Abstract
Background & Aims
Cirrhosis is associated with prominent changes in sinusoidal structure and function. While the resident pericyte in liver, the hepatic stellate cell (HSC), is well characterized in the process of fibrogenesis, signaling pathways that regulate HSC vascular function are less developed. Since pericyte populations outside the liver are increasingly being recognized as a key cell-type for angiogenesis and changes in vascular structure, in this study, we explore new HSC signaling pathways that regulate sinusoidal structure and function.
Methods
Real-time video microscopy and quantitative software analysis of vascular tube formation were used to measure HSC angiogenesis in vitro. Platelet-derived growth factor (PDGF) and ephrin signaling pathways were modulated using molecular and pharmacologic techniques. Complementary whole animal studies were performed to correlate in vitro findings with pericyte functions in vivo.
Results
We show that PDGF promotes a phenotype of HSC evidenced by enhanced HSC driven vascular tube formation in vitro, and enhanced HSC coverage of sinusoids in vivo. This angiogenic phenotype modulates specific pericyte vascular functions including permeability and pressure regulation. Furthermore, we identify a key role for ephrin-B2 as a downstream effector of PDGF signaling.
Conclusion
These studies elucidate novel HSC signaling pathways that regulate microvascular structure and function in liver.
Keywords: Vascular remodeling, angiogenesis, portal hypertension, ephrin, platelet-derived growth factor
Introduction
The hepatic microcirculation, termed hepatic sinusoids, has unique anatomic and functional characteristics 1. For example, the sinusoids consist of specialized fenestrated endothelial cells which lack a basement membrane, thereby facilitating macromolecular transport to the parenchyma. In liver, the local pericyte populations are termed hepatic stellate cells (HSC). These cells embrace abluminal sinusoidal endothelial cells (SEC), by virtue of long cytoplasmic processes and extensions, which ideally position HSC for paracrine signaling with SEC. Profound changes occur within the sinusoidal vascular network, during chronic liver injury and cirrhosis 2. For example, the architecture of hepatic microvasculature in the cirrhotic liver is characterized by distorted vessels of varying diameter that perfuse nodules of parenchymal cells. Additionally, cirrhosis is associated with intrahepatic angiogenesis, the formation of new blood vessels from pre-existing vasculature especially within bands of fibrous scar 3. Further, SEC lose their fenestrations and develop a deficiency in the production of vasodilator molecules such as nitric oxide (NO) that culminates in endothelial dysfunction 4. This remodeling and reorganization of the sinusoidal structure contributes to increased hepatic vascular resistance and portal hypertension, a major cause of morbidity and mortality in patients with cirrhosis 2. Although major investigations of HSC in liver have been focused on their unique capacity to deposit collagen matrix and the ensuing wound healing response 5, the contribution of HSC to hepatic microvascular structure and function has only recently become recognized 1. Furthermore, the role of HSC in angiogenesis and vascular remodeling, though speculated 1, has remained largely undefined.
Pericyte populations outside the liver are increasingly recognized to play a central role in remodeling of microvessels. This occurs through signaling cross talk with neighboring endothelial cells, with failure of such signals leading to severe vascular defects 6. A key molecule responsible for endothelial cell/pericyte interactions is platelet-derived growth factor-B (PDGF), which is secreted by endothelial cells and binds to its cognate PDGF receptor (PDGFR-β) on pericytes. Thus, PDGF/PDGFR-β signaling is crucial for the recruitment of pericytes during angiogenesis and vascular remodeling 6, however relevant signaling mechanisms are yet to be fully defined. These concepts lead us to explore the role of HSC as a liver-specific pericyte with angiogenic properties that contribute to sinusoidal remodeling in liver.
In the present study, we show that PDGF promotes an angiogenic phenotype of HSC that regulates HSC driven vascular tube formation in vitro and enhanced coverage of sinusoids in vivo. These regulatory events influence vascular functions that are mediated through pericytes including vascular permeability and pressure regulation. Furthermore, we identify a novel and essential role for the multi-functional ephrin-B2 receptor tyrosine kinase in the process of angiogenic PDGF signaling. In summary, these studies identify HSC as a key target for modulating microvascular structure and function thereby highlighting an important and likely generalizable role for pericytes in these processes.
Methods
Cell Culture
Isolated primary human HSC and primary human SEC (ScienCell) were used between passages 2 and 6. Their phenotypic characterization has been previously described 7, 8. HSC and SEC were cultured in defined medium (ScienCell), and supplemented with 10% fetal bovine serum (where indicated), L-glutamine (1 mM), penicillin (100 IU/ml), and streptomycin (100 μg/ml). In some experiments, primary HSC were isolated from rats after sham or bile duct ligation (BDL) surgery, or after 6-week administration of carbon tetrachloride (CCl4; 1 mg/kg body weight) or vehicle 8.
