4 (SFigure 3A). The promoter DNA hairpin comprises a 5-bp stem and 3-nt loop (Figure 2A), which includes DNA sequences from −5 to −9 forming a double-stranded DNA stem with their −17 to −13 complementary sequences. Within the 3 bases in the loop (−10 to −12), bases −10 and −11 stack with each other and also with the neighboring −9 base to mimic a double-stranded DNA helix conformation. There is a sharp turn between bases −11 and −12 that enables base stacking between bases −12 and −13 (Figure 2B).
Figure 2. The interaction between the promoter hairpin and the N4 vRNAP.
(A) P2 promoter DNA structure in the binary complex. The hairpin-stem promoter consists of a double-stranded stem (−5 to 9, and −17 to −13) and a 3-nt loop (−10 to −12). Template DNA contains bases from −4 to +2 with the transcription start site at +1.
(B) Promoter hairpin loop recognition. R119 and K114 interact with −11G (N7 and 6-keto) and −10G (N7), respectively. W129 participates in a stacking interaction with −11G. The Fingers residues K849 and K850 form salt-bridges with the phosphate backbone at −12 and −13, respectively. Hydrogen bonds and salt-bridges are depicted by red and orange dashed lines, respectively. Color code of the structure motifs is indicated.
(C) Promoter recognition by the specificity loop (cyan) and the β-intercalating hairpin (orange) in the P2_7a binary complex. D901 and R904 of the specificity loop recognize bases −9/−10 and −8, respectively, from the major grove. R902 interacts with the phosphate backbone at −7 and −6. Residues K267 and K268 in the β-intercalating hairpin face the DNA stem to separate the last 2 bp of the 7 bp stem yielding a 5 bp stem, and direct the template DNA toward the active site.
(D) Difference of −11 and R119/W129 interactions between P1 (blue) and P2 (pink) promoters in the binary complexes. Only residues R119 and W129 in the P2 binary complex are shown. The bifurcate hydrogen bonds between −11G (P2 promoter) and R119 are depicted by dashed red lines.
DNA-hairpin Promoter Recognition
Among the three vRNAP promoters, promoter P2 has the highest affinity and its binding to mini-vRNAP yields the most strongly salt-resistant binary complex (Davydova et al., 2007). The P2 binary complex structure revealed that hairpin promoter binding is achieved by four structural motifs: 1) the N-terminal domain −11 recognition site (Figure 2B); 2) the Fingers specificity loop (Figure 2C); 3) the N-terminal domain β-intercalating hairpin (Figure 2C); and 4) the C-terminus of the Fingers long α-helix (α35) (Figure 2B) (Murakami et al., 2008). Base-specific interactions by the −11 recognition site include K114 and R119 that interact with −10G (N7) and −11G (N7 and 6-keto), respectively. The stacking interaction between W129 and −11G is critical for the salt-resistance of the binary complex (Davydova et al., 2007). The specificity loop contacts the hairpin stem DNA from the major groove with base specific interactions that include D901 (bases −9 and −10) and R904 (−8G, N7 and 6-keto) (Figure 2C). Besides the base-specific interactions, K849 and K850, at the C-terminus of Fingers long α-helix (α35), form salt-bridges with the phosphate backbone at −12 and −13, respectively (Figure 2B). The −6 and −7 bases of the promoter and their complementary −15 and −16 bases have no base-specific contacts with vRNAP; rather, vRNAP fits the DNA stem structure on its surface with some interactions with the phosphate and sugar backbone.
N4 vRNAP and DNA Interactions that Define the Transcription Start Site
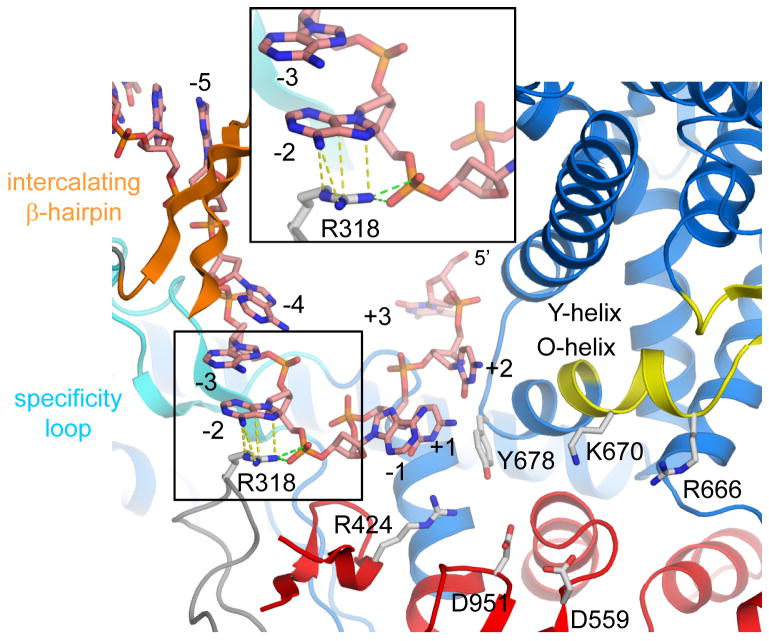
Primer-independent (de novo) initiation of transcription at a defined start site requires the DNA template strand to be positioned accurately at the active site for base-pairing with the first two nucleotide substrates. N4 vRNAP initiates 11 bases downstream of the central base of the hairpin loop with minimum preference for the initiation sequence (Davydova et al., 2007). Our binary complex, which has template DNA structured to position +3, allows us to understand the mechanism of transcription start site selection by this enzyme. N4 vRNAP uses the following combinations of DNA base contacts at the promoter hairpin and sequence-independent DNA contacts with bending of the template DNA to define the transcription start site: 1) a stacking interaction between residue W129 (−11 recognition site) and the −11 base of the hairpin loop defines the upstream edge of promoter (Figure 2B); 2) the β-intercalating hairpin defines the junction between double-and single-stranded DNA between bases −5 and −4 (Figure 2C); 3) R318 participates in dual interactions including cation-π interaction with base −2 and a salt bridge with the −2/−1 phosphate, which introduces unstacking leading to a sharp bend (~59°) between bases −2 and −1 (Figure 3); and 4) the phosphate backbone is turned ~90° between bases +2 and +3 to prevent transcription initiation at +2 or downstream (Figure 3). These interactions restrict the position only of bases −1 to +2 at the vRNAP active site. The four A residues of the template DNA (from −4 to −1) interact with vRNAP only through the phosphate backbone, suggesting that these bases serve as a molecular ruler to determine the transcription start site. There are no extensive mini-vRNAP-base specific contacts at or near the transcription start site of the DNA template (Figure 3), which is consistent with biochemical findings regarding promoter specificity (Davydova et al., 2007).
