Abstract
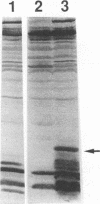
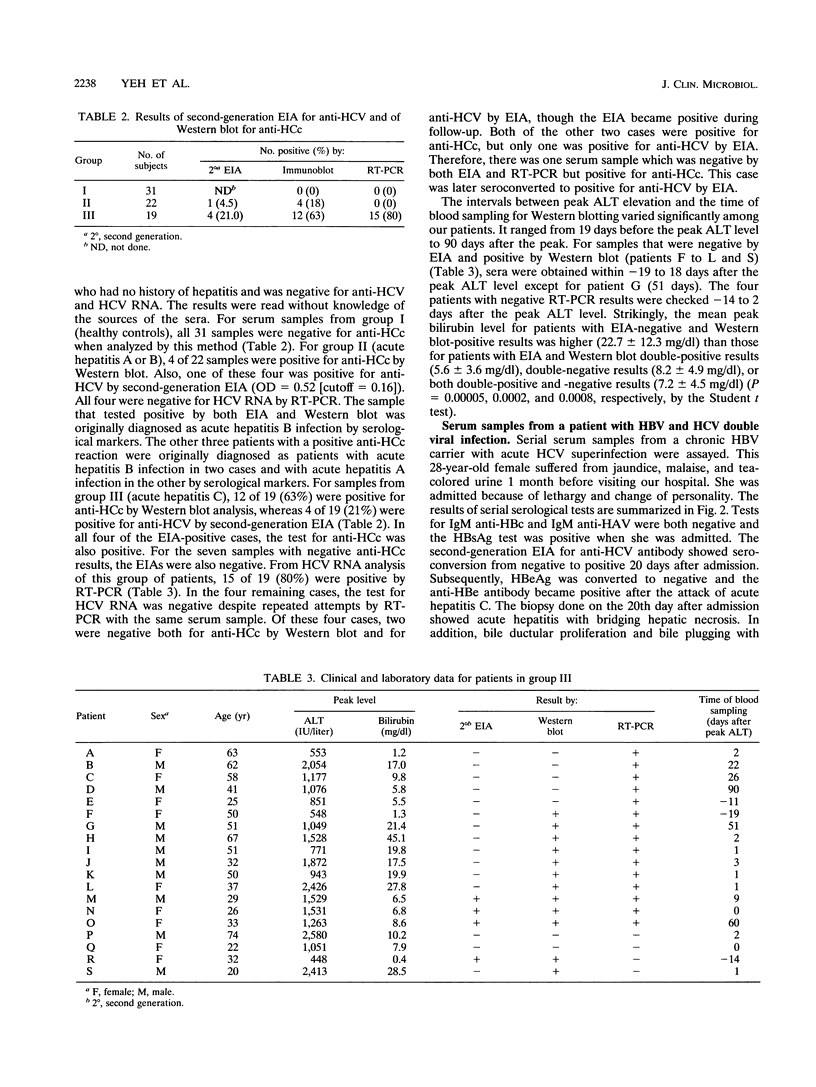
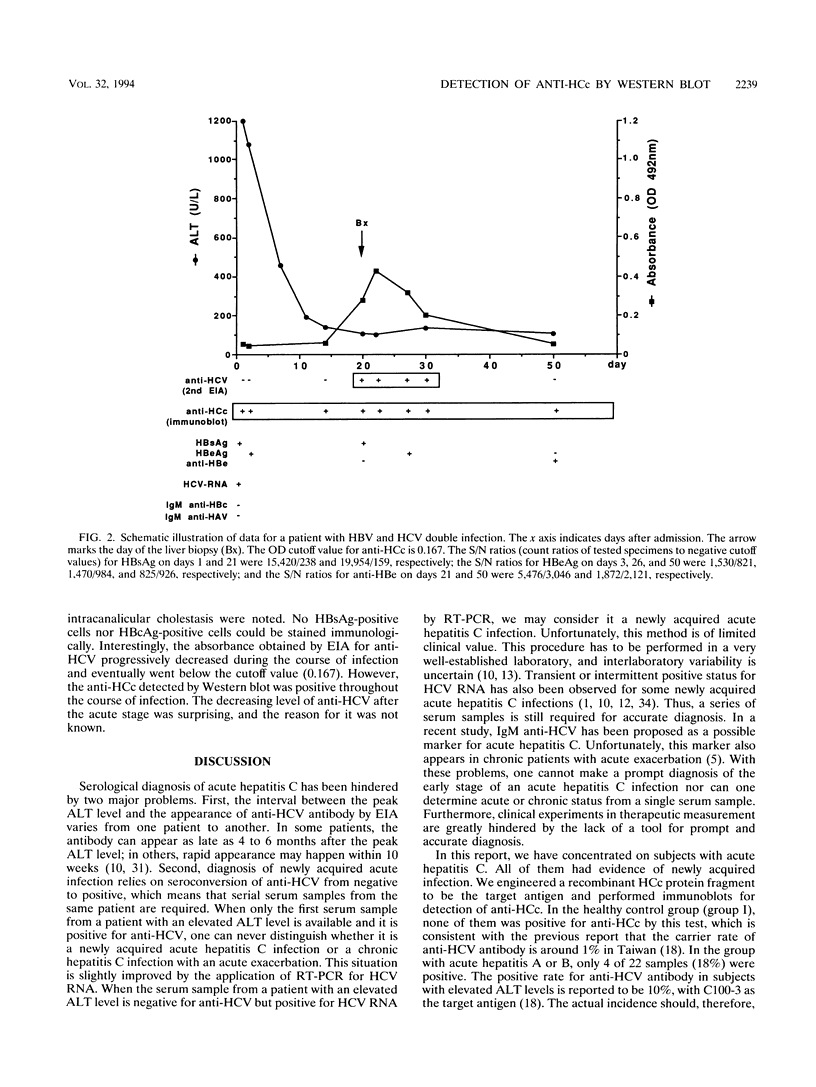
Crude extract from Escherichia coli which expressed a recombinant protein containing amino acids 2 to 127 of the hepatitis C virus (HCV) core protein was used to detect the antibody against HCV core protein (anti-HCc). After electrophoretic separation of proteins from the extract, Western blot (immunoblot) analysis was performed with the serum samples. This method was compared with a commercially available second-generation enzyme immunoassay (EIA) which employed synthetic peptides corresponding to highly antigenic segments of both structural and nonstructural portions of HCV. Also, reverse transcription PCR for HCV RNA was used for comparison. Seventy-two serum samples from three groups of patients were tested. Groups I and II represented healthy subjects and subjects with acute hepatitis A or B, respectively. Group III included patients with newly acquired acute hepatitis C. By Western blot analysis, 31 of 31 (100%) samples from group I were negative for anti-HCc antibody, whereas 4 of 22 (18%) samples from group II were positive for anti-HCc. One of these four samples was also positive for anti-HCV antibody by the second-generation EIA (1 of 22 [4.5%]). Among 19 patients diagnosed with newly acquired acute hepatitis C, 4 (21%) were positive for anti-HCV by the second-generation EIA, whereas 12 of 19 (63%) were positive for anti-HCc by Western blot analysis. Of EIA-positive subjects, 4 of 4 (100%) were also positive for anti-HCc by Western blot analysis, whereas among EIA-negative subjects, 8 of 15 (53%) were positive. For HCV RNA detected by reverse transcription PCR, 15 of 19 (80%) of this group of samples were positive. Strikingly, the peak bilirubin level for patients with EIA-negative and Western blot-positive results is significantly higher than that for patients with consistent EIA and Western blot results (22.7 versus 7.2 mg/dl). A series of serum samples from a patient with concurrent hepatitis B and C viral infection was also studied by both tests. Although anti-HCc persisted throughout the course of infection, anti-HCV by EIA converted from negative to positive 20 days after admission and then converted back to negative 30 days later.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Inchauspe G., Shikata T., Prince A. M. Three different patterns of hepatitis C virus infection in chimpanzees. Hepatology. 1992 Apr;15(4):690–695. doi: 10.1002/hep.1840150423. [DOI] [PubMed] [Google Scholar]
