Abstract
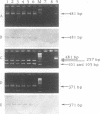
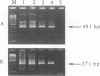
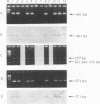
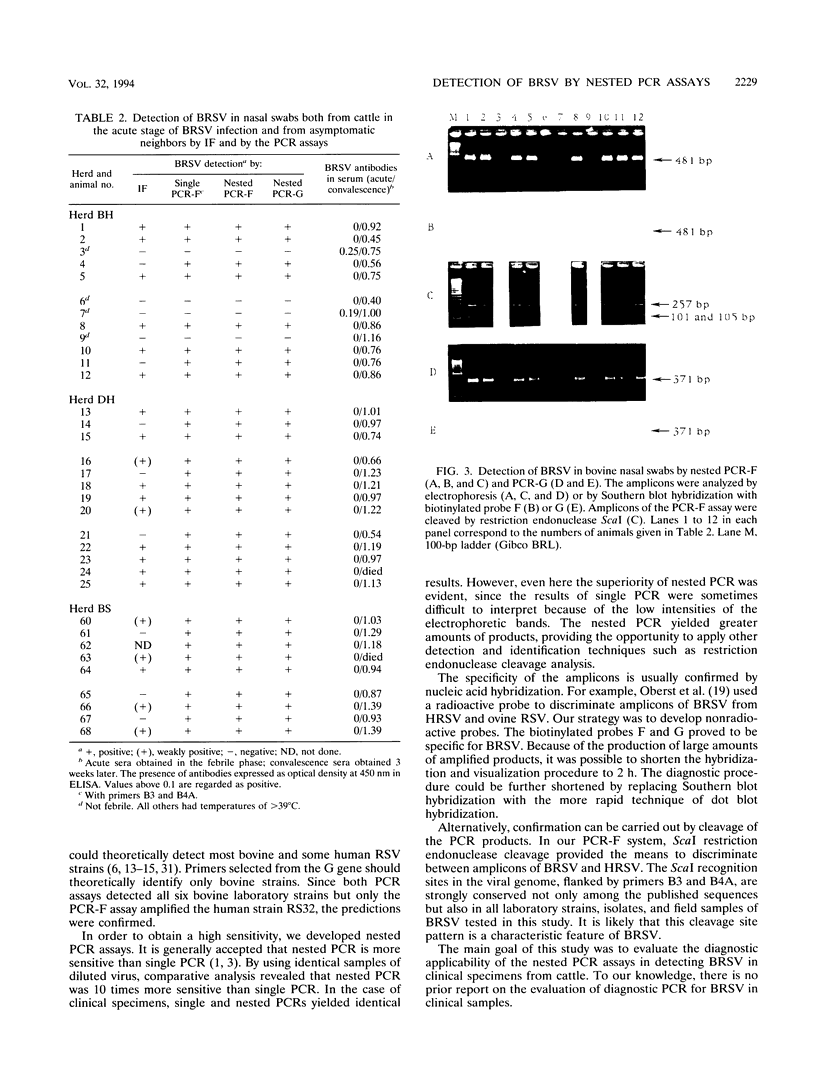
Two nested PCR assays were developed for the detection of bovine respiratory syncytial virus (BRSV). Primers were selected from the gene encoding the F fusion protein (PCR-F) and the gene encoding the G attachment protein (PCR-G). Biotinylated oligonucleotide probes, termed F and G, were selected for the hybridization of the respective PCR products. The sensitivities of the PCR-F and PCR-G assays were similar, both detecting 0.1 tissue culture infective dose of the virus. The PCR-F assay amplified all bovine strains and one human strain (RS32) tested. No cross-reactions were observed with nine heterologous respiratory viruses. PCR-F products of bovine and human RSV strains were discriminated by using endonuclease restriction enzyme ScaI, which specifically cleaved, products of BRSV. Oligonucleotide probe F was also specific for products of BRSV. The PCR-G assay detected all bovine strains and none of the human strains tested. A faint electrophoretic band was also observed with products of Sendai virus. However, probe G did not hybridize with this product, only with products of BRSV. Nasal swabs collected from cattle with no symptoms and cattle in the acute stage of respiratory disease were analyzed for BRSV by the immunofluorescence (IF) method and by the PCR-F and PCR-G assays. The virus was detected by the PCR assays in 31 of 35 (89%) samples tested. Only 23 samples (66%) were positive by the IF method, and these samples were also positive by both the PCR-F and PCR-G assays. The 31 samples detected as positive by PCR originated from cattle presenting clinical signs of acute respiratory disease; the four PCR-negative samples originated from clinically asymptomatic neighboring cattle. All sampled animals subsequently seroconverted and became reactive to BRSV. Thus, the detection of BRSV by PCR correlated with clinical observations and was considerably more sensitive (66 versus 89%) than IF. These results indicate that both nested PCR assays provide rapid and sensitive means for the detection of BRSV infection in cattle. Considering its higher specificity, the PCR-F assay can be recommended as the method of choice in the analysis of clinical specimens of BRSV.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alansari H., Brock K. V., Potgieter L. N. Single and double polymerase chain reaction for detection of bovine viral diarrhea virus in tissue culture and sera. J Vet Diagn Invest. 1993 Apr;5(2):148–153. doi: 10.1177/104063879300500201. [DOI] [PubMed] [Google Scholar]
- Belák S., Ballagi-Pordány A. Application of the polymerase chain reaction (PCR) in veterinary diagnostic virology. Vet Res Commun. 1993;17(1):55–72. doi: 10.1007/BF01839180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belák S., Ballagi-Pordány A. Bovine viral diarrhea virus infection: rapid diagnosis by the polymerase chain reaction. Arch Virol Suppl. 1991;3:181–190. doi: 10.1007/978-3-7091-9153-8_22. [DOI] [PubMed] [Google Scholar]