Figure 3. Positioning of the transcription start site at the vRNAP active site.
A view of the P2_7a promoter binary complex active center. Thumb and plug are removed to see the active center. Residue R318 in the N-terminal domain has a cation-π interaction with base −2 and salt-bridges with the phosphate backbone (depicted by yellow and green dashed lines, respectively) that induce a DNA kink between bases −2 and −1. The +3 base is rotated by ~90° presenting only DNA bases from −1 to +2 to the active site. Amino acid residues essential for activity at the active site are shown: R424 (T/DxxGR motif) for substrate binding; D559 (motif A) and D951 (motif C) for chelating the catalytically essential Mg2+ ions; R666, K670 and Y678 (motif B) for substrate binding. The boxed area is magnified.
We used two approaches to characterize the interaction of R318 with the template DNA and its role in transcription start site selection by N4 mini-vRNAP. First, we compared dinucleotide and trinucleotide synthesis supported by wild-type, R318A and R318K mini-vRNAPs using two templates, P2-4A-8 and P2-4T-8 containing 4 adenines and 4 thymines, respectively, preceding the transcription start site (Figure 4A). The wild-type enzyme showed an increase in transcription initiation at +2 on the P2-4T-8 template (25 %) compared with on the P2-4A-8 template (5%). In contrast, both mutant enzymes showed increased use of +2 (R318A, 40% and R318K, 25%) as a start site on the P2-4A-8 template, and a preference for initiation at +2 on the P2-4T-8 template (R318A, 95% and R318K, 85%).
Figure 4. Role of R318 in determining the site of transcription initiation.
(A) Effect of mini-vRNAP R318 substitutions on di- and tri-nucleotide synthesis on P2-4A-8 and P2-4T-8 templates. The percentage of GA synthesis is shown below each lane. The sequences of P2-4A-8 and P2-4T-8 are shown. Inverted repeat sequences that form the hairpin stem are indicated by arrows. The center of the hairpin loop (−11), and transcription start site (+1) with transcripts (GGA, from +1; GA, from +2) in the presence of GTP and ATP are shown.
(B) Phosphodiester bond formation by catalytic autolabeling using templates with increasing number (n) of As (P2-An-CTA) or Ts (P2-Tn-CTA) between the promoter hairpin and CTA and the hydrozybenzaldehyde derivatives of ATP (bATP) or GTP (bGTP). The sequences of the templates are shown at the top (n corresponds to the number of As and Ts between the hairpin and C in the P2-An-CTA and P2-Tn-CTA templates, respectively). Bottom left: bATP; Bottom right: bGTP. %: activity. TI: transcription initiation site (+1 corresponds to 11 nucleotides from the center of the hairpin loop).
(C) Selection of the transcription initiation site by wild-type and R318 mutant enzymes on templates containing different combinations of As and Ts between −4 and −1. %GA: transcription initiation at +2.
We have previously used catalytic autolabeling, to analyze the site of vRNAP transcription initiation (Davydova et al., 2007). The enzyme is cross-linked to the hydroxybenzaldehyde esters of GTP (bGTP) or ATP (bATP) as the initiating nucleotide followed by the addition of [α-32P]ATP or [α-32P]UTP as the next nucleotide with P2-A3-5-CTA as a DNA template. To confirm the role of R318 in transcription initiation, we measured the ability of the wild-type and mutant enzymes to undergo catalytic autolabeling using templates containing 3–5 adenines (P2-A3-5-CTA) or thymines (P2-T3-5-CTA) preceding the transcription initiation start site (Figure 4B). As expected, the wild-type enzyme initiated mainly at +1 on the P2-An-CTA templates (n=3 with bATP + [α-32P]UTP; n=4 with bGTP + [α-32P]ATP); in contrast, the mutant enzymes initiated at +1 and +2 with nearly equal efficiency. Transcriptional initiation by the mutant enzymes shifted to position +2 on the P2-Tn-CTA templates (n=4 with bATP + [α-32P]UTP; n=5 with bGTP + [α-32P]ATP). On these templates, the wild-type enzyme was less selective indicating that both R318 and the run of adenines are required for positioning the +1 template base at the active site. Analysis of the effect of T substitutions between positions −4 and −1 on selection of the initiation site indicates that: 1) initiation is shifted to +2 whenever a T is present at −2, highlighting the role of π-cation interaction between R318 and −2A (Figure 4C, compare AAAA and AATA); 2) with the exclusion of substitution at −4, substitutions at all other positions lead to a shift in initiation to +2, which is pronounced in the case of the mutant enzymes (Figure 4C).
Differences between the P1 and P2 Promoter-Binary Complexes
Compared with promoter P2, promoter P1 has lower affinity and the resulting binary complex is salt-sensitive (Davydova et al., 2007). Position −11 (A at P1, G at P2, Figure 1A) is the most critical determinant of affinity and complex salt-resistance. A purine at this position is essential for formation of a stable binary complex even in low salt conditions; replacement with a pyrimidine drastically changes the dissociation constant (Kd) from the nanomolar (−11G: 2 nM; −11A: 40 nM) to the micromolar (−11T: 1 μM; −11C: 2 μM) range (Davydova et al., 2007). To structurally address the formation of the salt-resistant complex, we solved the structure of the P1 binary complex (STable 2) and compared it with the P2 binary complex. There are no structural differences between these binary complexes except for the orientation of base −11 and its interaction with R119 and W129 in the −11 recognition site (Figure 2D). It appears that the salt-resistant P2 complex achieves tighter binding due to the −11G 6-keto group that is absent in the −11A of the P1 promoter, consistent with the results of biochemical experiments (Davydova et al., 2007). In the P2 binary complex, the N-terminal domain R119 residue makes bifurcating hydrogen bonds with the −11 G 6-keto and N7 groups that swing the −11G base ~20° with respect to −11A of the P1 binary complex. This structural arrangement has not been observed previously in any other protein-DNA complex.
