Abstract
Canola (Brassica napus L.) is one of the most important oilseed crops in the world and its seed yield and quality are significantly affected by drought stress. As an innate and adaptive response to water deficit, land plants avoid potential damage by rapid biosynthesis of the phytohormone abscisic acid (ABA), which triggers stomatal closure to reduce transpirational water loss. The ABA-mediated stomatal response is a dosage-dependent process; thus, one genetic engineering approach for achieving drought avoidance could be to sensitize the guard cell's responsiveness to this hormone. Recent genetic studies have pinpointed protein farnesyltransferase as a key negative regulator controlling ABA sensitivity in the guard cells. We have previously shown that down-regulation of the gene encoding Arabidopsis β-subunit of farnesyltransferase (ERA1) enhances the plant's sensitivity to ABA and drought tolerance. Although the α-subunit of farnesyltransferase (AtFTA) is also implicated in ABA sensing, the effectiveness of using such a gene target for improving drought tolerance in a crop plant has not been validated. Here, we report the identification and characterization of the promoter of Arabidopsis hydroxypyruvate reductase (AtHPR1), which expresses specifically in the shoot and not in non-photosynthetic tissues such as root. The promoter region of AtHPR1 contains the core motif of the well characterized dehydration-responsive cis-acting element and we have confirmed that AtHPR1 expression is inducible by drought stress. Conditional and specific down-regulation of FTA in canola using the AtHPR1 promoter driving an RNAi construct resulted in yield protection against drought stress in the field. Using this molecular strategy, we have made significant progress in engineering drought tolerance in this important crop species.
Keywords: ABA signaling, protein farnesylation, conditional promoter, drought, stomatal response, seed yield, field trial
INTRODUCTION
Drought is one of the environmental stresses that has a profound negative effect on the growth and development of plants, and, in most regions of the world, the productivity of major crops is defined by their ability to cope with this stress (Boyer, 1982; Tollenaar and Wu, 1999). In the case of canola, it has been demonstrated that water stress that occurs during flowering and grain filling periods results in a severe reduction in both seed yield and seed quality (Mingeau, 1974; Champolivier and Merrien, 1996; Sinaki et al., 2007). With the rising demand for plant products as food and biofuel material, development of drought-tolerant crops has become the focal point of research for crop improvement.
Dicot plants lose greater than 90% of available soil water through the process of transpiration via the stomatal apparatus. One of the best understood mechanisms of a plant's adaptation to water deficit is the modulation of stomatal aperture by the stress-inducible hormone ABA. It has been demonstrated that ABA-mediated stomatal closure is a dosage-dependent process that can be achieved by either up-regulation of de novo biosynthesis of ABA, or by sensitizing the guard cell's responsiveness to the hormone. Since stomatal movement is a reversible process, it appears that engineering for drought tolerance through genetic tailoring of ABA sensitivity would be a metabolically efficient approach.
Initial genetic screens identified several classical Arabidopsis ABA insensitive mutants such as abi1, abi2, and abi3, which are resistant to the inhibition of germination and early seedling growth by relatively high concentrations of exogenous ABA in the growth medium (Koornneef et al., 1984, 1989). These abi mutants are normal in ABA biosynthesis; however, their guard cells are insensitive to ABA, resulting in a wilty phenotype due to excessive transpiration under water deficit growth conditions. The first ABA hypersensitive mutant, termed era1, was identified in a forward genetic screen with low concentrations of the hormone in the seed germination growth medium (Cutler et al., 1996). Subsequent germination screens revealed a series of mutants that are hypersensitive to ABA. Interestingly, although seed dormancy is affected in all of these mutants, only a subset of them exhibits an altered ABA response in their guard cells. These include loss-of-function mutations in genes encoding a protein phosphatase 2C, ABI1 (Gosti et al., 1999; Saez et al., 2006), a nuclear mRNA cap-binding protein, ABH1 (Hugouvieux et al., 2001), a Ca2+ binding protein, ScaBP5, and its interacting protein kinase, PKS3 (Guo et al., 2002), the α- subunit of protein farnesyltransferase, AtFTA (Running et al., 2004; Wang et al., 2005), a subunit of holo-elongator, ABO1/ELO2 (Chen et al., 2006), and gain-of-function mutations in genes encoding a stress responsive transcription factor, SNAC1 (Hu et al., 2006), a RING finger E3 ligase, SDIR1 (Zhang et al., 2007), and a R2R3 Myb transcription factor, AtMYB44 (Jung et al., 2008), which confer ABA hypersensitivity at both germination and guard cell levels. The mutations in these genes cause reduced transpiration rate through a greater reduction in stomatal conductance; thus, they might be promising targets for further genetic manipulation for drought tolerance.