- Belák S., Ballagi-Pordány A. Experiences on the application of the polymerase chain reaction in a diagnostic laboratory. Mol Cell Probes. 1993 Jun;7(3):241–248. doi: 10.1006/mcpr.1993.1035. [DOI] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubie H. A., Inglis J. M., Leslie E. E., Edmunds A. T., Totapally B. Detection of respiratory syncytial virus in acute bronchiolitis in infants. J Med Virol. 1992 Dec;38(4):283–287. doi: 10.1002/jmv.1890380410. [DOI] [PubMed] [Google Scholar]
- Cubie H. A., Inglis J. M., McGowan A. M. Detection of respiratory syncytial virus antigen and nucleic acid in clinical specimens using synthetic oligonucleotides. J Virol Methods. 1991 Sep;34(1):27–35. doi: 10.1016/0166-0934(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Edwards S., Newman R. H., Stanley M. Respiratory syncytial virus diagnosis. Vet Rec. 1984 Jan 28;114(4):101–101. doi: 10.1136/vr.114.4.101. [DOI] [PubMed] [Google Scholar]
- Inaba Y., Tanaka Y., Sato K., Omori T., Matumoto M. Bovine respiratory syncytial virus. Studies on an outbreak in Japan, 1968-1969. Jpn J Microbiol. 1972 Sep;16(5):373–383. [PubMed] [Google Scholar]
- Johnson P. R., Collins P. L. The fusion glycoproteins of human respiratory syncytial virus of subgroups A and B: sequence conservation provides a structural basis for antigenic relatedness. J Gen Virol. 1988 Oct;69(Pt 10):2623–2628. doi: 10.1099/0022-1317-69-10-2623. [DOI] [PubMed] [Google Scholar]
- Lerch R. A., Anderson K., Amann V. L., Wertz G. W. Nucleotide sequence analysis of the bovine respiratory syncytial virus fusion protein mRNA and expression from a recombinant vaccinia virus. Virology. 1991 Mar;181(1):118–131. doi: 10.1016/0042-6822(91)90476-r. [DOI] [PubMed] [Google Scholar]
- López J. A., Villanueva N., Melero J. A., Portela A. Nucleotide sequence of the fusion and phosphoprotein genes of human respiratory syncytial (RS) virus Long strain: evidence of subtype genetic heterogeneity. Virus Res. 1988 May;10(2-3):249–261. doi: 10.1016/0168-1702(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Mallipeddi S. K., Samal S. K., Mohanty S. B. Analysis of polypeptides synthesized in bovine respiratory syncytial virus-infected cells. Arch Virol. 1990;115(1-2):23–36. doi: 10.1007/BF01310620. [DOI] [PubMed] [Google Scholar]
- Mallipeddi S. K., Samal S. K. Sequence variability of the glycoprotein gene of bovine respiratory syncytial virus. J Gen Virol. 1993 Sep;74(Pt 9):2001–2004. doi: 10.1099/0022-1317-74-9-2001. [DOI] [PubMed] [Google Scholar]
- Mufson M. A., Orvell C., Rafnar B., Norrby E. Two distinct subtypes of human respiratory syncytial virus. J Gen Virol. 1985 Oct;66(Pt 10):2111–2124. doi: 10.1099/0022-1317-66-10-2111. [DOI] [PubMed] [Google Scholar]
- Oberst R. D., Hays M. P., Evermann J. F., Kelling C. L. Characteristic differences in reverse transcription-polymerase chain reaction products of ovine, bovine, and human respiratory syncytial viruses. J Vet Diagn Invest. 1993 Jul;5(3):322–328. doi: 10.1177/104063879300500303. [DOI] [PubMed] [Google Scholar]