To investigate the role of R119 in promoter binding, we substituted R119 with either Ala (R119A) or Lys (R119K) and compared these enzymes for their in vivo transcription activity, binding affinity to the promoter DNA and salt-sensitivity of the binary complexes to the wild-type enzyme. Both R119A and R119K enzymes were inactive in vivo (data not shown). Promoter binding was greatly decreased when R119 was substituted with A and the resulting complex was salt sensitive; R119 substitution with K did not restore either affinity or salt resistance (SFigure 4) indicating that a bidentate interaction with the −11G 6-keto and N7 groups is required (Figure 2D). As expected, substitutions of R119 with A or K had no effect on the affinities or salt resistances of a −11A promoter-RNAP complexes (data not shown).
Theoretical calculations have indicated that the stacking between guanine and tryptophan is more stable than between adenine and tryptophan, with stacking energies of 11.5 and 8.2 kcal/mol, respectively (Kumar and Govil, 1984). In addition, the polar contact between R119 and −11G (P2) places −11G closer to W129 than −11A (Figure 2D, 3.41 Å between −11G(C6) and W129(CH2), 4.09 Å between −11A(C6) and W129(CH2)); therefore, we speculate that differences in the intrinsic stacking energies of the −11 base may play an important role in the high affinity and salt resistance of the P2 promoter-vRNAP complex.
Differences between the Binary Complexes with 5 or 7 bp Hairpin-Stem Promoters
The N4 vRNAP promoters differ in their hairpin stem lengths (5 bp in wt-P1, 7 bp in wt-P2 and wt-P3; Figure 1A). Extension of the hairpin stem beyond 7 bp decreases promoter binding by N4 mini-vRNAP (Davydova et al., 2007). To understand how N4 vRNAP accommodates different hairpin-stem lengths, we solved the structure of the P2_7a DNA (7-bp stem) binary complex (Figures 1B, STable 2). There are no vRNAP and DNA structural differences between the 5- and 7-bp stem-length binary complexes because the 7-bp hairpin-stem is melted to 5 bp in the binary complex structure, and the remaining 3′ bases (−18 and −19) are flipped away from the stem. These results indicate that the vRNAP has a DNA melting activity that can unwind only up to 2 bp to generate a 5 bp stem. Indeed, single-strandedness at positions −2, −1 and +1 are required for efficient mini-vRNAP binding to the promoter (Davydova et al., 2007). The β-intercalating hairpin plays a role in this DNA melting: two Lys side chains at 267 and 268 form a hydrophobic wall, which stacks against the −5/−17 bp to make the double- and single-stranded DNA junction (Figure 2C); the relevance of K268 is reflected in reduced promoter binding of the K268A enzyme (Murakami et al., 2008). These Lys residues in N4 vRNAP are the structural and functional counterparts to the β-intercalating hairpin residue V237 of T7 RNAP (Brieba and Sousa, 2001; Cheetham et al., 1999; Stano and Patel, 2002).
Structural Transition of vRNAP from the Apo-form to Binary Complex
While conserving the “right-hand” overall structure that is common to the T7-RNAP and DNA polymerase I families, the plug and motif B loop that occupy the DNA template-binding channel and the active site are unique in N4 vRNAP. Structural comparison between the apo-form (Murakami et al., 2008) and the binary complex of mini-vRNAP presented here identifies major movements of these two motifs (Figure 5 and Supplemental Movie), which are required to accommodate the binding of template DNA to establish a functional active site (Figure 3). These movements include: 1) a 6.8° rigid-body movement of the N-terminal domain (with the exception of the plug and β-intercalating hairpin) and the C-terminal one-third of the Fingers (residues 806–927); 2) another 25.1° rigid body rotation of the plug and intercalating β-hairpin (Figure 5A); and 3) a third 12.8° rigid movement of the N-terminal two thirds of Fingers (residues 608–805) (Figure 5B). With this third movement, there is a significant conformational change of the motif B loop including a translation of 32.6 Å of N659 from the interior of the protein (apo-form) to the exterior (binary complex). In the apo-structure (Murakami et al., 2008), motif B is sandwiched by the plug and the active site whereas in the binary complex it becomes a part of the O-helix and further extends to form a short β hairpin that presumably helps stabilize this structure. Motif B contains R666 and K670 that are absolutely conserved in single-subunit polymerases and participate in binding of the incoming nucleotide. These residues face toward the enzyme active site in the binary complex, to position the site of binding of the incoming nucleotide (Figure 3). These conformational changes would allow the polymerase to transition between the apo-form in an inactive state to an active form binary complex, only upon proper promoter recognition.
Figure 5. Conformational changes.
(A) Superimposed ribbon representations of N4 mini-vRNAP alone (black) and mini-vRNAP (color-coded as in Figure 1C) in the binary complex. The two structures were aligned by superimposing their Palm cores. The movements of the mobile modules from the apo-form structure to their positions in the binary complex are indicated by the arrows (green: a 6.8° rigid body movement including the N-terminal domain, except for the plug and the intercalating β-hairpin, and the C-terminal one-third of the Fingers, residues 806–927; orange: a rigid 25.1° body motion of the plug with intercalating β-hairpin).
(B) A 12.8° rigid body movement of the N-terminal two thirds of Fingers (residues 608–805) with a large structural transition of motif B. The structures in the binary complex and the apo-form are colored in light blue/yellow and black, respectively. Motif B rearranges its structure from a loop (apo-form) to a short anti-parallel β-hairpin (binary complex) with the largest transition (32.6 Å) at residue N659 (red dashed arrow). The positions of R666 and K670 in motif B that participate in substrate binding are also shown.