In plants, protein farnesylation was first implicated in ABA signaling through the molecular and physiological characterization of ERA1, a gene that encodes the β-subunit of farnesyltransferase (Cutler et al., 1996). Deletion of ERA1 or specific inhibition of farnesyltransferase in Arabidopsis caused greater ABA-induced guard cell S-type anion-channel activation and a rapid increase in the cytosolic Ca2+ concentration, resulting in significantly tighter stomatal closure at a wide range of physiologically relevant ABA concentrations (Pei et al., 1998; Allen et al., 2002). The usefulness of ERA1 as a biotechnological target for drought tolerance has been extensively examined (Wang et al., 2005). Consistent with the era1 phenotypes, constitutive down-regulation of ERA1 resulted in both increased ABA sensitivity and improved drought stress tolerance in Arabidopsis. Furthermore, data obtained from multiple years of field trials conducted in different locations suggest that conditional down-regulation of the gene in canola provided the crop with a significant increase in yield compared to the wild-type control under drought stress conditions at the time of flowering (Wang et al., 2005).
Results from recent studies indicate that AtFTA may also be involved in the regulation of ABA sensing, as complete loss-of-function of the gene caused enhanced ABA response during germination and reduced transpiration in Arabidopsis (Running et al., 2004; Wang et al., 2005). In comparison to ERA1, AtFTA is thought to be functionally more essential in that it is the common subunit shared by farnesyltransferase and its related enzyme geranylgeranyltransferase type I, which uses a distinct β-subunit to form an active heterodimeric enzyme to catalyze a similar protein modification reaction (Caldelari et al., 2001; Lane and Beese, 2006). Like the era1 mutants, Arabidopsis AtFTA knockout mutants display various developmental defects including enlarged meristems, increased floral organ number, and delayed growth (Running et al., 2004). Therefore, the combination of the above results casts some doubts about the effectiveness of this α-subunit for genetic improvement of crop stress tolerance. In the present study, we set out to design a novel approach to separate the possible pleiotropic effects from the drought-tolerant properties displayed by the AtFTA loss-of-function mutants. This was achieved through the use of a novel Arabidopsis shoot-specific, drought-inducible promoter to down-regulate FTA expression in canola. Our data obtained from field trials indicated that there was no significant difference in growth and agronomic performance between the genetically engineered transgenic canola and its wild-type control. However, under moderate drought stress conditions at flowering, the transgenic plants produced significantly higher seed yield. Together with our previous success using ERA1 as a gene target for yield protection against drought, we have established a functional paradigm by using genes that control plant ABA sensing for improvement of crop productivity.
RESULTS AND DISCUSSION
In recent years, tremendous progress has been made towards the understanding of various molecular mechanisms that plants have evolved to cope with environmental stresses such as extreme temperatures, salinity, and drought (Thomashow, 1999; Zhu, 2002; Umezawa et al., 2006). Many components have been implicated in the signaling pathways of water stress response; however, relatively few of them have been successfully engineered to meet the crop agronomic performance standards for growth conditions in the field (Ishitani et al., 2004; Sinclair et al., 2004; Zhang et al., 2004). This is likely due to two main reasons. Firstly, as illustrated in the case of ERA1, most genes that are involved in the regulation of stress responses also have important functions in the regulation of multiple growth and developmental processes. Thus, constitutive loss-of-function of these genes would often result in severe growth retardation even under optimal growth conditions. Secondly, drought stresses occur mostly in a transient manner in the field, so, in order to minimize any possible metabolic trade-off, genetically modified plants need to include a sensitive molecular switch to cope with the changing environmental conditions when needed.
Under well-watered conditions, plants open their stomates in the light for maximum CO2 uptake for photosynthesis and close them in the dark to prevent water loss. However, plants have naturally developed an over-riding survival mechanism that will trigger stomatal closure even in the presence of light when a drought signal is perceived (Luan, 2002). This suggests that the use of a promoter that preferentially specifies the expression of a gene in the photosynthetic shoot tissues and is also inducible by drought could be efficacious in engineering drought protection through modulation of the ABA-mediated stomatal response. Using these criteria to search targets, we focused on a small group of genes in the photorespiratory pathway in Arabidopsis, as they express and function in concert with the C3 plants’ photosynthetic genes, and yet they have substantially lower basal levels of expression. We were able to identify a gene termed AtHPR1, which belongs to a single-gene family in Arabidopsis and encodes hydroxypyruvate reductase. In photorespiration, AtHPR1 converts hydroxypyruvate to glycerate, which is then shuttled from peroxisomes to chloroplasts for participation in the Calvin cycle.
To determine the precise expression pattern of AtHPR1, we performed Northern blot analyses with different wild-type Arabidopsis tissues. The results indicate that AtHPR1 expresses in all above-ground tissues with predominant mRNA accumulation in the photosynthetic tissues such as leaves and stem, and with minimal expression in the roots (Figure 1). Our results are consistent with microarray data from diverse organs in Arabidopsis, in which expression of AtHPR1 was absent in roots and highest in leaves and stems (Schmid et al., 2005). Interestingly, a relatively high level of AtHPR1 expression was detected in the reproductive tissues.
Figure 1.
Expression of AtHPR1 in Various Wild-Type Arabidopsis Tissues.
Northern blot analysis was performed using total RNA isolated from young leaf, fully developed leaf (old leaf), stem, flower bud, open flower, root and silique. The blot was probed with a [32P]-labeled, double-stranded, full-length AtHPR1 cDNA probe. The transcript levels were normalized using β-tubulin as an internal control.