Vascular tube formation assay
HSC were stained with CellTracker Green (Molecular Probes), while SEC were stained with CM-DiI Red (Molecular Probes) for 30 minutes, then washed with PBS, counted, and seeded on Matrigel-coated chamber slides. For co-culture experiments, the ratio of SEC and HSC was designed to simulate in vivo ratios of the cells in normal liver 1; 1 part HSC (3,000) and 3 parts SEC (9,000), with a total of 12,000 cells per well. Cells were placed together on 100 μl Matrigel after 30 minutes of preincubation at 37°C. Wells were photographed at different time points at random fields with the use of a confocal microscope (Zeiss LSM Pascale Axiovert). The length of the vascular tubes after 16 hours was digitally analyzed using the software AnalySISD (Olympus Biosystems). In some experiments, HSC were incubated with PDGF-BB (0.1–25 ng/ml) and/or the PDGF receptor inhibitor, imatinib (0.1–25 μM), or with ephrin-B2 siRNA or agonistic antibody (described below).
Real-time video microscopy
HSC were seeded in glass-bottom Petri dishes (MatTek) and recorded at 3 min lapse intervals for 20 hours by Zeiss microscope equipped with phase-contrast objective lenses and F-View Soft Imaging System. The Petri dish on the stage was kept in a chamber supplied with 5% CO2 and 37° C. Acquired time-lapse images of HSC were acquired and analyzed with AnalySISD (Olympus Biosystems)9.
siRNA Gene Silencing
siRNA targeting human ephrin-B2 and a scrambled control were obtained from Qiagen (Valencia). Cells were transfected with siRNA using oligofectamine (Invitrogen) as described previously 9. Conditions required for specificity of knock-down with ephrin-B2 with high transfection efficiency was established (Supplementary Figure 1). In some experiments, reconstitution of functional ephrin-B2 signaling was achieved by addition of the agonistic recombinant ephrin-B2 Fc chimera, which activates the cognate EphB4 receptor10 (R and D Biosystems) (2 μg/ml).
Microarray analysis
HSC were incubated with vehicle or PDGF-BB (10 ng/ml) for 48 h. GEArray Q Series Human Angiogenesis Gene Array (HS-009) membrane was used for hybridization with the synthesized probe and detected by the Chemiluminescent detection kit (Super Array Bioscience Corporation) according to the manufacturer’s protocol. Total RNA isolation, probe preparation, and changes in expression were done as we described previously 11.
Quantitative Real time- PCR
Levels of ephrin-B2 or EphB4 mRNA were expressed as fold difference of compound-treated cells compared to vehicle treated cells using conditions and controls identical to what we have described previously 11.
Western blot analysis
HSC were lysed and prepared for Western blot analysis as we have previously described with antibody specifically recognizing ephrin-B2 or β-actin control (Sigma) 11.
Bile duct ligation and imatinib administration in vivo
Liver fibrosis and portal hypertension were induced by bile duct ligation (BDL), a well characterized model of cholestatic fibrosis 4. One week after sham operation or BDL, animals received imatinib or vehicle (normal saline) on a daily basis for four weeks (50 mg/kg body weight by intraperitoneal injection), after which animals were anesthetized for portal pressure measurement and sacrifice. Another series of rats was used to study survival after BDL with or without treatment with imatinib (2 groups: BDL vs. BDL+ imatinib). As in the first series, treatment was started one week after BDL and consisted of daily intraperitoneal injections of imatinib or vehicle at 50 mg/kg body weight. Imatinib used in these studies was derived by purification from commercially purchased tablets with purity of >99% as we have previously described 12. In an additional protocol, rats were administered CCl4 (CCl4; 1 mg/kg body weight) or olive oil vehicle for 6 weeks, after which CCl4 administered animals received one dose of imatinib (50 mg/kg body weight) or vehicle on the day prior to measurement of portal pressure.
Measurement of portal vein pressure
Hemodynamic measurements were performed on anesthetized rats after 4 weeks of imatinib treatment. The abdomen was opened, and a 19 gauge catheter (connected to a TXD 310 pressure transducer) was introduced into the portal vein via cannulation of an ileocolic vein. After pressure measurement, rats were killed by exsanguination and visceral organs were removed, weighed, and liver was aliquotted for snap-freezing in liquid nitrogen for Western blotting, Tissue-Tek (Sakura Finetek) fixation for frozen sectioning or fixed in formaldehyde for histology.