The Plug and Motif B loop Inactivate vRNAP
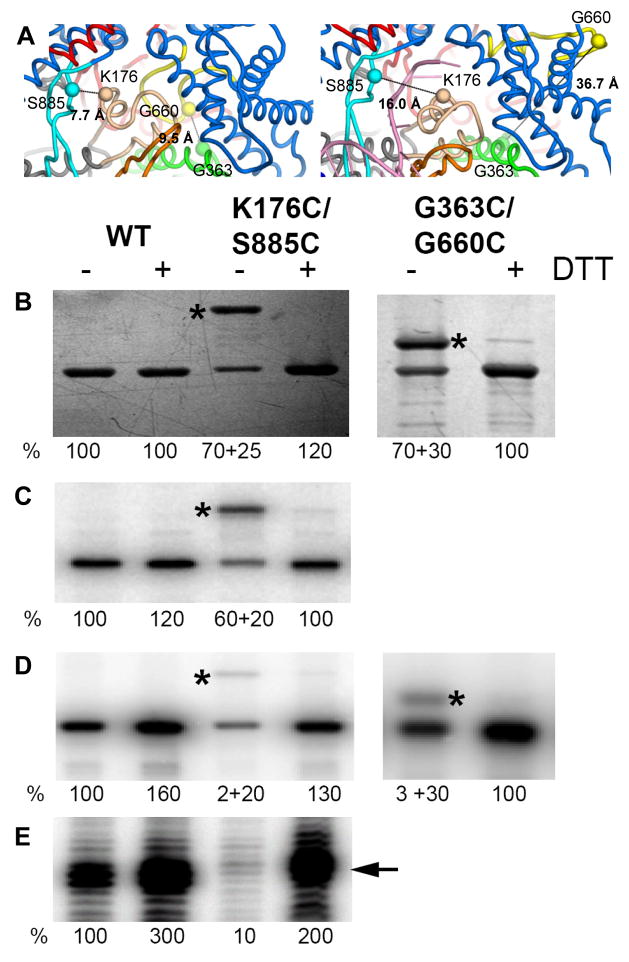
We reasoned that restriction of plug and motif B movements before promoter binding would inactivate the enzyme. Therefore, we substituted residues K176 and S885, which are located 7.7 Å apart (Cα-Cα distance) in the plug and in the specificity loop of the apo-form mini-vRNAP structure, respectively, with cysteines (Figure 6A, left). Disulfide bond formation between these two residues, which are located across the DNA binding channel, would result in immobilization of the plug module and blockage of template DNA binding into the RNAP active center. Wild-type and K176C/S885C proteins were purified in the absence of dithiotreitol (DTT), and tested before and after incubation with DTT 1) for their ability to bind to the promoter hairpin, 2) for formation of the first two phosphodiester bonds, and 3) in run-off transcription (Figure 6). Analysis of the K176C/S885C enzyme by SDS-PAGE revealed the presence of two migrating species (Figure 6B left, K176C/S885C); upon DTT treatment, only a single band with wild-type mobility was detected indicating that the slower migrating band contained a disulfide bond. Incubation of the respective pairs of single cysteine-substituted enzymes (mini-vRNAP K176C with mini-vRNAP S885C; mini-vRNAP G363C with mini-vRNAP G660C) in the presence of cystine did not give rise to the slower migrating species (data not shown), indicating that the slower migrating species did not arise from intermolecular disulfide bond formation. The ability of both forms (with or without disulfide bond) of the K176C/S885C enzyme to interact with the promoter hairpin was determined by UV-cross-linking to a −11 IdU-substituted 13mer oligonuclotide encompassing the promoter hairpin from −17 to −5 sequence; both species interacted with the promoter hairpin to the same extent (Figure 6C, left). In contrast, the disulfide bond-containing protein was deficient in transcription initiation and elongation, as measured by catalytic autolabeling (Figure 6D, left) and run-off transcription (Figure 6E, left), respectively.
Figure 6. The plug and motif B loop inactivate mini-vRNAP.
(A) The S885-K176 and G6363-G660 distances in the apo form (left panel) and the binary complex (right panel) are shown. Wild-type, K176C/S885C and G363C/G660C enzymes were purified in the absence of DTT, preincubated with cystine (−DTT), then with DTT (+DTT), and analyzed: by SDS-PAGE and silver staining (B), for their ability to bind to the promoter hairpin (C), catalyze autolabeling (D) and run off transcription (E). Disulfide bond containing species is denoted by *. The percentage of enzyme in each species, and their respective activities are shown below each lane.
Immobilization of the motif B loop through replacement of G363 (the Thumb) and G660 (motif B loop), which are located 9.5 Å apart (Cα-Cα distance) in the apo structure (Figure 6A, left), with cysteines followed by disulfide bond formation yielded a slower migrating species (Figure 6B, right) that was deficient in catalytic autolabeling (Figure 6D right, G363C/G660C). These results indicate that: 1) the position of the plug and motif B loop in the mini-vRNAP apo structure are not artifacts of crystallization; 2) the plug and motif B loop block the pathway of the DNA template to the active center; and 3) the plug and motif B loop must move away from the DNA-binding channel for catalysis upon promoter binding, as observed in the structures of the binary complexes.
Discussion
We have determined the crystal structures of three phage N4 mini-vRNAP-hairpin DNA promoter complexes. The high-resolution structures of the binary complex, along with structure-based biochemical experiments provide insights into: 1) the recognition of the hairpin-form DNA promoter by vRNAP; 2) the formation of the high affinity and salt-resistant binary complex; 3) the structural transition of vRNAP from its apo-form to the binary complex; 4) the mechanism for selecting the transcriptional initiation site. The results presented highlight the impact of subtle differences in protein-DNA interactions on their affinity and salt stability as well as the large vRNAP conformational changes that are associated with the phage life cycle and infection strategy.