There is a 487-bp stretch of intergenic genomic sequence between the TGA stop codon of the gene encoding a phosphatidylinositol synthase and the ATG start codon of AtHPR1 on chromosome 1 of Arabidopsis (Figure 2A, additional genomic sequence information available at www.arabidopsis.org/servlets/sv). We identified an Arabidopsis T-DNA insertion line with the insertion site 256-bp upstream of the AtHPR1 start codon. Northern expression analysis indicates that there was an approximately 50% reduction in AtHPR1 mRNA transcript in leaves of this T-DNA insertion line in comparison to that of the wild-type (data not shown). Furthermore, in vitro enzymatic assay results indicated that the T-DNA insertion caused about a 45% reduction in HPR activity in the leaves (data not shown). The positive correlation between the reduction in AtHPR1 transcript and its enzymatic activity in the T-DNA insertion line suggests that the 487-bp genomic sequence contains the promoter elements of AtHPR.
Figure 2.
Sequence Structure of the Arabidopsis AtHPR1 Promoter and Expression Analysis of AtHPR1.
(A) Several cis-acting elements in the nucleotide sequence of the AtHPR1 promoter were identified using the computer program PLACE. The two light-regulatory elements (I-box) are highlighted in red, the two Dof motifs are highlighted in purple, a core drought-responsive element (DRE) is marked with blue, and the ATG start codon of AtHPR1 is highlighted in green. The putative root expression repressing region of the promoter is highlighted in grey.
(B–E) show the histochemical localization of GUS expression in the T2 seedling of Arabidopsis driven by the CaMV 35S promoter (B), the full-length AtHPR1 promoter (C), the truncated AtHPR1 promoter (D), and in the wild-type and the T1 seedling of AtHPR1::GUS transgenic canola (E).
(F) Change in expression of AtHPR1 (open square), RD29A (open circle), and AtNCED3 (solid circle) in the leaf of wild-type Arabidopsis during a course of drought stress. Total RNA was isolated from the leaves of the plants on each of the water-withholding days for Northern blot analysis. The blot was probed with specific radio-labeled AtHPR1, RD29A, and AtNCED3 double-stranded cDNA probes. The transcript levels were normalized using β-tubulin as an internal control.
An HPR promoter isolated from cucumber has previously been characterized (Schwartz et al., 1991); however, comparison between the promoters of AtHPR1 and the cucumber HPR indicates that there is little sequence homology. Promoter motif analysis was performed on the AtHPR1 promoter using the program PLACE (Higo et al., 1999), and the results indicate the presence of several plant cis-acting regulatory DNA elements (Figure 2A). Among them, the I-box, which was defined as GATAA, was reported to be the regulatory element that is functionally important in many light-regulated promoters of both monocot and dicot species (Terzaghi and Cashmore, 1995). The Dof motif, which is defined as AAAG, was identified by functional analysis as the essential sequence element for binding by the DNA-Dof proteins, which are plant-specific transcription factors involved in diverse plant-specific biological processes, such as light-induction and tissue-specific gene expression (Yanagisawa, 2004). Experimental evidence accumulated so far suggests that hydroxypyruvate reductase is light-inducible. According to Michael et al. (2008), the expression level of AtHPR1 peaks 4 h after exposure to light, with a 1.5-fold induction compared to the control. In contrast, within 4 h of darkness, the expression level drops, and then slowly recovers. In agreement with this finding, AtHPR1 expression was induced between 1.5 and 3.5-fold by blue, far red, red, UV-A, UV-AB, and white light (Kilian et al., 2007).
In order to examine the pattern of gene expression regulated by the AtHPR1 promoter, two GUS constructs driven by either the full-length or approximately half of the length (487 and 279 bp upstream of the AtHPR1 translation start codon ATG, respectively) of the AtHPR1 promoter were transformed in Arabidopsis and canola. A GUS construct driven by the CaMV 35S promoter was also introduced into Arabidopsis for comparative gene expression study. Histochemical analysis indicated that GUS expression driven by the 35S promoter was strong in all tissues of Arabidopsis, especially in the root (Figure 2B). Although not as intensive, GUS expression was detected in the rosette leaves of transgenics of both full-length and partial-length AtHPR1 promoter–GUS constructs (Figure 2C and 2D). GUS expression driven by the full-length AtHPR1 promoter in the transgenic Arabidopsis and canola plants was detected only in the shoots and not in the roots (Figure 2C and 2E). Interestingly, removal of the first 208 bp (highlighted in grey in Figure 2B) from the AtHPR1 promoter resulted in GUS expression in the root (Figure 2D). This suggests that a root expression repressing element might be present within the 208-bp sequence, as observed in the case of the cucumber HPR promoter (Daniel and Becker, 1995).