Miles Assay
Male Sprague Dawley rats (approximately 275g) were anesthetized with a single i.p. injection of ketamine/xylazine (100 mg/ml) and a solution of filtered 1% Evan’s blue dye was injected (600 μl) via tail vein. Five minutes later, rats received intradermal injections of 100 μl of saline, VEGF (100 ng), or imatinib (100 mg/kg) and 30 minutes later, rats were killed, and the skin was dissected and removed. Wheals were resected and the Evan’s blue was extracted by incubating the skin samples in 1 ml of formamide at 60°C for 20–24 hrs. The absorbance of the samples was measured spectromorphometrically at 610 nm. Experiments were also performed in mice with genetic deletion of endothelial NO synthase (eNOS) or strain-specific control mice using conditions identical to those described for rats, with appropriate weight adjustments of compounds.
Immunohistochemistry and morphometry
Immunohistochemistry was performed from paraffin embedded and frozen tissue sections. Sinusoidal HSC density was quantified from liver sections stained with HSC selective marker, α-smooth muscle actin (SMA), respectively. All HSC in five random areas were counted for positive cells by light microscopy (200x) under blinded conditions.
Statistical Analysis
Experiments were performed with a minimum of three independent experiments from different HSC preparations. Data are depicted as mean +/− SEM. Comparisons were carried out by Student t or one-way ANOVA test, when comparing more than two sample groups, with * designating statistical significance when p<0.05.
Results
PDGF promotes an angiogenic phenotype of HSC that facilitates vascular tube formation
In liver, HSC are best characterized by their capacity to deposit collagen matrix; other traditional functions frequently ascribed to pericytes in other organ beds, are less defined in the context of HSC, including interaction with EC to form angiogenic vascular networks. To explore this concept, human HSC and human SEC were labeled with complementary vital dyes and then co-cultured on Matrigel. Co-culture of HSC and EC (ratio 1:3) on Matrigel leads to formation of a vascular network, a common in vitro measure of cell angiogenic capacity 9 (Figure 1A, with higher magnification view and Z-stack confocal images in Figure 1B), whereby endothelial cells associate with cellular extensions of HSC and form lumen-like structures in vitro. Additional images are available in Supplementary Figure 2. In contrast, human fibroblasts do not associate with SEC to form vascular networks (Figure 1C), suggesting specificity for HSC-EC interactions. Time lapse video microscopy images depict a time dependent development of vascular tubes over a period of 24 hours (Figure 1D–G). Furthermore, as depicted graphically in Figure 1H; while SEC monocultures (Figure 1H, white bars) show rapid decay of vascular tubes after maximum tube development at 24 hours, SEC tubes formed with HSC co-culture (Figure 1H, black bars) and remained durable, even past 96 hours of culture. Thus, these studies demonstrate that HSC support maintenance of EC-based vascular tube formation through close apposition and interdigitation with EC in the process of vascular tube formation.
Figure 1. HSC promote SEC driven vascular tube formation in vitro.
Human HSC or NIH 3T3 cells, and human SEC were labeled with fluorescent dyes and cocultured in Matrigel. A. Co-cultured HSC (green) and SEC (red) form a vascular tube network (white arrowhead points to a prominent site of SEC-HSC interdigitation, white box is an area depicted as Z stack and zoom in panel B, magnification 250 x). Additional images are also provided in the Supplementary data. B. Top, confocal slices reveal the close apposition of SEC (red) with HSC (green) in the Z-plane. Bottom, higher magnification view shows SEC (red) interdigitating with HSC (green) with a lumen-like structure (white asterisk, magnification 650x). C. Control experiments with human fibroblasts shows no engagement of fibroblasts (green) with tubes formed by SEC only (red) (magnification 250x). D–G. Sequential time lapse images are shown of co-cultured SEC and HSC at 0–24 hours from a single field. Co-culture of HSC (green) and SEC (red) leads to stabilization of EC driven vascular tube formation (magnification 250x), with well developed vascular tube formation by 24 hours. (D; time zero, E; 6h, F; 12h, G; 24h). H. SEC monoculture (white bars) shows decay of vascular tubes after 24 hours. SEC-HSC co-cultures (black bars) formed tubes that were stable over 96 hours (*p<0.05 monoculture vs co-culture; n=3).