DNA Hairpin-Stem Promoter Recognition by N4 vRNAP
The high-resolution crystal structures of binary complexes revealed that the N4 vRNAP uses four structural motifs to accommodate the promoter hairpin on the protein surface through sequence-specific interactions (Figures 2B–D): 1) the −11 recognition site contacts bases (−10 and −11) of the hairpin loop; 2) the specificity loop makes base-specific interactions to the hairpin stem (−8, −9 and −10) from its major groove; 3) the β-intercalating hairpin defines the junction between double- and single-stranded DNA with base recognition at −5G; and 4) K849 and K850 form salt-bridges with the phosphate backbone at −12 and −13. These base-specific contacts explain the sequence conservation of the N4 vRNAP promoters at positions −5, −8, −9, −10 and −11. Insights obtained from the binary complex structures are consistent with biochemical studies including DNA and promoter mutations as well as cross-linking experiments (Davydova et al., 2007).
The lack of sequence-specific interactions just upstream of the transcription initiation site has been puzzling. Our results indicate that the track of adenines, and specifically the identity of the bases upstream of position +1, serves as a ruler to define the site of transcription initiation. We speculate that the ability of adenines, in contrast to thymines, to stack (Goddard et al., 2000), and the interaction of R318 with the phosphate backbone that induces a kink between positions −2 and −1 are essential to mark the transcription initiation site.
Multi-subunit cellular RNAPs most likely use an alternative strategy to select the transcription start site. These enzymes utilize an additional polypeptide and change their structures for specific, de novo transcription initiation. For example, the region3.2 of σ factor (bacterial RNAP) and the B-finger of TFIIB (eukaryotic RNAPII) associate nearby the active site to determine the transcription start site and to support nucleotide binding (Bushnell et al., 2004; Campbell et al., 2002; Murakami et al., 2002).
Comparison between N4 and T7 Single-Subunit Polymerase Promoter Complex Structures
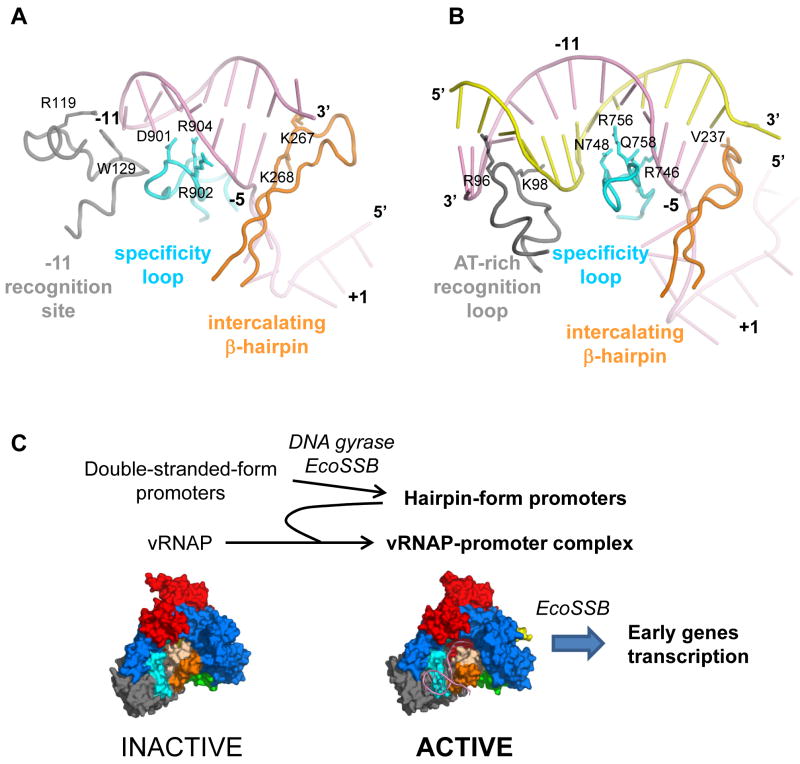
N4 vRNAP and T7 RNAP share striking structural similarity despite their low sequence similarity. In addition, although there is a critical difference of promoter DNA shape for these enzymes – the N4 and T7 RNAPs recognize a DNA hairpin and double-stranded DNA duplex, respectively – these enzymes recognize their promoters with similar domains and motifs (Figures 7A and 7B). The structures of the specificity loop and β-intercalating hairpin as well as their interactions with DNA are very similar. However, the N4 vRNAP −11 recognition site has higher architectural complexity when compared with its counterpart (AT-rich recognition loop in T7 RNAP), which may reflect the functional difference of these motifs. In the case of N4 vRNAP, it recognizes the tip of the hairpin loop through sequence-specific interactions (at −11 and −12) whereas in T7 RNAP, the AT-rich recognition loop fits into a wider and shallower minor groove of the AT-rich DNA sequence (between −13 and −17) without any base recognition (Cheetham et al., 1999). In addition to these three motifs, N4 vRNAP has extra contacts between the hairpin loop phosphate backbone (at −12 and −13) and Lys residues (K849 and K850) at the end of the long α-helix, that further improve the stability of the complex.
Figure 7. The three promoter-recognition structural motifs of N4 and T7 RNAPs.
(A) The −11 recognition site (gray), specificity loop (cyan) and β-intercalating hairpin (orange) of N4 vRNAP in the binary complex. The promoter hairpin is depicted by a pink ribbon.
(B) The AT-rich recognition loop (gray), specific loop (cyan) and β-intercalating hairpin of T7 RNAP in the binary complex (PDB: 2PI5). Template and non-template DNA strands are depicted by pink and yellow ribbons, respectively. The orientation of the T7 RNAP binary complex has been aligned with the N4 mini-vRNAP binary complex in panel A by superimposing their Palm cores. Amino acid residues involved in promoter recognition are shown as stick and labeled.
(C) Sequential remodeling of the N4 phage genome DNA and vRNAP to achieve phage early gene transcription. Both N4 vRNAP promoters and vRNAP are injected into the host cell in inactive conformations. Host factors, DNA gyrase and EcoSSB, play a role in remodeling the N4 genome DNA to produce three hairpin-form promoters (P1, P2 and P3) that, upon binding to vRNAP, remodel the vRNAP conformation to form the transcription-ready binary complexes. EcoSSB also participates in transcription elongation as a DNA-template recycling factor. Structures of N4 mini-vRNAP in the apo-form (left, inactive) and binary complex (right, active) are shown. Color scheme is the same as in Fig. 1C.