The DRE motif is defined as the dehydration response element and is present in the promoter regions of many drought and cold-inducible genes (Yamaguchi-Shinozaki and Shinozaki, 1994). Due to the presence of a core DRE motif, ACCGAC, in the putative AtHPR promoter region (Figure 2A), we decided to examine whether the AtHPR1 expression was inducible by drought stress. In this study, wild-type Arabidopsis plants were grown under optimal conditions in the growth chamber for 3 weeks before withholding water. RNA was extracted from leaf samples for Northern blot analysis on each of the 6 consecutive days after withholding water. Two days after water was withdrawn, a steady increase in the transcripts of RD29A and AtNCED3 was found (Figure 2F). These two genes are known to be inducible by drought stress (Yamaguchi-Shinozaki and Shinozaki, 1993; Iuchi et al., 2001), and the above result suggests that the plants started to experience water deficiency after 2 days. As drought stress progressed, the AtHPR1 expression started to increase on day 2 and reached a maximum of about a two-fold increase on day 4 before declining gradually over the next 2 days to the basal level (Figure 2F). This suggests the AtHPR expression is up-regulated by drought; however, the kinetics of the stress-inducible expression is different from that of other drought-inducible genes such as RD29A and AtNCED3. As observed in the light studies, the expression of AtHPR1 appears to be first up-regulated by the environmental cues, followed by a decrease through an unknown mechanism. Although expression of AtHPR1 was not inducible under all types of drought-imposed conditions such as desiccation of plants on filter paper, it was found to be up-regulated when drought was applied through the addition of mannitol (200 mM), or by withdrawing water (Seki et al., 2002; Kreps et al., 2002). For example, when drought stress was applied by withholding water until the relative water content was 70%, the expression level of HPR increased 1.6-fold compared to non-stressed plants (Rizhsky et al., 2004).
In order to examine the possible effects of conditional, tissue-specific down-regulation of FTA in canola for improved drought tolerance using the AtHPR1 promoter, we first cloned the α-subunit of farnesyltransferase from Brassica napus (BnFTA) using RT–PCR with a pair of primers generated from sequence homology comparison between FTA from Arabidopsis and other species (sequence data not shown). Results of a pairwise sequence alignment analysis using the Clustal W2 program (EMBO-EBI) indicated that the BnFTA gene is highly homologous to AtFTA, showing 88 and 87% identity in nucleotide and amino acid sequences, respectively. Because canola is an amphidiploid rapeseed species formed by the cross between the genomes of Brassica oleracea and Brassica rapa (Rakow, 2004), we went on to examine the possible number of expressed FTA orthologs in this species. Blast search against the 596 240 EST sequences deposited in NCBI's Brassica napus EST database (www.ncbi.nlm.nih.gov/dbEST/) identified multiple BnFTA ESTs that assembled into a single BnFTA sequence, suggesting there is only one active form of FTA in canola.
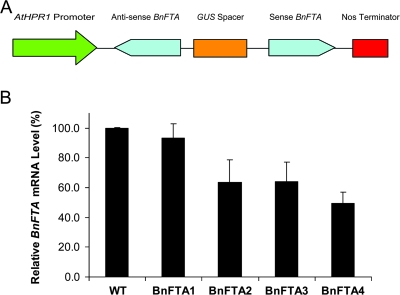
An AtHPR1 promoter-driven BnFTA hairpin RNAi expression cassette was assembled (Figure 3A) and subsequently transformed into the double-haploid canola variety (DH12075). A total of 13 independent, single-locus insertion transgenic lines were generated and advanced to homozygozity (the transgenic lines were designated as BnFTA1 to 13). ABA germination tests were performed according to the protocol described previously (Wang et al., 2005), and the results indicated that there was no significant difference in the ABA sensitivity between these transgenics and the DH12075 control (data not shown). This suggests that either the AtHPR1 promoter does not operate in the germinating seeds or the level of BnFTA down-regulation is not enough to trigger an enhanced ABA response in the tissues. Indeed, it is known that there is little photorespiratory activity in the peroxisomes of germinating seeds (Pracharoenwattana and Smith, 2008). The use of the AtHPR1 promoter might offer a means to restrict the expression of a transgene in the tissues where transpiration actually takes place.
Figure 3.
Shoot-Specific Down-Regulation of BnFTA in Canola.
(A) Diagram of the AtHPR::hairpin-BnFTA construct.
(B) Northern analysis of relative levels of endogenous BnFTA transcript in the wild-type (DH12075) and four T4 homozygous transgenic canola lines (BnFTA1–4). The blots were probed with a double-stranded BnFTA cDNA probe and the transcript levels were normalized using β-tubulin as an internal control. The error bars represent the standard errors from triplicate samples in three independent experiments.
We selected 4 transgenic canola lines, BnFTA1, BnFTA2, BnFTA3, and BnFTA4, for further molecular and agronomic analysis. Northern blot analysis with total RNA isolated from the developing young leaves of plants grown under optimal conditions was carried out to detect changes in the BnFTA transcript level in these 4 transgenic lines as well as the wild-type control. As shown in Figure 3B, the BnFTA transcript level was reduced by 6–50% in the transgenic lines, suggesting that the hairpin RNAi construct worked effectively to reduce the target gene expression.
In comparison to the wild-type Arabidopsis plants, transgenic Arabidopsis with constitutively down-regulated AtFTA showed an enhanced ABA response at germination and a significant reduction in water loss during a drought treatment (Wang, et al., 2005). This suggests that, like ERA1, FTA might also function to modulate a plant's ABA-mediated stomatal response to control the rate of transpiration. Indeed, gas-exchange experiments were carried out on the wild-type and BnFTA RNAi canola plants at the sixth leaf stage in the growth chamber, and the transgenic lines showed a clear trend of greater reduction in stomatal conductance and transpiration rate under drought conditions (data not shown).