- Bresters D., Cuypers H. T., Reesink H. W., Schaasberg W. P., van der Poel C. L., Mauser-Bunschoten E. P., Houghton M., Choo Q. L., Kuo G., Lesniewski R. Enhanced sensitivity of a second generation ELISA for antibody to hepatitis C virus. Vox Sang. 1992;62(4):213–217. doi: 10.1111/j.1423-0410.1992.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Lin M. H., Tai K. F., Liu P. C., Lin C. J., Chen D. S. The Taiwanese hepatitis C virus genome: sequence determination and mapping the 5' termini of viral genomic and antigenomic RNA. Virology. 1992 May;188(1):102–113. doi: 10.1016/0042-6822(92)90739-c. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Wang J. T., Hwang L. H., Yang Y. H., Hsieh C. L., Kao J. H., Sheu J. C., Lai M. Y., Wang T. H., Chen D. S. Transient immunoglobulin M antibody response to hepatitis C virus capsid antigen in posttransfusion hepatitis C: putative serological marker for acute viral infection. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5971–5975. doi: 10.1073/pnas.89.13.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba J., Ohba H., Matsuura Y., Watanabe Y., Katayama T., Kikuchi S., Saito I., Miyamura T. Serodiagnosis of hepatitis C virus (HCV) infection with an HCV core protein molecularly expressed by a recombinant baculovirus. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4641–4645. doi: 10.1073/pnas.88.11.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien D. Y., Choo Q. L., Tabrizi A., Kuo C., McFarland J., Berger K., Lee C., Shuster J. R., Nguyen T., Moyer D. L. Diagnosis of hepatitis C virus (HCV) infection using an immunodominant chimeric polyprotein to capture circulating antibodies: reevaluation of the role of HCV in liver disease. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10011–10015. doi: 10.1073/pnas.89.21.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Claeys H., Volckaerts A., De Beenhouwer H., Vermylen C. Association of hepatitis C virus carrier state with the occurrence of hepatitis C virus core antibodies. J Med Virol. 1992 Apr;36(4):259–264. doi: 10.1002/jmv.1890360405. [DOI] [PubMed] [Google Scholar]
- Dourakis S., Brown J., Kumar U., Karayiannis P., Kernoff P., Chiba J., Ohba H., Miyamura T., Saito I., Monjardino J. Serological response and detection of viraemia in acute hepatitis C virus infection. J Hepatol. 1992 Mar;14(2-3):370–376. doi: 10.1016/0168-8278(92)90185-r. [DOI] [PubMed] [Google Scholar]
- Garson J. A., Tuke P. W., Makris M., Briggs M., Machin S. J., Preston F. E., Tedder R. S. Demonstration of viraemia patterns in haemophiliacs treated with hepatitis-C-virus-contaminated factor VIII concentrates. Lancet. 1990 Oct 27;336(8722):1022–1025. doi: 10.1016/0140-6736(90)92487-3. [DOI] [PubMed] [Google Scholar]
- Gretch D., Lee W., Corey L. Use of aminotransferase, hepatitis C antibody, and hepatitis C polymerase chain reaction RNA assays to establish the diagnosis of hepatitis C virus infection in a diagnostic virology laboratory. J Clin Microbiol. 1992 Aug;30(8):2145–2149. doi: 10.1128/jcm.30.8.2145-2149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege A., Kurz M., Weinbeck M., Gerok W. Excretion of caffeine and its primary degradation products into bile. J Hepatol. 1993 Jan;17(1):67–73. doi: 10.1016/s0168-8278(05)80523-9. [DOI] [PubMed] [Google Scholar]