Role of the Promoter Hairpin in the Transition from the Inactive Apo-Form Enzyme to the “Transcription Ready” Binary Complex
The crystal structure of apo-form N4 mini-vRNAP revealed that the enzyme is inactivated by the plug module and motif B loop interactions that prevent the access of the DNA template to the active site and the binding of the incoming nucleotides (Murakami et al., 2008). The inactive conformation found in the apo-form crystal structure was validated by the engineered disulfide linkage experiments presented here (Figure 6). The binary complex structures show how vRNAP changes its structure to accommodate a promoter and template DNA as well as nucleotides at the active site. The template DNA binding channel is completely closed in the apo-form structure, although the promoter hairpin-binding region is surface exposed (Figure 1C). Therefore, we propose that an establishment of the promoter hairpin binding to three motifs (−11 recognition site, specificity loop and β-intercalating hairpin) partially opens the template DNA binding channel, which would be opened further by inserting the template DNA into the cleft. The promoter hairpin may act as the “key” to transform the inactive apo-form enzyme to the “transcription ready” binary complex. This hypothesis is supported by the fact that the N4 vRNAP is inactive on single-stranded DNA templates lacking hairpin-form promoters (Falco et al., 1977). It is worth noting the functional similarity of the N4 mini-vRNAP “plug” with σ70 region 1.1, which occupies the DNA binding channel before E. coli RNAP holoenzyme interacts with its cognate promoters (Mekler et al., 2002; Murakami et al., 2002; Nagai and Shimamoto, 1997).
Phage N4 early transcription is carried out by ~4 copies of vRNAP, which are injected from the phage virion (Choi et al., 2008). A lack of tight specificity toward the hairpin-form promoter DNA would allow vRNAP to randomly bind to the host’s genomic DNA, which would compete with the binding to the three N4 early promoters. We suggest that to achieve strict specificity, promoter DNA and vRNAP are delivered into the host in inactive conformations and undergo sequential remodeling (Figure 7C). First, the promoter DNA undergoes a supercoiled-induced structural transition that leads to hairpin extrusion, followed by EcoSSB binding to yield an active promoter (Dai and Rothman-Denes, 1998). Next, binding of the activated promoter remodels vRNAP into an active conformation. In contrast, T7 RNAP does not undergo a large conformational transition between the apo-form and the binary complex. We surmise that the difference between these enzymes (transcription-inactive N4 vRNAP vs transcription-ready T7 RNAP) originates from a difference in their infection strategies. The phage T7 early genes are transcribed by the host E. coli RNAP (Chamberlin et al., 1970), while T7 RNAP is responsible for transcription of the middle and late genes. This transcriptional program allows T7 RNAP to be abundant, without the need for such strict promoter specificity.
We have shown that the plug and motif B loop undergo conformational changes upon binding of the promoter to vRNAP. Future challenges include the identification of the RNAP “sensor” and specific promoter “signal” that trigger such conformational changes and the intermediate states of the structural transition pathway.
Mini-vRNAP is the most evolutionary-diverged member of the T7-like RNAP family (Kazmierczak et al., 2002), which comprises the closely related phage-encoded, nuclear-encoded mitochondrial and some chloroplast, and the linear plasmid-encoded enzymes. The results presented reveal a remarkable plasticity of the protein-DNA interaction where, through a limited number of adaptations in the protein, mini-vRNAP is able to recognize a hairpin stem promoter that is generated through a unique mode and has unique structure to fulfill the phage physiological requirements. Other biochemically-characterized members of the family – N4 RNAPII, the human and yeast mitochondrial RNAPs – require transcription factors for promoter recognition (Carter et al., 2003; Falkenberg et al., 2002; Mangus et al., 1994). Further insights into their mechanisms of promoter recognition await the resolution of the structures of these enzymes.
Experimental Procedures
Mini-vRNAP Purification
N-terminal His6-tag mini-vRNAP was over-expressed in E. coli BL21 cells transformed with the expression plasmid pKMK56 (Kazmierczak et al., 2002). Proteins were purified as described (Davydova et al., 2003).
DNA Purification
DNA oligonucleotides (Figure 1B) were purchased from Integrated DNA Technologies and purified by denaturing polyacrylamide gel electrophoresis. To form the hairpin DNA structure, the DNA in the annealing solution (5 mM Na-cacodylate pH 7.4, 0.5 mM EDTA, and 0.2 M NaCl) was heated to 95 °C for 5 min followed by cooling to 25 °C at a rate of 0.01 °C/s.
Crystallization of Binary Complexes
The binary complex was formed by mixing protein and DNA (1:1 molar ratio) in 10 mM Tris-HCl (pH 8 at 21 °C), 50 mM NaCl, 0.1 mM EDTA. Initial crystals were obtained by hanging drop vapor diffusion. The binary complex solution (10 mg/ml of mini-vRNAP) was mixed with an equal volume of the crystallization solution (20 % PEG 3350, 0.1 M Tris-HCl (pH 8), 0.2 M dibasic ammonium citrate) and incubated at 21 °C over the same crystallization solution. The binary complex crystals used for data collection were grown by the hanging drop vapor diffusion method with microseeding. Solutions used for each of the binary complexes are summarized in STable 1. Briefly, the mini-vRNAP/DNA mixture was mixed with an equal volume of crystallization solution including microseeds, and incubated as a hanging drop over a reservoir solution. The crystals were harvested in a stabilization solution, moved to a cryo-protection solution, and then flash frozen by liquid nitrogen. P2_7a binary crystals were soaked in crystallization solution with 10 mM MgCl2 overnight followed by the same cryo and freezing protocol as without the metal, but with the addition of the respective 10mM MgCl2 in each step.