Although yield is the single most important trait for a crop, it is seldom used as the performance criterion for assessment of drought tolerance conducted in laboratories. This is partly due to the fact that yield is a complex trait that is difficult to be accurately quantified, especially when biological replication is limited. In order to be able to assess the yield performance of the AtHPR1 promoter-hairpin BnFTA transgenic canola under different watering conditions, standard confined canola yield trials were carried out in the summer of 2006 in 2 separate locations (Kipp and Taber) in Alberta, Canada. The 4 transgenic lines, BnFTA1 to 4, were tested along with their wild-type parental control (DH12075). The seasonal temperatures and the amounts of water received from natural precipitation and irrigation at the 2 sites during the trials are summarized in Table 1. It appears that it was a hot season for both Kipp and Taber, as the monthly maximum temperatures for the growth season were higher than that of the 30-year averages. Both locations received noticeably more rainfall in the month of June, just before flowering. However, during the flowering, grain filling, and seed maturation months of July through September, the fields in Kipp and Taber only received a total of 13 and 38 mm of precipitation, respectively, instead of their average amounts of 120 and 134 mm (Table 1A and 1B). The drier than normal weather conditions during the time of flowering in July allowed us to manipulate the amounts of irrigation so that possible differences in yield performance could be observed. As shown in Table 1A, the limited irrigation field in Kipp received 51 mm of water in July, while the optimal irrigation field in the same location received double that amount of water (102 mm). Due to the higher ground moisture level in Taber, no irrigation was applied to the limited irrigation field, while its optimal irrigation field received 38 mm of water in July (Table 1B).
Table 1.
Summary of the climatic conditions during the 2006 summer field trials in Kipp (A) and Taber (B) of Alberta, Canada. The data were provided by the Department of Environment Canada. The ‘normal’ temperature and precipitation represent the average values from the past 30 years in the specified locations.
| A | ||||||
| Maximum temperature |
Precipitation |
Irrigation Treatments |
||||
| Normal | Actual | Normal | Actual | Optimal | Limited | |
| Month | (oC) | (oC) | (mm) | (mm) | (mm) | (mm) |
| May | 18.4 | 22 | 51 | 30 | ||
| June | 22 | 22 | 75 | 103 | ||
| July | 24.8 | 29 | 39 | 5 | 102 | 51 |
| August | 24.9 | 27 | 45 | 7 | ||
| September | 19.3 | 28 | 36 | 1 | ||
| Total | 145 | 102 | 51 | |||
| B | ||||||
| Maximum temperature |
Precipitation |
Irrigation Treatments |
||||
| Normal | Actual | Normal | Actual | Optimal | Limited | |
| Month | (oC) | (oC) | (mm) | (mm) | (mm) | (mm) |
| May | 18 | 24 | 54 | 21 | 38 | 38 |
| June | 22 | 24 | 63 | 114 | ||
| July | 26 | 29 | 48 | 10 | 38 | |
| August | 25 | 27 | 46 | 28 | ||
| September | 20 | 28 | 40 | 0 | ||
| Total | 172 | 76 | 38 | |||
Statistical analysis of the final seed yields from both sites indicated that under optimal irrigation conditions, no significant difference between the four transgenic canola lines and DH12075 was found (Figure 4). Reduction in irrigation in Kipp resulted in a 21% decrease in yield of the control, while the yield of the BnFTA transgenic lines was only reduced by 1–11% (Figure 4A). Under limited irrigation conditions, lines BnFTA1, BnFTA2, and BnFTA3 showed a significantly (p < 0.1) higher yield of 11–20% than that of the control. In Taber, reduction in irrigation caused an 18% yield reduction for the control, and 10 and 7% reduction for BnFTA1 and BnFTA3, respectively. There was no reduction for BnFTA2, and BnFTA4, in fact, showed 5% increase in yield under limited irrigation conditions as compared to that under optimal conditions (Figure 4B). Under limited irrigation conditions, the yields of BnFTA1, BnFTA2, and BnFTA4 were 14–16% higher than the control, and the differences were statistically significant (p < 0.1). Together, these results suggest that water deficit stress during flowering and pod filling had a significantly greater negative impact on the yield of the control than that of the BnFTA transgenic lines. The introduction of the hairpin BnFTA construct did not provide a yield drag under well-watered conditions; however, it prevented the transgenic plants from having a significant yield reduction when the availability of water in the field became limited.
Figure 4.
Seed Yield (kg ha−1) of DH12075 Wild-Type Canola (WT) and Independent Transgenic Lines of BnFTA1, BnFTA2, BnFTA3, and BnFTA4 in the 2006 Summer Field Trials Conducted in Kipp (A) and Taber (B).
The open bars represent mean seed yield for the optimal irrigation conditions, and the solid bars represent mean seed yield for the limited irrigation conditions for each entry. Standard errors of six replicates per field trial entry are shown for each mean. Asterisks above a bar indicate a significant difference (p < 0.1) between the trangenic BnFTA lines and the wild-type canola (LSD test).