Recent work suggests that pericytes may evidence angiogenic capacity; especially in liver which maintain a large and well defined pericyte population co-existent with an EC population that is sparse and maintains a porous fenestrated phenotype 1, 10. Therefore, HSC tube formation was next examined in HSC monocultures. Indeed, PDGF-BB promoted vascular tube formation by HSC in vitro as depicted in representative confocal microscopy images and quantitative image analysis (Figure 2A; videomicroscopy movie showing PDGF induced vascular tube formation at higher magnification is available in online Supplementary Figure 3). Additionally, PDGF driven HSC tube formation was entirely inhibited by pharmacologic inhibition of PDGF-R with imatinib (Figure 2A). Interestingly, imatinib not only inhibited tube formation basally but furthermore, its inhibitory capacity reduced tube formation to below basal levels in the presence or absence of PDGF, suggesting that imatinib may be acting through other pathways downstream from PDGF as well 12, or alternatively that imatinib may be inhibiting endogenous PDGF within the system. Lastly, to test the pathobiologic relevance of the proposed pathways, we next examined the angiogenic capacity of HSC isolated from sham rats and rats after BDL, a well characterized model of hepatic vascular dysfunction 4. HSC isolated from BDL rats evidenced enhanced vascular tube forming capacity as compared to HSC isolated from sham rats (Figure 2B). Similar results were obtained with HSC isolated from rats treated chronically with CCl4 as well (Supplementary Figure 4).
Figure 2. PDGF signaling pathway regulates HSC driven vascular tube formation.
Human HSC or HSC isolated from sham, or BDL animals were labeled with fluorescent dye and analyzed for vascular tube formation in Matrigel in presence of PDGF (10 ng/ml), imatinib (10 μM), or vehicle, using confocal microscopy (magnification 100x) (Panel A) or phase contrast microscopy (Panel B). A. Vascular tubes were quantified from confocal images after 16 hours from human HSC incubated with vehicle, PDGF, imatinib, or both. PDGF promotes vascular tube formation while imatinib inhibits basal and PDGF driven vascular tube formation (*p<0.05; vs vehicle control n=6). B. HSC isolated from rats after BDL evidence increased tube formation compared to HSC from sham animals (upper panel; representative micrographs, 100x; lower panel; graph from 3 independent cell preparations; *p<0.05; sham vs BDL).
Modulation of HSC coverage regulates pressure and permeability in liver injury and portal hypertension
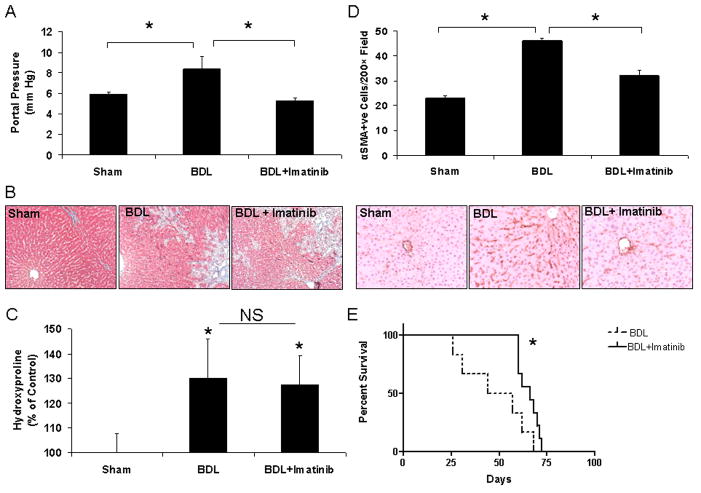
Next, to explore the effects of PDGF on vascular relevant physiologic functions of HSC in vivo, we again utilized the BDL rat model, which evidences portal hypertension. As expected, BDL rats evidenced a significant increase in portal pressure and increased fibrosis in comparison to sham operated animals (Figure 3A and B). In contrast, BDL rats administered imatinib evidenced significantly lower portal pressure measurements compared to BDL rats receiving vehicle. Interestingly this was not accompanied by a reduction of fibrosis in BDL rats receiving imatinib as quantitated by trichrome staining (Figure 3B). This finding was also confirmed by measurement of tissue hydroxyproline levels (Figure 3C). Interestingly, however, imatinib treatment of BDL rats was associated with a significantly reduced level of sinusoidal α-SMA positive HSC as compared to BDL rats that received vehicle (Figure 3D). Lastly, imatinib also conferred a statistically significant improvement in survival in BDL rats (Figure 3E). The ability of imatinib to limit the development of portal hypertension appeared to be mediated through chronic structural changes in the sinusoidal vasculature rather than an acute hemodynamic effect because acute administration of a single dose of imatinib did not reduce portal pressure (Supplementary Figure 5). These studies indicate that HSC evidence a vascular phenotype in vivo that is PDGF-dependent and is associated with enhanced pericyte coverage of sinusoidal vessels and ensuing increase in portal pressure. Furthermore, inhibition of this vascular phenotype of HSC limits the development of portal hypertension in absence of effects on fibrosis.
Figure 3. HSC recruitment to vessels regulates portal pressure.