- Hosein B., Fang C. T., Popovsky M. A., Ye J., Zhang M., Wang C. Y. Improved serodiagnosis of hepatitis C virus infection with synthetic peptide antigen from capsid protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3647–3651. doi: 10.1073/pnas.88.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida C., Matsumoto K., Fukada K., Matsushita K., Shiraki H., Maeda Y. Detection of antibodies to hepatitis C virus (HCV) structural proteins in anti-HCV-positive sera by an enzyme-linked immunosorbent assay using synthetic peptides as antigens. J Clin Microbiol. 1993 Apr;31(4):936–940. doi: 10.1128/jcm.31.4.936-940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T., Mazda T., Kikuchi S., Harada S., Matsuura Y., Chiba J., Ohba H., Saito I., Miyamura T. Improved serodiagnosis of non-A, non-B hepatitis by an assay detecting antibody to hepatitis C virus core antigen. Hepatology. 1992 Mar;15(3):391–394. doi: 10.1002/hep.1840150306. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Lazizi Y., Elfassi E., Pillot J. Detection of hepatitis C virus sequences in sera with controversial serology by nested polymerase chain reaction. J Clin Microbiol. 1992 Apr;30(4):931–934. doi: 10.1128/jcm.30.4.931-934.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw Y. F., Lin S. M., Sheen I. S., Chu C. M. Acute hepatitis C virus superinfection followed by spontaneous HBeAg seroconversion and HBsAg elimination. Infection. 1991 Jul-Aug;19(4):250–251. doi: 10.1007/BF01644957. [DOI] [PubMed] [Google Scholar]
- Lin H. J., Shi N., Mizokami M., Hollinger F. B. Polymerase chain reaction assay for hepatitis C virus RNA using a single tube for reverse transcription and serial rounds of amplification with nested primer pairs. J Med Virol. 1992 Nov;38(3):220–225. doi: 10.1002/jmv.1890380312. [DOI] [PubMed] [Google Scholar]
- Nakatsuji Y., Matsumoto A., Tanaka E., Ogata H., Kiyosawa K. Detection of chronic hepatitis C virus infection by four diagnostic systems: first-generation and second-generation enzyme-linked immunosorbent assay, second-generation recombinant immunoblot assay and nested polymerase chain reaction analysis. Hepatology. 1992 Aug;16(2):300–305. doi: 10.1002/hep.1840160204. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S., Kuroki T., Ueda T., Fukuda K., Takeda T., Nakajima S., Shiomi S., Kobayashi K., Otani S., Hayashi N. Detection of hepatitis C virus antibody in the absence of viral RNA in patients with autoimmune hepatitis. Ann Intern Med. 1992 Jan 1;116(1):21–25. doi: 10.7326/0003-4819-116-1-21. [DOI] [PubMed] [Google Scholar]