In the current study, the BnFTA expression levels in the transgenic canola plants during drought stress in the field were not monitored. Under optimal growth conditions, there were various degrees of reduction in BnFTA transcript in the transgenic lines (Figure 3B), and we presume further reduction in the expression levels would occur under water-stressed conditions because of the drought inducibility of the AtHPR1 promoter. However, additional work is required to correlate the level of the BnFTA expression with some key physiological indicators such as stomatal conductance and transpiration rate during drought stress. Interestingly, it has been observed that under both optimal and drought conditions, small reductions in stomatal conductance and transpiration rate, as a result of enhanced ABA sensitivity, do not necessarily affect photosynthetic rate significantly in the drought-tolerant SNAC1-overexpressing rice (Hu et al., 2006). This suggests that plants might transpire more than necessary and stomatal apertures could be slightly reduced without having an overall effect on the rate of CO2 uptake and growth of plants. Consistent with this, no significant pleiotropic phenotypes were detected in the BnFTA transgenic canola lines, suggesting that the reduction in BnFTA expression under optimal growth conditions was not enough to impact plant growth and development. In addition, because these results show yield protection against drought stress during flowering and pod filling in 2 different field locations and in 4 independent transgenic events, it is concluded that the tissue-specific, conditional reduction in BnFTA via the AtHPR1 promoter was successful in engineering drought tolerance into canola without a negative impact on seed yield under water sufficient conditions.
Figure 5 shows a schematic model of the use of the guard cell ABA response regulators for enhanced drought tolerance. ERA1 is one target that has previously been validated in transgenic canola in the field (Wang et al., 2005). Results from the present study suggest FTA could also be effective if the expression of the transgene is controlled by a conditional promoter. As described above, a large number of genes have now been identified to act on different signaling pathways for modulation of the ABA-mediated stomatal response and transpiration. Careful selection and genetic engineering with these ABA sensing regulators will help solve the perennial problem of drought stress in many crops so that agricultural productivity can be increased.
Figure 5.
A Schematic Model for Engineering Drought Tolerance and Yield Protection through Genetic Modulation of ABA-Mediated Stomatal Response.
Enhanced ABA responsiveness in the guard cells can be achieved through inhibition of either the α-subunit (FTA) or the β-subunit (ERA1) of farnesyltransferase, or by down-regulation of the unknown substrates of farnesyltransferase (CaaX proteins). A number of positive and negative regulators of ABA hypersensitivity have been identified so for; however, it is not clear how they work together to regulate ABA sensing. Enhancement in guard cell ABA response can promote stomatal closure and direct reduction of transpiration, resulting in increased plant drought tolerance.
METHODS
Plant Growth and Drought Stress Treatment
The Columbia ecotype of Arabidopsis thaliana was used as wild-type and is the background of an Arabidopsis T-DNA insertion line identified in-house. The T-DNA insertion site was identified to be 256 bp upstream of the ATG start codon of the gene encoding HPR by the PCR-based genome walking method (Huang, 1994), and the line was subsequently advanced to the T3 homozygous generation. Brassica napus (canola cv. DH12075) was used as wild-type and is the background of all transformed canola constructs. Normally, plants were grown in a controlled environment growth chamber (BioChambers, Winnipeg, MB, Canada) at 22°C, 70% relative humidity on a daily 16 h light/8 h dark cycle. Wild-type Arabidopsis plants subjected to the drought stress treatment were grown in 3-inch pots under optimal conditions to the early bolting stage before water was withheld for 6 consecutive days.
Cloning and Plant Transformation
For the GUS reporter constructs, the full-length and the partial AtHPR1 promoters (intergenic genomic sequences of 487 and 279 bp upstream of the ATG start codon of AtHPR, respectively) plus the first 6 bp of AtHPR1 coding sequence were fused with the GUS reporter gene in the promoterless binary plasmid pBI101 (Clontech, Palo Alto, California). These constructs and the plasmid pBI121 containing the GUS reporter gene driven by the 35S promoter were transformed into Arabidopsis wild-type plants by the floral dipping method (Clough and Bent, 1998). For the hairpin BnFTA construct, first, a near full-length BnFTA sequence was isolated by RT–PCR and its sequence identity was further confirmed by sequence alignment analysis against other known plant FTA sequences. A 530-bp DNA fragment encoding partial-length BnFTA was then placed directly downstream of the AtHPR promoter in sense and antisense orientations spaced by a truncated GUS gene as previously described (Chuang and Meyerowitz, 2000). Thus, the 35S:GUS fragment of pBI121 was replaced by this hairpin BnFTA fragment. The resultant plasmid was subsequently transformed into canola as described (Moloney et al., 1989).
RNA Gel Blot Analysis
Plant total RNA was isolated from various Arabidopsis and canola tissues using the RNeasy Plant Mini Kit (Qiagen, Mississauga, ON, Canada), separated by agarose gel electrophoresis, and blotted to a nylon membrane (Roche) as described (Ausubel et al., 1996). The blots containing the Arabidopsis and canola samples were hybridized in Clontech's ExpressHyb solution with a [32P]-labeled, double-stranded AtHPR1 or BnFTA cDNA probe, respectively. Blots containing samples isolated from the drought-treated Arabidopsis tissues were stripped and probed with radio-labeled AtNCED3 first and subsequently with RD29A cDNA probes. The signals were quantified using the Personal Molecular Imager system (Bio-Rad, Hercules, CA, USA). The relative expression levels were calculated by normalizing each signal against that of β-tubulin from the respective species.