Rats underwent sham or BDL surgery and 1 week after surgery, received imatinib or vehicle for 4 weeks. Portal pressure was measured and tissues were prepared for further analysis. A. BDL rats evidenced significant increase in portal pressure versus sham animals. BDL rats administered imatinib showed attenuation of portal pressure elevation (*p<0.05; n=6–10). B. Fibrosis, assessed by trichrome stain, is increased in BDL rats compared to sham rats. No difference in fibrosis is detected between BDL rats receiving vehicle and imatinib after 4 weeks (100x). C. Hydroxyproline levels from liver lysates were significantly increased in rats after BDL and not attenuated in animals receiving imatinib (*p<0.05 compared to sham; n=6–10). D. Sinusoidal HSC were quantified after immunohistochemistry for the HSC marker alpha-SMA which revealed increased centrilobular SMA positive HSC in BDL rats, which was attenuated in BDL rats administered imatinib (upper panel; quantification and lower panel; representative micrographs). E. One week after BDL surgery, rats received vehicle or imatinib and survival rate was determined. Administration of imatinib improved survival in BDL rats as compared to administration of vehicle (*p<0.05; BDL vs BDL + imatinib; n=6 and 9 respectively).
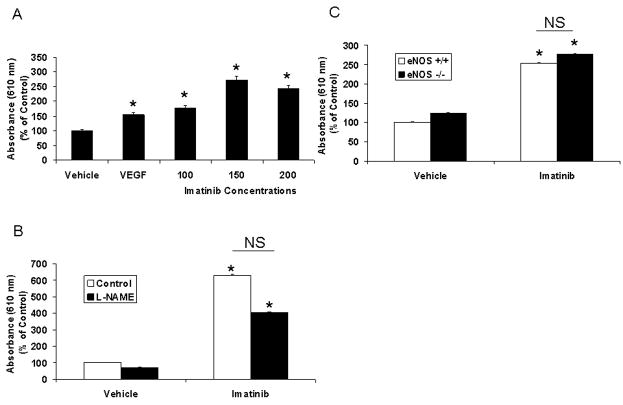
Although amelioration of portal hypertension would be expected to prevent ascites formation, paradoxically, we also observed that imatinib treatment of rats was associated with increased formation of ascites despite reduction in portal pressure. Indeed, all (9/9) imatinib-treated animals evidenced ascites while ascites was not detected in animals from other experimental groups. As a common dose limiting side effect of imatinib in patients is edema and third-spacing of fluid 13, we hypothesized that imatinib may promote a vascular leakage syndrome through disruption of pericyte coverage of vessels. To explore this further, we performed a modified Miles assay to directly measure changes in vascular permeability in response to imatinib in vivo. As seen in Figure 4A, intradermal administration of imatinib, as well as a positive control, VEGF, but not vehicle, increased Evans Blue extravasation in rats, indicative of plasma protein leakage from the micro-vascular space. To verify that plasma leak from vasculature was mediated through pericytes rather than EC in these experiments, we next performed studies in presence of NOS inhibition since eNOS contributes importantly to endothelial cell-based vascular permeability 14. Even in the presence of the NOS inhibitor, L-NAME (N(G)-nitro-L-arginine methyl ester), imatinib increased Evans Blue extravasation (Figure 4B). This result was also confirmed in eNOS −/− mice; the permeability increase in response to imatinib was similar in both eNOS −/− and eNOS +/+ mice (Figure 4C). These studies indicate that a significant portion of the actions of imatinib occurred independent of NO and endothelium and support the concept that disruption of PDGF signaling regulates pericyte vascular functions in vivo.
Figure 4. Inhibition of PDGF signaling disrupts vascular permeability.
eNOS −/− mice, eNOS +/+ mice, or rats, underwent Miles assay for measurement of vascular permeability. A. Intradermal administration of imatinib (0–200 mg/kg/body weight) in rats, increased permeability in a concentration dependent manner compared to vehicle (*p<0.05). VEGF (200 ng/ml) was used as a positive control. B. L-NAME did not significantly correct imatinib (100 mg/kg/body weight) induced increase in permeability in rats (n= 4; *p<0.05 vs respective vehicle). C. Permeability in response to imatinib (150 mg/kg/body weight) was similar in eNOS −/− and +/+ mice (*p<0.05 vs vehicle; n= 3).