- Nishizono A., Hiraga M., Mifune K., Terao H., Fujioka T., Nasu M., Goto T., Misumi J., Moriyama M., Arakawa Y. Correlation of serum antibody titers against hepatitis C virus core protein with clinical features by western blot (immunoblot) analysis using a recombinant vaccinia virus expression system. J Clin Microbiol. 1993 May;31(5):1173–1178. doi: 10.1128/jcm.31.5.1173-1178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Kurai K., Okada S., Yamamoto K., Lizuka H., Tanaka T., Fukuda S., Tsuda F., Mishiro S. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: comparative study of four distinct genotypes. Virology. 1992 May;188(1):331–341. doi: 10.1016/0042-6822(92)90762-e. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Scheuer P. J. Non-A, non-B hepatitis. Virchows Arch A Pathol Anat Histopathol. 1989;415(4):301–303. doi: 10.1007/BF00718632. [DOI] [PubMed] [Google Scholar]
- Stahl S., MacKay P., Magazin M., Bruce S. A., Murray K. Hepatitis B virus core antigen: synthesis in Escherichia coli and application in diagnosis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1606–1610. doi: 10.1073/pnas.79.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991 May 5;219(1):37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- Tremolada F., Casarin C., Alberti A., Drago C., Tagger A., Ribero M. L., Realdi G. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol. 1992 Nov;16(3):273–281. doi: 10.1016/s0168-8278(05)80657-9. [DOI] [PubMed] [Google Scholar]
- Yokosuka O., Ito Y., Sakuma J., Imazeki F., Ohto M., Omata M. Expression of hepatitis C virus core protein as a fusion protein with maltose binding protein. Detection of anti-hepatitis C core antibody by western blot. Dig Dis Sci. 1993 Apr;38(4):626–630. doi: 10.1007/BF01316791. [DOI] [PubMed] [Google Scholar]
- Yoshikawa A., Takahashi K., Kishimoto S., Tsuda F., Akahane Y., Naito S., Tanaka T., Yoshizawa H., Yamasaki M., Okamoto H. Serodiagnosis of hepatitis C virus infection by ELISA for antibodies against the putative core protein (p20c) expressed in Escherichia coli. J Immunol Methods. 1992 Apr 8;148(1-2):143–150. doi: 10.1016/0022-1759(92)90167-r. [DOI] [PubMed] [Google Scholar]
- Young K. K., Resnick R. M., Myers T. W. Detection of hepatitis C virus RNA by a combined reverse transcription-polymerase chain reaction assay. J Clin Microbiol. 1993 Apr;31(4):882–886. doi: 10.1128/jcm.31.4.882-886.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. Y., Kuramoto I. K., Mamish D., Sazama K., Holland P. V., Zeldis J. B. Hepatitis C virus in blood samples from volunteer donors. J Clin Microbiol. 1993 Mar;31(3):606–609. doi: 10.1128/jcm.31.3.606-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel C. L., Bresters D., Reesink H. W., Plaisier A. A., Schaasberg W., Leentvaar-Kuypers A., Choo Q. L., Quan S., Polito A., Houghton M. Early antihepatitis C virus response with second-generation C200/C22 ELISA. Vox Sang. 1992;62(4):208–212. doi: 10.1111/j.1423-0410.1992.tb01200.x. [DOI] [PubMed] [Google Scholar]