Histochemical GUS Expression
Plants from the T2 generation of transgenic Arabidopsis and T1 transgenic Canola along with their wild-type control samples harvested at various stages of development were incubated in the GUS staining solution (50 mM NaPO4, pH 7.0, 10 mM EDTA; 0.1% v/v Triton X-100; 0.5 mg ml−1 5-bromo-4-chloro-3-indolyl-β-glucuronic acid) at 37°C for 16 h. The samples were fixed in with 3.7% formaldehyde and 50% ethanol for 30 min before further chlorophyll clearing by incubation in 50–100% ethanol. Localization of the blue precipitate in these tissues was observed using a Leica MZ 12.5 stereo microscope.
Confined Canola Field Trials
The bulk-harvested T4 homozygous seeds of transgenic lines along with the same generation seeds of the non-transgenic parent DH12075 were subjected to confined canola field trials. The trials were conducted simultaneously in 2 separate sites located at Kipp and Taber of Alberta, Canada, by Ag-Quest Inc. in the summer of 2006. Each site contained 2 identical fields to test the effects of different irrigation treatments on canola growth and yield. Each field was arranged in a randomized complete block design containing 6 replicated plots per trial entry. Each replicate contained approximately 1800 plants and was grown in a plot of 1.5 × 6.7 m in size with eight rows spaced 0.15 m apart. In Kipp, the fields for both optimal and limited watering treatments were irrigated with 51 mm of water on 5 July, and an additional 51 mm of water was applied only to the optimal irrigation field 2 weeks later. In Taber, both the optimal and limited irrigation fields were watered with 38 mm of water on 25 May, and an additional 38 mm of water was applied only to the optimal irrigation field on 8 July. During the field trials, recommended local crop management practices were employed to achieve maximum yield. Seedling vigor, plant stand establishment, days to flowering, plant height, lodging, and days to maturity were measured. Upon maturation, seeds were harvested from the middle 7.5 m2 area of each plot to determine seed yield (kg ha−1). Seasonal temperatures and total rainfall during the entire growth season in the field trial locations were recorded.
Statistical Analysis
All field data were subjected to separate analyses of variance (ANOVAs) using nearest neighbour analysis (release 5.1.2600, v.8.00, SAS Institute Inc., Cary, NC). The data collected from each irrigation treatment at each location were analyzed separately. Statistical analyses were performed, assuming that the experimental effects followed a linear model, that experimental errors were normally distributed, random, and had a common variance and zero mean, and that environmental effects were additive. Variation was partitioned into block and line effects or line, block, and plot effects. Residuals were combined over lines and analyzed to confirm that all error assumptions (homogeneous, normally distributed and sum to zero) were met. The randomness and independence of residuals were assessed by visual inspection of residual versus predicted, residual versus block, residual versus line, and residual versus plot graphs. Residual normality was evaluated using the Shapiro-Wilk goodness-of-fit test. Outliers were detected using Studentized residuals and Lund's critical value. If all error assumptions were met, LSD tests at α = 0.1 were conducted to identify significant differences between the means of transgenics and DH12075 for each agronomic trait measured.
Accession Numbers
The AGI locus accession numbers for the Arabidopsis genes discussed in this manuscript are At1g68010 for AtHPR1, At3g14440 for AtNCED3 and At5g52310 for RD29A.
Acknowledgments
The authors would like to thank Dr Gerhard Rakow of Agriculture and AgriFood Canada, Saskatoon, for providing the elite canola seed of DH12075 for our transformation. We also like to thank Dr Barbara Vanderbeld and Ms Delina Melo for proofreading of the manuscript. No conflict of interest declared.