Data Collections and Structure Determinations
Crystallographic data were collected by synchrotron radiation at the National Synchrotron Light Source (Brookhaven National Laboratory, Upton, NY) and processed with HKL2000 (Otwinowski and Minor, 1997) (STable 2). Binary complex structure containing P2-7a DNA (PDB code: 3C46) was solved by molecular replacement with the program CNS (Brunger et al., 1998) and with the entire apo-enzyme structure (Murakami et al., 2008) as a search model. The molecular replacement found two binary complexes in the asymmetric unit (SFigure 1), and the models of these complexes were refined by rigid body refinement followed by simulated annealing refinement using CNS (Brunger et al., 1998). After density modification, clear densities corresponding to DNA were obtained (SFigure 3B) in addition to the density maps corresponding to the mini-vRNAPs. There were substantial differences between the density modified map and parts of enzyme including the plug, β-intercalating hairpin and motif B loop (SFigure 5), and the models of these shifted parts were manually rebuild by O (Jones et al., 1991). The DNA models were also built by O, and the binary complex models were refined by right body refinement, energy minimization and individual B-factor refinements by CNS (Brunger et al., 1998) (STable 2). The refined binary complex structure was used as the search model for determining other binary complex structures.
Site-directed Mutagenesis of Mini-vRNAP
Site-directed mutagenesis was performed as described previously (Murakami et al., 2008). Mutant mini-vRNAP enzymes contain the following DNA sequence changes: R119A, CGT to GCT; R119K, CGT to AAA; K176C, AAA to TGC; R318A, CGT to GCT; R318K, CGT to AAA; G363C, GGT to TGT; G660C, GGT to TGT; S885C, TCA to TGT. The sequence of all polymerase expression constructs was confirmed. Enzymes were produced and purified as described (Davydova et al., 2003).
Salt-resistance of Promoter-vRNAP Complexes and Determination of Equilibrium dissociation Binding Constants
The salt resistance of mini-vRNAP-promoter complexes, determination of equilibrium dissociation constants and promoter-binding affinities were performed as described (Davydova et al., 2007).
Analysis of R318A and R318K Mini-vRNAPs
Transcription initiation reactions (5 μl) contained 1 μM template (P2-4A-8 or P2-4T-8, Figure 4A), 0.1 μM enzyme, 1 mM [γ-32P] GTP, 0.5 mM ATP, 10 mM Tris-HCl pH 7.8, 10 mM MgCl2, 50 mM NaCl, 1 mM DTT. Reactions were incubated at 37° C for 2 min, processed as described previously (Davydova et al., 2003) and analyzed by electrophoresis on 8 M urea/20% polyacrylamide gels and phosphorimaging. Catalytic autolabeling was performed using the hydroxylbenzaldehyde derivatives of GTP or ATP as the initiating nucleotide as described previously (Davydova et al., 2007).
Analysis of K176C/S885C and G363C/G660C Mini-vRNAPs
Wild-type, K176C/S885C and G363C/G660C enzymes were purified as described previously (Davydova et al., 2003), but in the absence of DTT. Proteins (8 μl of 10 μM enzyme in 10% glycerol, 20 mM Tris-HCl pH8, 500 mM NaCl) were incubated with L-cystine (Sigma) (2 μl of 0.5 mM in 0.2 M NaCl) overnight on ice to generate disulfide bond formation. Proteins were incubated with 10 mM DTT at 37° C for 15 min to reduce the disulfide bond. All reactions described below with the disulfide bond-containing enzymes lacked DTT. Cross-linking reactions of wild-type and mutant proteins to a 5′ 32P end-labeled 13mer oligonucleotide encompassing the −11 5 IdU-substituted promoter hairpin (5′GAAGCG-5IdU-AGCTTC3′) were performed as described previously (Davydova et al., 2007). Catalytic autolabeling reactions contained 1μM P2-3 oligonucleotide (5′TCCAAAAGAAGCGGAGCTTC3′, +1 underlined), 1 mM benzaldehyde derivative of GTP, and [α-32P]ATP (3,000 Ci/mmol) (Davydova et al., 2007). Crosslinking and catalytic autolabeling reactions were terminated by the addition of SDS-loading dye lacking DTT, and analyzed by 10% SDS-PAGE, silver staining and phophorimaging. Run-off transcription reactions were performed at 37 °C for 10 min using 0.1 μM 12-P2-52 DNA template in the presence of 1 μM EcoSSB, and analyzed by 8M urea-PAGE and phosphorimaging as described previously (Davydova et al., 2003).
Supplementary Material
Acknowledgments
We thank E. Davydova for technical assistance, and the staff at X25 of the National Synchrotron Light Source and H. Yennawar for supports. We thank E. P. Geiduschek for critiques of the manuscript. We thank Hauptman-Woodward Institute for screening crystallization conditions. Figures were prepared using PyMOL. This work was supported by NIH grants AI12575 to L.B.R-D., and GM071897 to K.S.M.