PDGF regulation of the HSC capillary-tube formation occurs through the ephrin-B2 system
Prior studies have delineated the role of c-abl in PDGF-dependent signaling in contractile cells 12; however other complementary signaling pathways remain less well defined. In order to explore new targets that may mediate PDGF signaling in pericytes, we first pursued a focused microarray approach by studying gene expression in HSC that were incubated with PDGF or vehicle. Microarray analysis for angiogenesis genes in human HSC revealed that the receptor tyrosine kinase, ephrin-B2 is upregulated by PDGF in comparison to unstimulated HSC (Figure 5A). These observations were confirmed by RT-PCR which revealed significant upregulation of ephrin-B2 mRNA (Figure 5B, white bars) in a concentration-dependent manner in HSC after incubation with PDGF for 20 hours. Conversely, the PDGF receptor antagonist imatinib downregulated ephrin-B2 mRNA levels (Figure 5B, black bars). Interestingly, the ephrin-B2 cognate receptor, EphB4, was also detected as a PDGF target in the microarray analysis and the increase was confirmed by PCR analysis (Supplementary Figure 6).
Figure 5. PDGF upregulates ephrin-B2 in human HSC.
A. Human HSC were incubated with vehicle or PDGF for 48 hours and RNA was isolated for angiogenesis microarray. Upregulation of ephrin-B2 (EFNB2) was observed in response to PDGF as compared to vehicle. GAPDH RNA levels were similar between the two groups. B. Human HSC were incubated for 20 h with vehicle or PDGF (0–10 ng/ml) or imatinib (0–10 μM). Ephrin-B2 transcripts were measured using qRT-PCR. Ephrin-B2 mRNA levels increased and decreased in a concentration dependent manner in response to PDGF (0.1–10 ng/ml) and imatinib (0.1–10 μM), respectively (*p<0.05 vs vehicle; n=3). C. Tube formation was analysed in human HSC transfected with ephrin-B2 siRNA or control siRNA. In some experiments, ephrin-B2 signaling was reconstituted with ephrin-B2 Fc agonistic antibody (2 μg/ml). Ephrin-B2 silencing significantly reduced tube length versus scrambled siRNA; ephrin-B2 Fc rescued tube formation in cells transfected with ephrin-B2 siRNA (*p<0.05 vs vehicle; n=3). D. Ephrin-B2 is upregulated in HSC from BDL rats as compared to sham rats (representative Western blot radiograph; upper panel and densitometric analysis from 3 independent experiments; lower panel). E. Vascular tube formation was analysed in HSC from sham and BDL rats, transfected with ephrin-B2 siRNA or control siRNA. Ephrin-B2 silencing attenuated PDGF induced increase in tube length versus scrambled siRNA in sham rats and attenuated basal and PDGF induced tube formation in HSC from BDL rats (*p<0.05 vs sham with vehicle; n=3).
To delineate if ephrin-B2 can mediate PDGF-dependent angiogenic signaling in HSC, vascular tube formation of human HSC was examined after siRNA silencing of ephrin-B2. Ephrin-B2 siRNA attenuated vascular tube formation of HSC in response to PDGF (Figure 5C). To establish further specificity of effect, ephrin-B2 siRNA transfected cells were incubated with ephrin-B2 Fc agonistic antibody. Ephrin-B2 siRNA inhibition of vascular tube formation was rescued by incubation with ephrin-B2 Fc (Figure 5C). Furthermore, ephrin-B2 Fc also rescued the inhibition of vascular tube formation that occurred in response to imatinib, supporting the concept that ephrin-B2 may signal downstream from PDGF-R (Supplementary Figure 7). Lastly, since HSC were evidenced to be angiogenic in the BDL model of liver injury, we examined ephrin-B2 levels in HSC isolated from sham and BDL rats. Western blot analysis of whole cell lysates of HSC isolated from BDL rats showed upregulation of ephrin-B2 as compared to cells from sham rats (Figure 5D). This was corroborated by tube formation assays in HSC isolated from sham and BDL rats which revealed that ephrin-B2-siRNA attenuated the enhanced tube formation observed in HSC from BDL rats in the presence or absence of PDGF (Figure 5E). Thus, these studies support a role for ephrin-B2 as a downstream mediator of PDGF in the process of HSC driven angiogenesis.