References
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell. 2002;14:1649–1662. doi: 10.1105/tpc.010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1996. [Google Scholar]
- Boyer JS. Plant productivity and environment. Sci. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Caldelari D, Sternberg H, Rodríguez-Concepción M, Gruissem W, Yalovsky S. Efficient prenylation by a plant geranylgeranyltransferase-I requires a functional CaaL box motif and a proximal polybasic domain. Plant Physiol. 2001;126:1416–1429. doi: 10.1104/pp.126.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champolivier L, Merrien A. Effects of water stress applied at different growth stages to Brassica napus L. oleifera on yield, yield components and seed quality. Eur. J. Agron. 1996;5:153–160. [Google Scholar]
- Chen Z, Zhang H, Jablonowski D, Zhou X, Ren X, Hong X, Schaffrath R, Zhu J-K, Gong Z. Mutations in ABO1/ELO2, a subunit of holo-elongator, increase abscisic acid sensitivity and drought tolerance in Arabidopsis thaliana. Mol. Cell. Biol. 2006;26:6902–6912. doi: 10.1128/MCB.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C, Meyerowitz EM. Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc. Natl Acad. Sci. U S A. 2000;97:4985–4990. doi: 10.1073/pnas.060034297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farneyl transferase involved in abscisic acid signal transduction in Arabidopsis. Sci. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- Daniel SG, Becker WM. Transgenic analysis of the 5′- and 3′-flanking regions of the NADH-dependent hydroxypyruvate reductase gene from Cucumis sativus L. Plant Mol. Biol. 1995;28:821–836. doi: 10.1007/BF00042068. [DOI] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell. 1999;11:1897–1909. doi: 10.1105/tpc.11.10.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xiong L, Song C, Gong D, Halfter U, Zhu J-K. A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signaling in Arabidopsis. Dev. Cell. 2002;3:233–244. doi: 10.1016/s1534-5807(02)00229-0. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl Acad. Sci. U S A. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH. Inverse polymerase chain reaction: an efficient approach to cloning cDNA ends. Mol. Biotechnol. 1994;2:15–22. doi: 10.1007/BF02789286. [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell. 2001;106:477–487. doi: 10.1016/s0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Ishitani M, Rao I, Wenzl P, Beebe S, Tohme J. Integration of genomics approach with traditional breeding towards improving abiotic stress adaptation: drought and aluminum toxicity as case studies. Field Crops Res. 2004;90:35–45. [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong J-J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008;146:623–635. doi: 10.1104/pp.107.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, et al. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007;50:347–363. doi: 10.1111/j.1365-313X.2007.03052.x. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, Hilhorst HWM, Karssen CM. In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol. 1989;90:463–469. doi: 10.1104/pp.90.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 1984;61:377–383. [Google Scholar]
- Kreps JA, Wu Y, Chang H-S, Zhu T, Wang X, Harper JF. Transcriptome changes for arabidopsis in response to salt, osmotic, and cold Stress. Plant Physiol. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane KT, Beese LS. Structural biology of protein farnesyltransferase and gernanylgeranyltransferase type I.J. Lipid Res. 2006;47:681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Luan S. Signaling drought in guard cells. Plant Cell Environ. 2002;25:229–237. doi: 10.1046/j.1365-3040.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- Michael MP, Breton G, Hazen SP, Priest H, Mockler TC, Kay SA, Chory J. A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Bio. 2008;6:e225. doi: 10.1371/journal.pbio.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingeau M. Comportement du colza de printemps a la secheresse. Inf. Tech. 1974;36:1–11. Paris. [Google Scholar]
- Moloney MM, Walker JM, Sharma KK. High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep. 1989;8:238–242. doi: 10.1007/BF00778542. [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Sci. 1998;282:287–290. doi: 10.1126/science.282.5387.287. [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I, Smith SM. When is a peroxisome not a peroxisomes? Trends Plant Sci. 2008;13:522–525. doi: 10.1016/j.tplants.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Rakow G. Species origin and economic importance of Brassica. In: Pua EC, Douglas CJ, editors. Biotechnology in Agriculture and Forestry. Vol. 54. New York: Springer-Verlag Berlin Heidelberg; 2004. pp. 3–11. [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. When defense pathways collide: the response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004;134:1683–1696. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Running MP, Lavy M, Sternberg H, Galichet A, Gruissem W, Hake S, Ori N, Yalovsky S. Enlarged meristems and delayed growth in plp mutants result from lack of CaaX prenyltransferases. Proc. Natl Acad. Sci. U S A. 2004;101:7815–7820. doi: 10.1073/pnas.0402385101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A, Robert N, Maktabi MH, Schroeder JI, Serrano R, Rodriguez PL. Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatases type 2C ABI1 and HAB1. Plant Physiol. 2006;141:1389–1399. doi: 10.1104/pp.106.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nat. Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- Schwartz BW, Sloan JS, Becker WM. Characterization of genes encoding hydroxypyruvate reductase in cucumber. Plant Mol. Biol. 1991;17:941–947. doi: 10.1007/BF00037078. [DOI] [PubMed] [Google Scholar]
- Seki M, et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002;31:279–292. doi: 10.1046/j.1365-313x.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- Sinaki JM, Heravan EM, Rad AHS, Noormohammadi G, Zarei G. The effects of water deficit during growth stages of canola (Brassica napus L.). American-Eurasian J. Agric. Environ. Sci. 2007;2:417–422. [Google Scholar]
- Sinclair TR, Purcell LC, Sneller CH. Crop transformation and the challenge to increase yield potential. Trends Plant Sci. 2004;9:70–75. doi: 10.1016/j.tplants.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Terzaghi WB, Cashmore AR. Light-regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:445–474. [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Tollenaar M, Wu J. Yield improvement in temperate maize is attributable to greater stress tolerance. Crop Sci. 1999;39:1597–1604. [Google Scholar]
- Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K. Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr. Opin. Biotech. 2006;17:113–122. doi: 10.1016/j.copbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J. 2005;43:413–424. doi: 10.1111/j.1365-313X.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol. Gen. Genet. 1993;236:331–340. doi: 10.1007/BF00277130. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S. Dof domain proteins: plant-specific transcription factors associated with diverse phenomena unique to plants. Plant Cell Physiol. 2004;45:386–391. doi: 10.1093/pcp/pch055. [DOI] [PubMed] [Google Scholar]
- Zhang J, Creelman RA, Zhu J-K. From laboratory to field: using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiol. 2004;135:615–621. doi: 10.1104/pp.104.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang C, Li Y, Zheng N, Chen H, Zhao Q, Gao T, Guo H, Xie Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell. 2007;19:1912–1929. doi: 10.1105/tpc.106.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]