Footnotes
Accession Number
Coordinates and structure factors have been deposited to the Protein Data Bank (STable 2).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brieba LG, Sousa R. The T7 RNA polymerase intercalating hairpin is important for promoter opening during initiation but not for RNA displacement or transcription bubble stability during elongation. Biochemistry. 2001;40:3882–3890. doi: 10.1021/bi002716c. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 5):905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Bushnell DA, Westover KD, Davis RE, Kornberg RD. Structural basis of transcription: an RNA polymerase II-TFIIB cocrystal at 4.5 Angstroms. Science. 2004;303:983–988. doi: 10.1126/science.1090838. [DOI] [PubMed] [Google Scholar]
- Campbell EA, Muzzin O, Chlenov M, Sun JL, Olson CA, Weinman O, Trester-Zedlitz ML, Darst SA. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol Cell. 2002;9:527–539. doi: 10.1016/s1097-2765(02)00470-7. [DOI] [PubMed] [Google Scholar]
- Carter RH, Demidenko AA, Hattingh-Willis S, Rothman-Denes LB. Phage N4 RNA polymerase II recruitment to DNA by a single-stranded DNA-binding protein. Genes Dev. 2003;17:2334–2345. doi: 10.1101/gad.1121403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M, McGrath J, Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970;228:227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Cheetham GM, Jeruzalmi D, Steitz TA. Structural basis for initiation of transcription from an RNA polymerase-promoter complex. Nature. 1999;399:80–83. doi: 10.1038/19999. [DOI] [PubMed] [Google Scholar]
- Choi KH, McPartland J, Kaganman I, Bowman VD, Rothman-Denes LB, Rossmann MG. Insight into DNA and protein transport in double-stranded DNA viruses: the structure of bacteriophage N4. J Mol Biol. 2008;378:726–736. doi: 10.1016/j.jmb.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Greizerstein MB, Nadas-Chinni K, Rothman-Denes LB. Supercoil-induced extrusion of a regulatory DNA hairpin. Proc Natl Acad Sci U S A. 1997;94:2174–2179. doi: 10.1073/pnas.94.6.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Rothman-Denes LB. Sequence and DNA structural determinants of N4 virion RNA polymerase-promoter recognition. Genes Dev. 1998;12:2782–2790. doi: 10.1101/gad.12.17.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydova EK, Kazmierczak KM, Rothman-Denes LB. Bacteriophage N4-coded, virion-encapsulated DNA-dependent RNA polymerase. Methods Enzymol. 2003;370:83–94. doi: 10.1016/S0076-6879(03)70008-1. [DOI] [PubMed] [Google Scholar]
- Davydova EK, Rothman-Denes LB. Escherichia coli single-stranded DNA-binding protein mediates template recycling during transcription by bacteriophage N4 virion RNA polymerase. Proc Natl Acad Sci U S A. 2003;100:9250–9255. doi: 10.1073/pnas.1133325100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydova EK, Santangelo TJ, Rothman-Denes LB. Bacteriophage N4 virion RNA polymerase interaction with its promoter DNA hairpin. Proc Natl Acad Sci U S A. 2007;104:7033–7038. doi: 10.1073/pnas.0610627104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco SC, Laan KV, Rothman-Denes LB. Virion-associated RNA polymerase required for bacteriophage N4 development. Proc Natl Acad Sci U S A. 1977;74:520–523. doi: 10.1073/pnas.74.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco SC, Zivin R, Rothman-Denes LB. Novel template requirements of N4 virion RNA polymerase. Proc Natl Acad Sci U S A. 1978;75:3220–3224. doi: 10.1073/pnas.75.7.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nature Genetics. 2002;31:289–294. doi: 10.1038/ng909. [DOI] [PubMed] [Google Scholar]
- Glucksmann MA, Markiewicz P, Malone C, Rothman-Denes LB. Specific sequences and a hairpin structure in the template strand are required for N4 virion RNA polymerase promoter recognition. Cell. 1992;70:491–500. doi: 10.1016/0092-8674(92)90173-a. [DOI] [PubMed] [Google Scholar]
- Goddard NL, Bonnet G, Krichevsky O, Libchaber A. Sequence dependent rigidity of single stranded DNA. Phys Rev Lett. 2000;85:2400–2403. doi: 10.1103/PhysRevLett.85.2400. [DOI] [PubMed] [Google Scholar]
- Haynes LL, Rothman-Denes LB. N4 virion RNA polymerase sites of transcription initiation. Cell. 1985;41:597–605. doi: 10.1016/s0092-8674(85)80032-5. [DOI] [PubMed] [Google Scholar]
- Jeruzalmi D, Steitz TA. Structure of T7 RNA polymerase complexed to the transcriptional inhibitor T7 lysozyme. EMBO J. 1998;17:4101–4113. doi: 10.1093/emboj/17.14.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kazmierczak KM, Davydova EK, Mustaev AA, Rothman-Denes LB. The phage N4 virion RNA polymerase catalytic domain is related to single-subunit RNA polymerases. EMBO J. 2002;21:5815–5823. doi: 10.1093/emboj/cdf584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NV, Govil G. Theoretical studies on protein-nucleic acid interactions. III. Stacking of aromatic amino acids with bases and base pairs of nucleic acids. Biopolymers. 1984;23:2009–2024. doi: 10.1002/bip.360231015. [DOI] [PubMed] [Google Scholar]
- Mangus DA, Jang SH, Jaehning JA. Release of the yeast mitochondrial RNA polymerase specificity factor from transcription complexes. J Biol Chem. 1994;269:26568–26574. [PubMed] [Google Scholar]
- Markiewicz P, Malone C, Chase JW, Rothman-Denes LB. Escherichia coli single-stranded DNA-binding protein is a supercoiled template-dependent transcriptional activator of N4 virion RNA polymerase. Genes Dev. 1992;6:2010–2019. doi: 10.1101/gad.6.10.2010. [DOI] [PubMed] [Google Scholar]
- Mekler V, Kortkhonjia E, Mukhopadhyay J, Knight J, Revyakin A, Kapanidis AN, Niu W, Ebright YW, Levy R, Ebright RH. Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase-promoter open complex. Cell. 2002;108:599–614. doi: 10.1016/s0092-8674(02)00667-0. [DOI] [PubMed] [Google Scholar]
- Murakami KS, Davydova EK, Rothman-Denes LB. X-ray crystal structure of the polymerase domain of the bacteriophage N4 virion RNA polymerase. Proc Natl Acad Sci U S A. 2008;105:5046–5051. doi: 10.1073/pnas.0712325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- Nagai H, Shimamoto N. Regions of the Escherichia coli primary sigma factor sigma70 that are involved in interaction with RNA polymerase core enzyme. Genes Cells. 1997;2:725–734. doi: 10.1046/j.1365-2443.1997.1600357.x. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Charles J, Carter W, editors. Macromolecular Crystallography Part A, Methods in Enzymology. Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Stano NM, Patel SS. The intercalating beta-hairpin of T7 RNA polymerase plays a role in promoter DNA melting and in stabilizing the melted DNA for efficient RNA synthesis. J Mol Biol. 2002;315:1009–1025. doi: 10.1006/jmbi.2001.5313. [DOI] [PubMed] [Google Scholar]
- Yin YW, Steitz TA. The structural mechanism of translocation and helicase activity in T7 RNA polymerase. Cell. 2004;116:393–404. doi: 10.1016/s0092-8674(04)00120-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.