Discussion
While pressure regulation within the sinusoids is highly dependent on vasoregulatory mediators, the ability of the sinusoids to contract requires the generation of a critical mass of HSC that align themselves in an effective manner around the vessel lumen. This process, which we refer to as pathological sinusoidal remodeling, requires the recruitment of “angiogenic” HSC to the vessel wall or activation of local HSC with extension of tentacle-like structures from these cells that encircle the lumen and adjacent EC. This process is evident in the BDL model of liver injury. Furthermore, our work demonstrates that inhibition of PDGF, a key signaling pathway upregulated in chronic liver disease 15, reduces sinusoidal coverage of HSC and lowers pressure in portal hypertensive rats. Since these in vivo effects of PDGF correlate with the ability of PDGF to promote HSC based vascular tube formation in vitro, we hypothesize that the angiogenic capacity of HSC may contribute to pathological sinusoidal remodeling in vivo with ensuing effects on pressure regulation. Indeed, the ability of imatinib to limit the development of portal hypertension in coordination with a reduction in sinusoidal HSC mass, supports this premise. Prior studies buttress the concept observed in our work, that antifibrotic effects of imatinib are rather limited in liver 16, 17, further supporting the idea that the predominant mechanism of action of imatinib in liver may be through regulation of pericyte coverage of sinusoids. This is an important point since one may have predicted that HSC mass would correlate directly with the degree of fibrosis. However, it is becoming increasingly recognized that there are heterogenous subpopulations of HSC 17–19; and thus it is tempting to speculate that while some HSC populations are fibrogenic, others may orient towards vascular functions such as contractility or angiogenesis. Indeed, even within our present study, some of the observed effects may be relevant to not only HSC but also to portal fibroblasts 18. Especially, since both HSC and portal fibroblasts may eventually reach a state of myofibroblast-like activation upon liver injury 18; further work will be needed to fully define the overlapping and distinct aspects of the activation phenotype as opposed to the angiogenic phenotype of these cell types.
Although our work here focuses on pericyte based remodeling of hepatic sinusoids, the broader role of angiogenesis in portal hypertension and cirrhosis has developed into an area of vigorous investigation. While VEGF dependent splanchnic angiogenesis is increasingly recognized to contribute to increased mass of portosystemic collateral circulation characteristic of cirrhosis 20, angiogenesis also occurs within the cirrhotic liver including the development of well developed “scar vessels” that course through fibrotic bands of the cirrhotic liver 1. Indeed, it has been postulated that hepatic angiogenesis may even contribute to fibrosis in liver, akin to the role of angiogenesis in tumor growth 21. Conversely, angiogenesis has also been implicated in the process of vascular repair in response to liver injury suggesting potentially divergent sequelae of hepatic angiogenesis that may have beneficial or detrimental consequences. Thus, the “angiogenic phenotype” of HSC described in the present work may contribute to diverse liver processes beyond the sinusoidal remodeling that is observed in liver in portal hypertension. In this regard, although endothelial cells are the cell type traditionally ascribed for angiogenesis, recruitment of pericytes is an equally essential step for angiogenesis. Especially in liver, where HSC reside in high numbers and in the microenvironment of a discontinuous, fenestrated endothelial layer, HSC are likely to play an important role in angiogenic sprouting at the sinusoidal level in vivo. Indeed, recent studies in combination with the present work, support the concept that inhibition of PDGF may improve portal hypertension, through regulation of angiogenic pathways in multiple vascular beds relevant to the development of portal hypertension and its complications 21, 22.
Emerging studies support the concept that PDGF signals are crucial for angiogenesis and vascular remodelling 6. In liver, PDGF is upregulated in chronic disease and is a potent mitogen for HSC 15. However, despite the important role of PDGF in pericyte mitogenesis and motogenesis, the downstream pathways which mediate these effects remain an area of active investigation. The present study significantly advances our understanding of pericyte/HSC biology and PDGF signaling by identifying ephrin-B2 as a key mediator of long-term PDGF signals that contribute to sinusoidal remodeling in liver. Ephrin-B2 and its cognate receptor, EphB4 are members of a large class of multifunctional receptor tyrosine kinases that are best characterized in the process of repulsion signaling that contributes to axon guidance 23. These observations have been subsequently extended into the vascular biology realm in which ephrin-B2 has been demonstrated to play an essential role in endothelial cell based angiogenesis and vasculogenesis, most likely through regulation by VEGF 23. However, ephrin-B2 expression was recently recognized within pericytes and vascular smooth muscle cells surrounding sites of active angiogenesis as well 23, whereby this pathway is required for assembly of vessel walls 10. While at first it may seem paradoxical that a repulsion molecule may contribute to vascular tube formation, upon further introspection, vascular tube formation clearly requires repulsion signals of equal importance to guidance cues, in order to coordinate formation of a lumen as opposed to a confluent monolayer in culture or disorganized masses of vascular cells in vivo. In the present study, we use complementary approaches including siRNA, to demonstrate a critical role for ephrin-B2 in the process of HSC vascular tube formation driven by PDGF. Furthermore, reconstitution of ephrin-B2 signaling in presence of agonistic antibody supports specificity of effect and implicates EphB4 receptor interactions with ephrin-B2 for signaling requisite for vascular tube formation. Thus, the present work identifies the ephrin class of proteins as key mediators downstream from PDGF in the process of HSC driven vascular tube formation and sinusoidal remodeling in vivo, thereby building on the emerging concept that PDGF-dependent recruitment of pericytes is essential for vascular remodeling that occurs in disease 6.