- Oberst R. D., Hays M. P., Hennessy K. J., Stine L. C., Evermann J. F., Kelling C. L. Identifying bovine respiratory syncytial virus by reverse transcription-polymerase chain reaction and oligonucleotide hybridizations. J Clin Microbiol. 1993 May;31(5):1237–1240. doi: 10.1128/jcm.31.5.1237-1240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Shirotori K., Kudo K., Ito E., Togawa K., Saito I., Moro I., Ogra P. L. Genomic sequences of respiratory syncytial virus in otitis media with effusion. Lancet. 1991 Oct 19;338(8773):1025–1026. doi: 10.1016/0140-6736(91)91894-z. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E., Mufson M. A. Preparation and characterization of monoclonal antibodies directed against five structural components of human respiratory syncytial virus subgroup B. J Gen Virol. 1987 Dec;68(Pt 12):3125–3135. doi: 10.1099/0022-1317-68-12-3125. [DOI] [PubMed] [Google Scholar]
- Osorio F. A., Anderson G. A., Sanders J., Grotelueschen D. Detection of bovine respiratory syncytial virus using a heterologous antigen-capture enzyme immunoassay. J Vet Diagn Invest. 1989 Jul;1(3):210–214. doi: 10.1177/104063878900100302. [DOI] [PubMed] [Google Scholar]
- Paccaud M. F., Jacquier C. A respiratory syncytial virus of bovine origin. Arch Gesamte Virusforsch. 1970;30(4):327–342. doi: 10.1007/BF01258363. [DOI] [PubMed] [Google Scholar]
- Paton A. W., Paton J. C., Lawrence A. J., Goldwater P. N., Harris R. J. Rapid detection of respiratory syncytial virus in nasopharyngeal aspirates by reverse transcription and polymerase chain reaction amplification. J Clin Microbiol. 1992 Apr;30(4):901–904. doi: 10.1128/jcm.30.4.901-904.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. H., Frey M. L., Dierks R. E. Isolation, characterization, and pathogenicity studies of a bovine respiratory syncytial virus. Arch Virol. 1975;47(3):237–247. doi: 10.1007/BF01317811. [DOI] [PubMed] [Google Scholar]
- Sullender W. M., Anderson L. J., Anderson K., Wertz G. W. Differentiation of respiratory syncytial virus subgroups with cDNA probes in a nucleic acid hybridization assay. J Clin Microbiol. 1990 Aug;28(8):1683–1687. doi: 10.1128/jcm.28.8.1683-1687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullender W. M., Wertz G. W. Synthetic oligonucleotide probes differentiate respiratory syncytial virus subgroups in a nucleic acid hybridization assay. J Clin Microbiol. 1991 Jun;29(6):1255–1257. doi: 10.1128/jcm.29.6.1255-1257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Stott E. J. Diagnosis of respiratory syncytial virus infection in the bovine respiratory tract by immunofluorescence. Vet Rec. 1981 May 16;108(20):432–435. doi: 10.1136/vr.108.20.432. [DOI] [PubMed] [Google Scholar]
- Walravens K., Kettmann R., Collard A., Coppe P., Burny A. Sequence comparison between the fusion protein of human and bovine respiratory syncytial viruses. J Gen Virol. 1990 Dec;71(Pt 12):3009–3014. doi: 10.1099/0022-1317-71-12-3009. [DOI] [PubMed] [Google Scholar]