Interestingly, the PDGFR inhibitor used in this study, imatinib, has been observed to promote fluid third-spacing such as edema during clinical use 13. Our present findings indicate that imatinib does indeed promote vascular permeability through effects on pericyte barrier function. This observation is consistent with the enhanced vascular leak that is observed in mice genetically deficient in PDGF and in mice genetically deficient in ephrin-B2 24, further supporting the key role for these molecules in pericyte recruitment and vascular formation observed in our studies. Although vascular permeability changes may be attributable to endothelial cells or underlying pericytes, imatinib increased permeability, even in the absence of endothelial cells derived NO signaling, suggesting that its ability to promote vascular leak is likely to occur through effects on pericyte coverage of vessels.
Thus, the present study identifies new and important signaling pathways in HSC that govern vascular structure and function in liver. They also identify pathologic sinusoidal remodeling as a potential novel target in the treatment of portal hypertension, a frequent and severe complication of liver cirrhosis. Lastly, we anticipate that the uncovering of new downstream targets of PDGF is likely to be generalizable to other extrahepatic pericyte populations as well.
Supplementary Material
Abbreviations
- BDL
bile duct ligation
- HSC
hepatic stellate cells
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDGF
platelet-derived growth factor
- SEC
sinusoidal endothelial cells
- VEGF
vascular endothelial growth factor
Footnotes
The contributors disclose no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee JS, Semela D, Iredale J, Shah VH. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–25. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 2.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–31. doi: 10.1002/hep.20993. [DOI] [PubMed] [Google Scholar]
- 3.Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–95. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL, Rockey DC, Bissell DM. Hepatic fibrosis 2006: report of the Third AASLD Single Topic Conference. Hepatology. 2007;45:242–9. doi: 10.1002/hep.21459. [DOI] [PubMed] [Google Scholar]
- 6.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 7.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–48. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 8.Perri RE, Langer DA, Chatterjee S, Gibbons SJ, Gadgil J, Cao S, Farrugia G, Shah VH. Defects in cGMP-PKG pathway contribute to impaired NO dependent responses in hepatic stellate cells upon activation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G535–42. doi: 10.1152/ajpgi.00297.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kang-Decker NCS, Chatterjee S, Yao J, Egan J, Semela D, Mukhopadhyay D, Shah V. Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin. J Cell Sci. 2007;120:492–501. doi: 10.1242/jcs.03361. [DOI] [PubMed] [Google Scholar]
- 10.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–73. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Das A, Fernandez Zapico M, Cao S, Yao J, Fiorucci S, Hebbel R, Urrutia R, Shah V. Disruption of an SP2/KLF6 Repression Complex by SHP is Required for Farnesoid X Receptor-Induced Endothelial Cell Migration. J Biol Chem. 2006;281:39105–13. doi: 10.1074/jbc.M607720200. [DOI] [PubMed] [Google Scholar]
- 12.Daniels C, Wilkes M, Edens M, Kottom T, Murphy S, Limper A, Leof E. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, Robak T, Khoroshko N, Masszi T, Skotnicki A, Hellmann A, Zaritsky A, Golenkov A, Radich J, Hughes T, Countouriotis A, Shah N. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–50. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 14.Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci U S A. 2005;102:904–8. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46:1064–74. doi: 10.1016/j.jhep.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Neef M, Ledermann M, Saegesser H, Schneider V, Widmer N, Decosterd LA, Rochat B, Reichen J. Oral imatinib treatment reduces early fibrogenesis but does not prevent progression in the long term. J Hepatol. 2006;44:167–75. doi: 10.1016/j.jhep.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Kinnman N, Goria O, Wendum D, Gendron MC, Rey C, Poupon R, Housset C. Hepatic stellate cell proliferation is an early platelet-derived growth factor-mediated cellular event in rat cholestatic liver injury. Lab Invest. 2001;81:1709–16. doi: 10.1038/labinvest.3780384. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007 doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 19.Magness ST, Bataller R, Yang L, Brenner DA. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology. 2004;40:1151–9. doi: 10.1002/hep.20427. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez M, Vizzutti F, Garcia-Pagan J, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, Friedman SL, Carmeliet P, Jimenez W, Morales-Ruiz M. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–26. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez M, Mejias M, Garcia-Pras E, Mendez R, Garcia-Pagan JC, Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007;46:1208–17. doi: 10.1002/hep.21785. [DOI] [PubMed] [Google Scholar]
- 23.Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA, Jr, Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230:139–50. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- 24.Kuijper S, Turner CJ, Adams RH. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med. 2007;17:145–51. doi: 10.1016/j.tcm.2007.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.